Abstract
Introduction
Skin involvement in patients with psoriatic arthritis (PsA) worsens the severity and burden of disease. Ixekizumab (IXE), a selective interleukin (IL)-17A antagonist, was compared to placebo (PBO) in the SPIRIT-P1 (NCT01695239) and SPIRIT-P2 (NCT02349295) studies in patients with PsA and evidence of plaque psoriasis. This post hoc analysis reports musculoskeletal, skin, and nail outcomes through week 24 in patients from SPIRIT-P1 and SPIRIT-P2, stratified by mild, moderate, or psoriasis at baseline.
Methods
This post hoc analysis pooled patients from SPIRIT-P1 and SPIRIT-P2 who were randomly assigned to PBO or IXE 80 mg every 4 weeks (Q4W) or every 2 weeks (Q2W). Efficacy outcomes were analyzed through week 24 by baseline psoriasis severity, defined by percent body surface area (BSA) affected; mild = BSA < 3%, moderate = 3% ≤ BSA ≤ 10%, severe = BSA > 10%. The primary outcomes assessed were the proportion of patients achieving American College of Rheumatology (ACR)20, ACR50, and ACR70 responses. Secondary outcomes included musculoskeletal, disease activity, skin and nail, and health-related quality-of-life measures.
Results
Similar proportions of patients achieved ACR20/ACR50/ACR70 over time across all severity subgroups and treatment arms. More than one-third of IXE-treated patients achieved ACR20 at week 4, or ACR50 at week 24, with no significant differences according to psoriasis severity at baseline. Disease activity outcomes were similar through week 24 with both IXEQ4W and IXEQ2W, regardless of psoriasis severity at baseline. There were no significant differences over 24 weeks in the proportions of IXE-treated patients with mild, moderate, or severe baseline psoriasis who achieved Minimal Disease Activity (MDA). Across all severity subgroups, IXE demonstrated Psoriasis Area Severity Index 100 response as early as week 4, and approximately one-third of IXE-treated patients achieved total skin clearance at week 24.
Conclusion
IXE demonstrated rapid and consistent efficacy in joint, skin, and nail for patients with PsA, regardless of baseline psoriasis severity.
Trial Registration
SPIRIT-P1 (NCT01695239), SPIRIT-P2 (NCT02349295).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Psoriatic arthritis (PsA) is a chronic inflammatory disease affecting joints with extra-articular manifestations that can involve entheses, nails, and skin. |
Greater skin involvement in PsA corresponds to a more severe overall disease state with worse clinical, patient-reported, and health-related quality-of-life outcomes. |
The objective of this post hoc analysis was to investigate whether the severity of concomitant psoriasis (mild, moderate, or severe) affects the response to Ixekizumab (IXE) treatment by using outcomes through week 24 from the SPIRIT-P1 and SPIRIT-P2 trials. |
What was learned from the study? |
In this post hoc subgroup analysis of SPIRIT-P1 and SPIRIT-P2, IXE showed rapid and consistent efficacy for patients with psoriatic arthritis, regardless of psoriasis severity at baseline. |
While all patients received an initial dose of 160 mg IXE at baseline (week 0), the therapeutic benefit of 80 mg IXE every 4 weeks (IXEQ4W) or every 2 weeks (IXEQ2W) was assessed in patients with PsA and appears to be consistent irrespective of the baseline percent body surface area (BSA) affected by psoriasis. Among the patient outcomes reported, total skin clearance was achieved by one-third of patients treated with IXE by week 24 and a Dermatology Quality of Life Index score of 0 or 1, DLQI (0,1), was achieved by similar proportions of IXE-treated patients, regardless of psoriasis severity. |
Introduction
Psoriatic arthritis (PsA) is a chronic inflammatory disease of the joints and tendons, typically associated with psoriasis of the skin and nails [1]. Approximately 20–30% of patients with psoriasis develop PsA, with the risk of developing PsA highest among those with the most severe psoriasis [2,3,4]. An estimated 75–85% of patients with PsA show signs of cutaneous psoriasis prior to arthritis development, with a delay of up to 13 years reported between the onset of psoriasis and PsA [5,6,7]. As such, dermatologists play a vital role in the early detection of PsA [3]. According to a recent study of patients with PsA from the CorEvitas PsA/Spondyloarthritis Registry, 24% of patients reported no skin involvement (BSA 0%), 44% of patients had a low percent body surface area (BSA; BSA ≤ 3%) affected by psoriasis, and 32% had moderate-to-high skin involvement (BSA > 3%) [8].
Patients with PsA involving both joint and skin symptoms exhibit a more severe overall disease state with worse clinical, patient-reported, and health-related quality-of-life outcomes compared to patients with joint-only involvement [9, 10]. Additionally, patients with PsA and comorbid skin psoriasis experience greater impairments in work productivity and increased healthcare resource utilization compared to patients with joint-only PsA [9, 10]. Skin involvement should thus be considered along with joint involvement when treating patients with PsA. In addition to joint and skin symptoms, fatigue is another important symptom relevant to patients with PsA [11]. Research indicates that approximately 50% of patients with PsA experience moderate-to-severe fatigue [12]. Rheumatologists believe that fatigue results from chronic inflammation affecting both the skin and joints, primarily through the release of cytokines [13, 14]; however, psychological stress associated with PsA-related conditions (nail conditions, joint deformities, etc.) cannot be ruled out [11, 13].
