Abstract
Nail psoriasis is a difficult-to-treat manifestation of psoriatic disease affecting up to 80% of patients with psoriatic arthritis (PsA) and 40–60% of patients with plaque psoriasis (PsO). Ixekizumab (IXE), a high-affinity monoclonal antibody that selectively targets interleukin-17A, is approved for the treatment of patients with PsA and patients with moderate-to-severe PsO. This narrative review aims to summarize nail psoriasis data generated from IXE clinical trials in patients with PsA (SPIRIT-P1, SPIRIT-P2, and SPIRIT-H2H) and/or moderate-to-severe PsO (UNCOVER-1, -2, -3, IXORA-R, IXORA-S, and IXORA-PEDS) with an emphasis on head-to-head clinical trial data. Across numerous trials explored, IXE treatment was associated with greater improvement in resolution of nail disease versus comparators at week 24, results which were maintained up to and beyond week 52. Additionally, patients experienced higher rates of resolution of nail disease versus comparators at week 24 and maintained high levels of resolution up to week 52 and beyond. In both PsA and PsO, IXE demonstrated efficacy in treating nail psoriasis, and therefore may be an effective therapy option. Trial Registration: ClinicalTrials.gov identifier UNCOVER-1 (NCT01474512), UNCOVER-2 (NCT01597245), UNCOVER-3 (NCT01646177), IXORA-PEDS (NCT03073200), IXORA-S (NCT02561806), IXORA-R (NCT03573323), SPIRIT-P1 (NCT01695239), SPIRIT-P2 (NCT02349295), SPIRIT-H2H (NCT03151551).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Nail psoriasis is a predictive factor for the development of psoriatic arthritis in patients with psoriasis and may be the first visible sign of psoriatic joint involvement. |
Ixekizumab, a high-affinity monoclonal antibody that selectively targets interleukin-17A, is approved for the treatment of patients with psoriatic arthritis or moderate-to-severe psoriasis. |
Numerous trials in both PsO and PsA have highlighted the efficacy of ixekizumab in clearing difficult-to-treat areas of psoriasis, including nail psoriasis. |
This narrative review highlights the rapid and sustained efficacy of ixekizumab in the treatment of nail psoriasis in patients with psoriasis and/or psoriatic arthritis. |
Introduction
Plaque psoriasis (PsO) is a chronic, immune-mediated, inflammatory skin condition characterized by erythematous plaques on the skin. Approximately 20–30% of patients with PsO develop psoriatic arthritis (PsA) [1, 2]. PsA is a chronic, progressive, inflammatory disease affecting the musculoskeletal system, skin, and nails. Nail psoriasis is a common and difficult-to-treat manifestation of psoriatic disease affecting 40–60% of patients with PsO and up to 80% of patients with PsA [3,4,5]. Nail psoriasis is a predictive factor for the development of PsA in patients with PsO and may be the first visible sign of psoriatic joint involvement. It is associated with significant physical and psychological burden in affected patients, causing pain, impairment of hand mobility, aesthetic problems, as well as an altered sense of touch [3, 6]. Nail involvement is also associated with greater disease severity and lower quality of life in patients with PsO and/or PsA [3].
Pathogenesis and Clinical Manifestations of Nail Psoriasis
The nail unit comprises the nail plate and the surrounding periungual folds, including the eponychium (proximal fold), the perionychium (lateral fold), and the hyponychium (distal fold). The nail matrix produces the keratinized nail plate while the nail bed attaches the nail plate to the distal phalanx. The hyponychium lies beneath the distal-free edge forming a natural barrier between the nail plate and nail bed [7].
Inflammation of the nail matrix or nail bed results in morphological changes characteristic of nail psoriasis. The nail unit is intimately connected to underlying bone through the enthesis network that is fused with the extensor tendon connecting the distal interphalangeal (DIP) joint [8]. Entheseal inflammation, known as enthesitis, occurs in many sites in patients with PsA, with the Achilles’ tendon and plantar fascia insertions most commonly affected [8]. Nail psoriasis is often associated with enthesitis, and as a result, nail changes often precede the development of inflammation of the DIP joint [9, 10]. The spread of inflammation into the entheseal tissues and DIP joint is considered an early indicator for the presence of PsA and is associated with increased disease severity [11]. There is evidence that sub-clinical enthesopathy in patients with PsO is a risk factor for the development of PsA [12].
Clinical manifestations of nail matrix involvement include pitting, leukonychia (white spots within the nail plate), Beau lines (transverse grooves), red spots in the lunula, and nail crumbling. Clinical manifestations of nail bed involvement include onycholysis (detachment of the nail plate from the nail bed), subungual hyperkeratosis, oil-spot discoloration, salmon patches, and splinter hemorrhages [13].