The pro-inflammatory interleukin (IL)-17 cytokines are key regulators of the pathogenesis of both psoriasis and PsA [5, 15, 16]. Ixekizumab (IXE) is a high-affinity monoclonal antibody that selectively targets IL-17A and is approved to treat moderate-to-severe psoriasis, active PsA, ankylosing spondylitis, and non-radiographic axial spondyloarthritis.
In psoriasis, the therapeutic efficacy of IXE compared to other biologics has been demonstrated across numerous head-to-head clinical trials [17,18,19]. The real-world effectiveness of IXE for the treatment of psoriasis has additionally been proven through real-world studies including the Psoriasis Study of Health Outcomes (PSoHO) [20,21,22]. Results from PSoHO showed that anti-IL-17A biologics, including IXE, have greater real-world effectiveness compared to other biologics for the treatment of moderate-to-severe psoriasis, especially among patients with comorbid PsA [21]. Conversely, this same study found that IL-23 p19 inhibitors (guselkumab and risankizumab) were proportionally less effective for the treatment of moderate-to-severe psoriasis among those with PsA compared to those without PsA.
In PsA, the placebo-controlled SPIRIT-P1 and SPIRIT-P2 registration studies respectively established the safety and efficacy of IXE up to 3 years in biologic-naïve and biologic-experienced patients with active PsA and evidence of plaque psoriasis [23,24,25,26]. The SPIRIT-H2H study (NCT03151551) additionally compared the efficacy and safety of IXE versus the tumor necrosis factor (TNF) alpha inhibitor adalimumab (ADA) through 52 weeks in biologic-naïve patients with active PsA and active plaque psoriasis [27, 28]. IXE demonstrated greater efficacy than ADA with respect to simultaneous achievement of American College of Rheumatology (ACR)50 and Psoriasis Area and Severity Index (PASI)100 responses, as well as the individual achievement of PASI75/90/100 responses [27, 28]. Both drugs were comparable across PsA-related domains [27, 28]. A post hoc subgroup analysis of SPIRIT-H2H subsequently established that IXE showed consistent efficacy compared with ADA in patients with PsA both with and without moderate-to-severe psoriasis at baseline [29].
The objective of this post hoc analysis was to evaluate joint, skin, and nail efficacy outcomes for IXE through 24 weeks in patients with mild, moderate, or severe psoriasis at baseline, integrated from SPIRIT-P1 and SPIRIT-P2.
Methods
Study Design and Participants
SPIRIT-P1 and SPIRIT-P2 were both phase 3, 24-week, multicenter, randomized, double-blinded studies that evaluated the efficacy and safety of IXE compared to placebo (PBO) in adults with active PsA and evidence of plaque psoriasis (current or personal history) who were, respectively, biologic-naïve and TNF inhibitor experienced. Each study was followed by long-term evaluation of the efficacy and safety of up to 156 weeks of IXE treatment [24, 26]. Details of the study design and patient eligibility criteria have already been published for SPIRIT-P1 [30] and SPIRIT-P2 [25]. This post hoc analysis pooled patients from the intent-to-treat populations of SPIRIT-P1 and SPIRIT-P2 who were randomly assigned to receive PBO or IXE 80 mg every 4 weeks (Q4W) or every 2 weeks (Q2W). Inadequate responders at week 16 received rescue therapy; patients who were initially randomized to PBO were re-randomized 1:1 to either IXE group, receiving a starting dose of IXE 160 mg at week 16 followed by 80 mg IXEQ4W or IXEQ2W.
SPIRIT-P1 and SPIRIT-P2 were conducted in accordance with Good Clinical Practice, the principles of the Declaration of Helsinki, and local laws and regulations. SPIRIT-P1 was approved by the Western Institutional Review Board (approval #1-838258-1), and SPIRIT-P2 was approved by the Bellberry Human Research Ethics Committee (Application #2015-01-049-AA). For both studies, approval was also obtained from each additional site. All patients in both studies gave written informed consent. The full lists of investigators and sites are provided in the primary manuscript supplements [25, 30].
Outcome Measures
In the current analysis, efficacy was assessed at each visit through week 24 in three subgroups of patients, those with (i) mild, (ii) moderate, or (iii) severe psoriasis at baseline. Psoriasis severity was defined by the percent BSA affected, per joint guidance from the American Academy of Dermatology and the National Psoriasis Foundation; mild = BSA < 3%, moderate = 3% ≤ BSA ≤ 10%, severe = BSA > 10% [31].
The primary musculoskeletal outcomes assessed were the proportions of patients who achieved ACR20, ACR50, and ACR70 responses; these are respectively defined by an improvement of at least 20/50/70% in both swollen and tender joint count and in at least three of five other measures: (i) patient pain assessment, (ii) patient global assessment of disease activity, (iii) physician global assessment of disease activity, (iv) patient physical function assessment, and (v) C-reactive protein level. Other musculoskeletal outcomes assessed were a Leeds Enthesitis Index score of 0 (LEI = 0), and a Leeds Dactylitis Index-Basic score of 0 (LDI-B = 0). Disease activity outcome measures included the achievement of Minimal Disease Activity (MDA) as measured with the Psoriasis Area and Severity Index (MDA-PASI) and Disease Activity in Psoriatic Arthritis Low Disease Activity (DAPSA-LDA; DAPSA ≤ 14). Skin outcomes included the proportions of patients achieving total skin clearance (PASI100), as well as the proportions of patients achieving an improvement in the PASI of at least 90% (PASI90) or 75% (PASI75) compared to baseline. Nail outcomes included the proportions of patients achieving a Nail Psoriasis Severity Index Score of 0 (NAPSI = 0). Quality of life was assessed by a Dermatology Quality of Life Index score of 0 or 1, DLQI (0,1), which is considered to have no effect on a patient’s life [32]. Fatigue was assessed using the Fatigue Severity Numeric Rating Scale (patient-administered single-item 11-point horizontal scale anchored at 1 and 10, with 0 representing “no fatigue” and 10 representing “as bad as you can imagine”).
Statistical Analyses
This post hoc analysis included the pooled intent-to-treat populations from SPIRIT-P1 and SPIRIT-P2 and used treatment (PBO, IXEQ4W, or IXEQ2W) and psoriasis severity at baseline as factors. For continuous variables, missing data were imputed using the modified baseline observation carried forward (mBOCF). For categorical variables, missing data were imputed using the non-responder imputation (NRI) method. Continuous variables are presented as mean with standard deviation, median with interquartile range, whereas categorical variables are reported using absolute numbers and percentages. A Shapiro–Wilk test was used to assess the normality for continuous parameters. For categorical variables, the differences in proportions achieving response between baseline mild (BSA < 3%), moderate (3% ≤ BSA ≤ 10%), and severe (BSA > 10%) psoriasis were assessed using a logistic regression with the mild category as a reference. Analysis of covariance (ANCOVA) was used for continuous variables. Both analyses were adjusted by geographic regions (North America and other), conventional DMARD experience at baseline (naïve, past use, and current use) and the outcome corresponding baseline value. Least squared means with 95% confidence intervals and standard errors were provided to report both the estimations and the change from baseline for each timepoint within each psoriasis severity category (mild, moderate, and severe). The least square means difference was used to compare psoriasis severity groups with the mild category used as a reference. The significance level of the tests was set to 0.05, so any p value < 0.05 was considered significant. No multiplicity testing adjustment was planned for this analysis.
Results
Patient Characteristics
In total, 655 patients were included in the analysis, with 219 patients receiving PBO, 221 patients receiving IXEQ4W, and 215 patients receiving IXEQ2W. At baseline, 253 (38.6%) patients had mild psoriasis (PBO, N = 85; IXEQ4W, N = 80; IXEQ2W, N = 88), 208 (31.8%) patients had moderate psoriasis (PBO, N = 76; IXEQ4W, N = 67; IXEQ2W, N = 65), and 194 (29.6%) patients had severe psoriasis (PBO, N = 58; IXEQ4W, N = 74; IXEQ2W, N = 62).
Table 1 presents the baseline demographics and disease characteristics of patients according to the treatment received and severity of psoriasis at baseline. Patients with severe psoriasis tended to be numerically younger, with numerically higher serum C-reactive protein levels, and higher LDI-B and NAPSI scores. Patients with severe psoriasis also reported DLQI scores more than double those of patients with mild psoriasis.
Efficacy
For all outcome measures described herein, response rates over time were numerically greater with IXE versus PBO, and similar overall with both IXEQ4W and IXEQ2W, regardless of psoriasis severity at baseline.
Musculoskeletal Outcomes
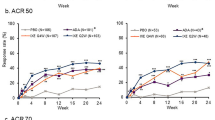
Similar proportions of IXEQ4W- and IXEQ2W-treated patients achieved ACR20/50/70 responses over time across all baseline psoriasis severity subgroups (Fig. 1). Within each treatment arm, there were few statistically significant differences in ACR response between patients with moderate or severe baseline psoriasis versus patients with mild psoriasis (Supplementary Table S1).
More than one-third of patients treated with IXE showed ACR20 response as early as week 4, increasing to about half at week 24 (Fig. 1). Table 2 highlights the proportions of patients achieving ACR20, ACR50 or ACR70 following treatment with IXEQ4W or IXEQ2W vs PBO. The results demonstrate an increase in the proportion of patients achieving these outcomes by week 24. Through weeks 4 to 24, there were no significant differences in ACR20 responses according to baseline psoriasis severity (Supplementary Table S1).
Between approximately 10% and 30% of IXE-treated patients achieved ACR50 as early as week 4 (Fig. 1, Table 2). Through weeks 4 to 24, there was only one statistically significant difference in ACR50 response according to baseline psoriasis severity (Supplementary Table S1); IXEQ2W-treated patients with severe baseline psoriasis had significantly higher ACR50 response rates at week 8 compared to those with mild baseline psoriasis (37% vs. 19%, p = 0.025).
At week 4, proportions of patients achieving ACR70 with IXEQ4W or IXEQ2W vs. PBO treatment were, respectively, 4% or 1% vs. 2% for mild psoriasis, 6% or 3% vs. 1% for moderate psoriasis, and 7% or 10% vs. 2% for severe psoriasis. At week 24, there was a notable increase in the proportion of patients with mild, moderate, or severe psoriasis who achieved an ACR70 response (Table 2). Through weeks 4 to 24, there were no significant differences in ACR70 responses according to baseline psoriasis severity (Supplementary Table S1).
At baseline, more than half the total patient population had enthesitis (LEI > 0). As early as week 4, approximately half of the patient population achieved complete resolution of enthesitis (LEI = 0) and this response was sustained at week 24 for patients treated with IXE, with no significant differences according to baseline psoriasis severity (Supplementary Table S2).
Similarly, approximately one-fifth of the total patient population had dactylitis (LDI-B > 0) at baseline, and around 70–80% of IXE-treated patients achieved dactylitis resolution (LDI-B = 0) at week 24, regardless of psoriasis severity at baseline (Supplementary Table S2).
Disease Activity Outcomes Defined by Composite Indices
Similar proportions of IXEQ4W- and IXEQ2W-treated patients achieved MDA-PASI and DAPSA-LDA through week 24 across all baseline psoriasis severity subgroups (Fig. 2). There were no significant differences over 24 weeks in the proportions of IXE-treated patients with mild, moderate, or severe baseline psoriasis who achieved MDA-PASI (Supplementary Table S3). There were few statistically significant differences in DAPSA-LDA response between patients with severe baseline psoriasis versus patients with mild psoriasis (Supplementary Table S3). These were noted at weeks 2 and 8 (p value for DAPSA-LDA response < 0.05 in patients with severe baseline psoriasis compared to patients with mild baseline psoriasis from the same treatment arm).
Disease activity outcomes defined by composite indices in patients with active PsA based on psoriasis severity at baseline. DAPSA-LDA Disease Activity in Psoriatic Arthritis Low Disease Activity, IXE ixekizumab, MDA Minimal Disease Activity, PASI Psoriasis Area and Severity Index, PBO placebo, PsA psoriatic arthritis, Q2W every 2 weeks, Q4W every 4 weeks, sPGA static Physician’s Global Assessment
At week 4, proportions of patients achieving MDA-PASI with IXEQ4W or IXEQ2W treatment vs. PBO were, respectively, 13% or 14% vs. 5% for mild psoriasis, 12% or 12% vs. 4% for moderate psoriasis, and 7% or 10% vs. 2% for severe psoriasis. At week 24, the proportions of patients achieving MDA response with IXEQ4W or IXEQ2W vs. PBO had increased for those with mild, moderate, or severe psoriasis at baseline (Table 3).
As early as week 4, proportions of patients achieving DAPSA-LDA with IXEQ4W or IXEQ2W vs. PBO treatment were, respectively, 21% or 22% vs. 9% for mild psoriasis, 30% or 15% vs. 12% for moderate psoriasis, and 20% or 21% vs. 14% for severe psoriasis. At week 24, there was a notable increase in the proportion of patients with mild, moderate, or severe psoriasis who achieved DAPSA-LDA (Table 3). Over 24 weeks, there were two statistically significant differences in DAPSA-LDA response according to baseline psoriasis severity (Supplementary Table S3); IXEQ2W-treated patients with severe baseline psoriasis had significantly higher DAPSA-LDA response rates compared to those with mild baseline psoriasis at week 2 (19% vs. 14%, p = 0.022) and week 8 (36% vs. 32%, p = 0.042).
Skin and Nail Outcomes
Across all baseline psoriasis severity subgroups, IXE demonstrated PASI75 response as early as week 4, and approximately one-third of IXE-treated patients achieved total skin clearance at week 24 (Fig. 3). There was overall consistency observed between nail and skin outcomes in patients regardless of baseline psoriasis severity, although there were a few exceptions (Supplementary Table S4).
More than one-third of patients treated with IXE showed PASI75 response as early as week 4, increasing to up to three-quarters of patients at week 24 (Fig. 3). At week 4, proportions of patients achieving PASI75 with IXEQ4W or IXEQ2W vs. PBO treatment were, respectively, 39% or 36% vs. 14% for mild psoriasis, 48% or 46% vs. 5% for moderate psoriasis, and 42% or 42% vs. 5% for severe psoriasis. At week 24, there was a notable increase in the proportion of patients with mild, moderate, or severe psoriasis who achieved PASI75 compared to week 4 (Table 4). Through weeks 4 to 24, there was only one statistically significant difference in PASI75 response according to baseline psoriasis severity (Supplementary Table S4); IXEQ4W-treated patients with moderate psoriasis had significantly higher PASI75 response rates at week 20 compared to those with mild baseline psoriasis (64% vs. 41%, p = 0.026).
Approximately one-fifth of IXE-treated patients achieved PASI90 as early as week 4 (Fig. 3). At week 4, proportions of patients achieving PASI90 with IXEQ4W or IXEQ2W vs. PBO treatment were, respectively, 25% or 23% vs. 13% for mild psoriasis, 22% or 28% vs. 4% for moderate psoriasis, and 19% or 19% vs. 3% for severe psoriasis. At week 24, there was a notable increase in the proportion of patients with mild, moderate, or severe psoriasis who achieved PASI90 compared to week 4 (Table 4). Over 24 weeks, there were no significant differences in PASI90 responses according to baseline psoriasis severity (Supplementary Table S4).
At week 4, proportions of patients achieving PASI100 with IXEQ4W or IXEQ2W vs. PBO treatment were, respectively, 23% or 23% vs. 12% for mild psoriasis, 15% or 12% vs. 4% for moderate psoriasis, and 5% or 7% vs. 2% for severe psoriasis. At week 24, there was a notable increase in the proportion of patients with mild, moderate, or severe psoriasis who achieved PASI100 (Table 4). PASI100 responses were consistent across all baseline psoriasis severity subgroups, although there were a few exceptions (Supplementary Table S4).
At baseline, approximately two-thirds of the total patient population had nail psoriasis (NAPSI > 0). Almost half of IXEQ2W-treated patients and at least one-quarter of IXEQ4W-treated patients achieved NAPSI = 0 at week 24, regardless of psoriasis severity at baseline (Table 5). The proportion of patients achieving NAPSI = 0 was comparable across all baseline psoriasis severity subgroups.
Quality-of-Life Outcomes
The mean baseline DLQI scores were around 4 for mild psoriasis (PBO = 4.7, IXEQ4W = 4.1, IXEQ2W = 3.8), 7 for moderate psoriasis (PBO = 7.3, IXEQ4W = 6.8, IXEQ2W = 8.0), and 10 for severe psoriasis (PBO = 10.3, IXEQ4W = 10.2, IXEQ2W = 10.1). At week 4, patients with moderate and severe psoriasis appeared to achieve DLQI (0,1) responses with IXE vs. PBO (Table 2). Approximately half of all IXE-treated patients achieved DLQI (0,1) at week 24, with no significant differences according to baseline psoriasis severity (Table 2).
Fatigue
Overall, the fatigue NRS scale LSM change from baseline observed in patients receiving IXEQ4W or IXEQ2W revealed a greater numerical reduction on the fatigue scale over 24 weeks of treatment compared to PBO. Consistent patterns of fatigue reduction were observed across the three levels of psoriasis severity (Fig. 4).
Fatigue NRS scale least squares mean change from baseline in patients with active PsA based on psoriasis severity at baseline. Fatigue was assessed using the Fatigue Severity Numeric Rating Scale (a patient-administered, single-item, 11-point, horizontal scale, anchored at 1 and 10, with 0 representing “no fatigue” and 10 representing “as bad as you can imagine”). IXE ixekizumab, NRS Numeric Rating Scale, PBO placebo, Q2W every 2 weeks, Q4W every 4 weeks
Discussion
Although it is known that greater skin involvement in PsA corresponds to a more severe overall disease state with worse clinical, patient-reported, and health-related quality-of-life outcomes [9, 10], few studies have investigated the impact of psoriasis severity on treatment response for patients with PsA [29]. This post hoc analysis demonstrates that IXE provided rapid and consistent therapeutic benefit for patients with PsA and concomitant psoriasis irrespective of whether skin involvement was mild, moderate, or severe. Responses to IXEQ4W and IXEQ2W were observed as early as week 4 and were consistent through 24 weeks across all psoriasis severity subgroups when considering ACR response rates, composite disease activity outcomes (MDA-PASI and DAPSA-LDA), PASI response rates, and health-related quality of life.
It has been shown that both joint and skin symptom improvements are required for patients with PsA to achieve optimal improvements in health-related quality-of-life [9, 33]. The present analysis shows that IXE treatment improved both joint and skin outcomes for patients with PsA, regardless of psoriasis severity at baseline. Moreover, similar proportions of these IXE-treated patients achieved DLQI (0,1) at week 24 across all psoriasis severity subgroups.
Rapid skin improvement is one of the most important treatment goals targeted by patients with psoriasis [34, 35]. At week 4, more than one-third of IXE-treated patients had achieved PASI75 response, and total skin clearance was already achieved by some patients across all baseline psoriasis severity subgroups. Furthermore, total skin clearance was achieved by week 24 in about one-third of patients treated with IXE, whether their baseline psoriasis was mild, moderate, or even severe.
We additionally observed that both dosing regimens of IXE were effective in reducing fatigue in patients across psoriasis severity groups, compared to treatment with PBO, over 24 weeks. Reducing fatigue among the patient population under study has the potential to improve patient well-being and daily function, which may encourage greater compliance with prescribed treatment regimens, fostering better long-term health outcomes [36].
The efficacy of IXEQ4W and IXEQ2W appeared largely similar with respect to joint, disease activity, and skin outcomes, regardless of baseline psoriasis severity. However, a limitation of this post hoc analysis is that it was not powered to compare the efficacy of different IXE dosing regimens by baseline psoriasis severity. We also acknowledge that the protocol-specified, cross-over nature of the study after week 16 may introduce some elements of bias in the analysis.
Compared to the previously published subgroup analysis of SPIRIT-H2H [29], which assessed the efficacy of IXE compared to ADA in patients with and without moderate-to-severe psoriasis (defined by PASI ≥ 12, sPGA ≥ 3, and BSA ≥ 10%), a strength of the current study is that it delineated between mild, moderate, and severe psoriasis. Additionally, while SPIRIT-H2H only included patients with active psoriatic skin lesions and BSA ≥ 3%, the present analysis included patients with evidence plaque psoriasis irrespective of severity or current activity. Therefore, this post hoc analysis represents a wider spectrum of psoriasis presentations among patients with PsA.
The present analysis is limited to the 24-week double-blind treatment period despite the availability of long-term safety and efficacy data from SPIRIT-P1 and SPIRIT-P2 [24, 26]. This is because the analysis of data from week 24 onwards would be complicated by the fact that, at week 24, all patients still receiving PBO were then re-randomized to IXE. In addition, patients initially randomized to PBO who were deemed inadequate responders at week 16 were re-randomized to IXE starting from week 16 onwards. Nevertheless, the data presented here show consistent results between week 16 and week 24.
The SPIRIT-P1 and SPIRIT-P2 trials were the registration studies whose results were the basis for the approved dosing of IXE in adults with PsA. Taken together with the fact that neither SPIRIT-P1 nor SPIRIT-P2 stratified patients by skin severity at baseline, another limitation of the present analysis is that many patients did not receive the subsequently approved dosing of IXE according to the presence or absence of comorbid moderate-to-severe plaque psoriasis; IXEQ4W-treated patients who had PsA without moderate-to-severe psoriasis received the on-label dose regimen through 24 weeks, while IXEQ2W-treated patients who had both PsA and moderate-to-severe psoriasis received on-label treatment through to week 12.
Conclusion
This analysis demonstrated that IXEQ4W and IXEQ2W provided rapid and consistent therapeutic benefit with respect to musculoskeletal outcomes for patients with PsA and plaque psoriasis, irrespective of the baseline percent BSA affected. Both IXE dosing regimens also showed rapid and consistent efficacy across multiple disease activity measures, regardless of psoriasis severity at baseline. In addition, IXE delivered rapid and consistent skin improvements for patients with PsA regardless of whether baseline psoriasis severity was mild, moderate, or severe. Together, these results highlight the efficacy of IXE in improving all PsA-related manifestations in patients with PsA and concomitant psoriasis, regardless of concomitant psoriasis severity.
Data Availability
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available for request 6 months after the indication studied has been approved in the USA and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
References
Peluso R, Iervolino S, Vitiello M, Bruner V, Lupoli G, Di Minno MN. Extra-articular manifestations in psoriatic arthritis patients. Clin Rheumatol. 2015;34(4):745–53.
Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64(Suppl 2):ii14–7.
Gottlieb AB, Merola JF. A clinical perspective on risk factors and signs of subclinical and early psoriatic arthritis among patients with psoriasis. J Dermatolog Treat. 2022;33(4):1907–15.
Merola JF, Tian H, Patil D, et al. Incidence and prevalence of psoriatic arthritis in patients with psoriasis stratified by psoriasis disease severity: retrospective analysis of an electronic health records database in the United States. J Am Acad Dermatol. 2022;86(4):748–57.
de Vlam K, Gottlieb AB, Mease PJ. Current concepts in psoriatic arthritis: pathogenesis and management. Acta Derm Venereol. 2014;94(6):627–34.
Goldenstein-Schainberg C, Favarato MH, Ranza R. Current and relevant concepts in psoriatic arthritis. Rev Bras Reumatol. 2012;52(1):98–106.
Tasçılar K, Aydın SZ, Akar S, et al. Delay between the onset of psoriasis and arthritis in PsA patients from the PsART international cohort. Arthritis Rheumatol. 2019;71:2854.
Mease PJ, Etzel CJ, Huster WJ, et al. Understanding the association between skin involvement and joint activity in patients with psoriatic arthritis: experience from the Corrona Registry. RMD Open. 2019;5(1):e000867.
Tillett W, Merola JF, Thaci D, et al. Disease characteristics and the burden of joint and skin involvement amongst people with psoriatic arthritis: a population survey. Rheumatol Ther. 2020;7(3):617–37.
de Vlam K, Merola JF, Birt JA, et al. Skin involvement in psoriatic arthritis worsens overall disease activity, patient-reported outcomes, and increases healthcare resource utilization: an observational, cross-sectional study. Rheumatol Ther. 2018;5(2):423–36.
Skoie IM, Ternowitz T, Jonsson G, Norheim K, Omdal R. Fatigue in psoriasis: a phenomenon to be explored. Br J Dermatol. 2015;172(5):1196–203.
Husted JA, Tom BD, Schentag CT, Farewell VT, Gladman DD. Occurrence and correlates of fatigue in psoriatic arthritis. Ann Rheum Dis. 2009;68(10):1553–8.
Overman CL, Kool MB, Da Silva JA, Geenen R. The prevalence of severe fatigue in rheumatic diseases: an international study. Clin Rheumatol. 2016;35(2):409–15.
Prins JB, van der Meer JW, Bleijenberg G. Chronic fatigue syndrome. Lancet. 2006;367(9507):346–55.
Suzuki E, Mellins ED, Gershwin ME, Nestle FO, Adamopoulos IE. The IL-23/IL-17 axis in psoriatic arthritis. Autoimmun Rev. 2014;13(4–5):496–502.
Raychaudhuri SK, Saxena A, Raychaudhuri SP. Role of IL-17 in the pathogenesis of psoriatic arthritis and axial spondyloarthritis. Clin Rheumatol. 2015;34(6):1019–23.
Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386(9993):541–51.
Blauvelt A, Leonardi C, Elewski B, et al. A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 24-week efficacy and safety results from a randomized, double-blinded trial. Br J Dermatol. 2021;184(6):1047–58.
Reich K, Pinter A, Lacour JP, et al. Comparison of ixekizumab with ustekinumab in moderate-to-severe psoriasis: 24-week results from IXORA-S, a phase III study. Br J Dermatol. 2017;177(4):1014–23.
Reich A, Reed C, Schuster C, Robert C, Treuer T, Lubrano E. Real-world evidence for ixekizumab in the treatment of psoriasis and psoriatic arthritis: literature review 2016–2021. J Dermatol Treat. 2023;34(1):2160196.
Lynde C, Riedl E, Maul JT, et al. Comparative effectiveness of biologics across subgroups of patients with moderate-to-severe plaque psoriasis: results at week 12 from the PSoHO study in a real-world setting. Adv Ther. 2023;40(3):869–86.
Pinter A, Puig L, Schakel K, et al. Comparative effectiveness of biologics in clinical practice: week 12 primary outcomes from an international observational psoriasis study of health outcomes (PSoHO). J Eur Acad Dermatol Venereol. 2022;36(11):2087–100.
van der Heijde D, Gladman DD, Kishimoto M, et al. Efficacy and safety of ixekizumab in patients with active psoriatic arthritis: 52-week results from a phase III study (SPIRIT-P1). J Rheumatol. 2018;45(3):367–77.
Orbai AM, Gratacos J, Turkiewicz A, et al. Efficacy and safety of ixekizumab in patients with psoriatic arthritis and inadequate response to TNF inhibitors: 3-year follow-up (SPIRIT-P2). Rheumatol Ther. 2021;8(1):199–217.
Nash P, Kirkham B, Okada M, et al. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet. 2017;389(10086):2317–27.
Chandran V, van der Heijde D, Fleischmann RM, et al. Ixekizumab treatment of biologic-naive patients with active psoriatic arthritis: 3-year results from a phase III clinical trial (SPIRIT-P1). Rheumatology (Oxford). 2020;59(10):2774–84.
Mease PJ, Smolen JS, Behrens F, et al. A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naive patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Ann Rheum Dis. 2020;79(1):123–31.
Smolen JS, Mease P, Tahir H, et al. Multicentre, randomised, open-label, parallel-group study evaluating the efficacy and safety of ixekizumab versus adalimumab in patients with psoriatic arthritis naive to biological disease-modifying antirheumatic drug: final results by week 52. Ann Rheum Dis. 2020;79(10):1310–9.
Kristensen LE, Okada M, Tillett W, et al. Ixekizumab demonstrates consistent efficacy versus adalimumab in biologic disease-modifying anti-rheumatic drug-naive psoriatic arthritis patients regardless of psoriasis severity: 52-week post hoc results from SPIRIT-H2H. Rheumatol Ther. 2022;9(1):109–25.
Mease PJ, van der Heijde D, Ritchlin CT, et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis. 2017;76(1):79–87.
Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–72.
Hongbo Y, Thomas CL, Harrison MA, Salek MS, Finlay AY. Translating the science of quality of life into practice: what do dermatology life quality index scores mean? J Invest Dermatol. 2005;125(4):659–64.
Kavanaugh A, Gottlieb A, Morita A, et al. The contribution of joint and skin improvements to the health-related quality of life of patients with psoriatic arthritis: a post hoc analysis of two randomised controlled studies. Ann Rheum Dis. 2019;78(9):1215–9.
Torbica A, Fattore G, Ayala F. Eliciting preferences to inform patient-centred policies: the case of psoriasis. Pharmacoeconomics. 2014;32(2):209–23.
Blome C, Gosau R, Radtke MA, et al. Patient-relevant treatment goals in psoriasis. Arch Dermatol Res. 2016;308(2):69–78.
Gossec L, Walsh JA, Michaud K, et al. Effect of fatigue on health-related quality of life and work productivity in psoriatic arthritis: findings from a real-world survey. J Rheumatol. 2022;49(11):1221–8.
Thanking Patient Participants
Eli Lilly and Company would like to thank the study participants and their caregivers, without whom this work would not be possible.
Medical Writing/Editorial Assistance
The authors wish to acknowledge Orla Deevy, PhD and Lynn Naughton, PhD of Eli Lilly and Company, for project management support and scientific communication expertise.
Authorship
All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Funding
Sponsorship for this work and Rapid Service Fee was funded by Eli Lilly and Company.
Author information
Authors and Affiliations
Contributions
Tarannum Jaleel, Cameron C. Helt, William N. Malatestinic, and Sarah E. Ross contributed to the study conceptualization and/or design. Data analyses were performed by Marcus E. Ngantcha, with contributions from Cameron C. Helt and Alice B. Gottlieb. April W. Armstrong, Tarannum Jaleel, Joseph F. Merola, Alice B. Gottlieb, Saakshi Khattri, Cameron C. Helt, William N. Malatestinic, Sarah E. Ross, Marcus E. Ngantcha, and Kurt de Vlam contributed to the acquisition or interpretation of the data. Marcus Ngantcha contributed to writing the original draft and all authors contributed to critical review and/or revision of the manuscript. All authors read, approved, and are accountable for the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
April W. Armstrong has received grants or contracts from, and served as a consultant, speaker and/or investigator for: AbbVie, Almirall, Arcutis, ASLAN, Beiersdorf, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant, Dermira, Eli Lilly and Company, EPI Health, Incyte, LEO Pharma, UCB, Janssen, Nimbus, Novartis, Ortho Dermatologics, Pfizer, Sun, Sanofi, and Regeneron; has participated on data safety monitoring or advisory boards for: Boehringer Ingelheim and Parexel; and has been elected to the Board of Directors for the American Academy of Dermatology. Tarannum Jaleel has served as a consultant and/or investigator for: Eli Lilly and Company, Novartis, and UCB Pharma; and has received grants from: Dermatology Foundation, NIH K12, Pfizer-SOCS Grant, Skin of Colour Society, and UCB Pharma. Joseph F. Merola is a consultant and/or investigator for: AbbVie, Amgen, Biogen, Bristol Myers Squibb, Dermavant, Eli Lilly and Company, Janssen, LEO Pharma, Novartis, Pfizer, Sanofi Regeneron, Sun Pharma, and UCB Pharma. Alice B. Gottlieb receives research/educational grants from: Highlights Therapeutics, Bristol Myers Squibb, Janssen and UCB Pharma—all of which were paid to Mount Sinai School of Medicine; and has received honoraria as an advisory board member and consultant for: Amgen, AnaptypsBio, Avotres Therapeutics, Boehringer Ingelheim, Bristol Myers Squibb, Dice Therapeutics, Eli Lilly and Company, Highlights Therapeutics, Janssen, Novartis, Sanofi, Teva, UCB, and Xbiotech (stock options for RA). Saakshi Khattri has received grants or contracts from, and/or served as a consultant and/or speaker for: AbbVie, Bristol Myers Squibb, Eli Lilly and Company, Incyte, Janssen, LEO Pharma, Pfizer, Regeneron, Sanofi, Arcutis, Bristol Myers Squibb, Incyte, Takeda, Boehringer Ingelheim, Novartis and UCB Pharma. Cameron C. Helt, William N. Malatestinic, Sarah E. Ross, and Marcus E. Ngantcha are employees and shareholders of Eli Lilly and Company. Kurt de Vlam has received grant and/or research support from: Celgene; and is a consultant for: Celgene, Eli Lilly and Company, Galapagos NV, Novartis, Pfizer, and UCB Pharma.
Ethical Approval
SPIRIT-P1 and SPIRIT-P2 were conducted in accordance with Good Clinical Practice, the principles of the Declaration of Helsinki of 1964 and its later amendments, and local laws and regulations. SPIRIT-P1 was approved by the Western Institutional Review Board (approval #1-838258-1), and SPIRIT-P2 was approved by the Bellberry Human Research Ethics Committee (Application #2015-01-049-AA). For both studies, approval was also obtained from each additional site the full lists of investigators and sites are provided in the primary manuscript supplements [25, 30]. All patients in both studies gave written informed consent to participate in the study.
Additional information
Prior Presentation: Parts of the analysis presented here were presented as a poster at the 2024 Winter Clinical Dermatology Conference—Hawaii (Honolulu, HI, USA).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Armstrong, A.W., Jaleel, T., Merola, J.F. et al. Ixekizumab Demonstrates Rapid and Consistent Efficacy for Patients with Psoriatic Arthritis, Regardless of Psoriasis Severity. Dermatol Ther (Heidelb) 14, 1615–1631 (2024). https://doi.org/10.1007/s13555-024-01188-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-024-01188-y