Diagnosis and Measurement of Nail Psoriasis
The assessment of nail psoriasis is based on the presence or absence of psoriatic changes in the nail. As an important predictor of disease evolution, nail psoriasis must be continuously monitored, even in the absence of skin manifestations [14]. Although nail psoriasis is an important factor in disease severity and progression, it is not evaluated by the most commonly used psoriasis assessment tool, the Psoriasis Area of Severity Index (PASI). As a result, multiple instruments have been developed to assess nail psoriasis. The most widely utilized system for measuring nail psoriasis in clinical trials is the Nail Psoriasis Severity Index (NAPSI) [15]. To calculate the NAPSI score, each nail is divided into quadrants and scored for the absence or the presence (0 or 1) of psoriasis manifestations in the nail bed and matrix [15]. Scores are summed to obtain total NAPSI nail score with a possible range of 0–160 (where a higher score indicates greater severity of nail psoriasis). NAPSI is usually used to assess the fingernails only and the toenails are often excluded (scored 0–80) [16]. For the purposes of this review and to be consistent with previously published reports, moderate-to-severe (“significant”) nail psoriasis is defined as a NAPSI score ≥ 16 with the involvement of at least four nails. A modified version of the NAPSI (mNAPSI) includes assessment of the degree of disease severity for each nail matrix and nail bed feature in the most severely affected nail. The mNAPSI allows a comprehensive assessment of nail psoriasis, but it can be difficult to complete on a routine basis in real-life settings. An alternative measure used to evaluate psoriatic changes is the Physician’s Global Assessment of Fingernail Psoriasis (PGA-F). A PGA-F score ≥ 3 is indicative of moderate-to-severe nail psoriasis. Other nail scores are available to assess clinical severity of nail psoriasis, including the Nail Area Severity score, the composite nail score, and the Nail Assessment in Psoriasis and Psoriatic Arthritis tool, as well as other subtypes of NAPSI [15].
Therapies for Management of Nail Psoriasis
Traditionally, nail psoriasis is slow to resolve due to the naturally slow growth of the nail and difficult to treat as many therapies are ineffective, painful to administer, or have undesirable side effects [17]. It often persists despite control of cutaneous manifestations of psoriasis. Topical treatment of nail psoriasis is challenging due to limited penetration through the nail plate to diseased tissue and poor patient adherence. Although traditional systemic therapies may result in complete skin clearance, nail psoriasis frequently persists. Also, physicians often consider systemic therapies as inappropriate for nail psoriasis, especially in the absence of severe cutaneous psoriasis [18]. Recent emergence of biologics has revolutionized the treatment of nail psoriasis with numerous clinical trials showing improvement in nail psoriasis in patients with PsO and/or PsA following biologic treatment. However, not all biologics have demonstrated the same level of efficacy or speed in resolving nail psoriasis [19,20,21].
Ixekizumab
Ixekizumab (IXE), a high-affinity monoclonal antibody that selectively targets interleukin-17A, is approved for the treatment of adult patients with PsA, non-radiographic axial spondyloarthritis, radiographic axial spondyloarthritis, and pediatric (≥ 6 years old) and adult patients with moderate-to-severe PsO. This narrative review will summarize nail psoriasis data generated from IXE clinical trials in patients with moderate-to-severe PsO (UNCOVER-1, UNCOVER-2, UNCOVER-3, IXORA-R, IXORA-S, and IXORA-PEDS) and patients with PsA (SPIRIT-P1, SPIRIT-P2, and SPIRIT-H2H) (Table 1), highlighting results from head-to-head trials and indirect evidence relating to IXE response rates. All phase 3 studies with ixekizumab data regarding nail psoriasis in either PsO or PsA at the time of this review were included. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Proportion of Patients with Nail Psoriasis and Severity of Nail Disease at Baseline Across IXE Studies
Across all IXE trials in PsO and PsA included in this review, a similar proportion of patients (59–73%) presented with nail psoriasis at baseline, except in IXORA-R and IXORA-PEDS (Table 2). In IXORA-R, a lower proportion of patients had nail psoriasis at baseline and it is important to note that an alternative nail psoriasis assessment tool (PGA-F) was used compared to all other clinical trials included here (Table 1). In IXORA-PEDS, a lower proportion of patients had nail psoriasis at baseline and unlike all other studies reviewed, IXORA-PEDS included only pediatric patients. Considering severity of nail disease, a similar proportion of patients presented with significant nail psoriasis (NAPSI score ≥ 16 with the involvement of at least four nails) across UNCOVER-3, IXORA-S and IXORA-PEDS [22,23,24]. In IXORA-R, 16% of IXE and 12% of guselkumab (GUS) patients had moderate-to-severe nail psoriasis as determined by a PGA-F score ≥ 3 [25]. Finally, an analysis of patients enrolled in SPIRIT-H2H reported that those with PsA and moderate-to-severe comorbid PsO more frequently reported nail psoriasis at baseline compared to those without moderate-to-severe PsO [IXE: 75.5% and adalimumab (ADA): 80.4% versus IXE: 65.8% and ADA: 58.9%] [26, 27].
A similar mean NAPSI score at baseline was reported across trials in PsO and PsA (Table 2). In SPIRIT-H2H, patients with PsA and concomitant moderate-to-severe PsO had numerically higher mean NAPSI score (IXE: 26.1 [standard deviation (SD) 21.6], ADA: 23.3 [SD 18.5]) at baseline compared to those without moderate-to severe PsO (IXE: 18.1 [SD 17.3], ADA: 17.8 [SD 15.4]) [26].
Changes in Nail Psoriasis Scores Over Time
PsO Trials
Early improvement of mean nail NAPSI scores with IXE treatment was noted in several trials in PsO. In the IXORA-S trial, compared to ustekinumab (UST), early mean improvements in NAPSI scores were noted with IXE treatment by week 8 (IXE: − 6.6; 95% confidence interval [CI] − 8.9, − 4.3, and UST: − 2.1; 95% CI − 4.1, − 0.1 [p = 0.002]) [22]. Similarly, in UNCOVER-3 significantly greater mean percent improvements from baseline in NAPSI scores versus comparator, etanercept (ETN), and placebo were reported by week 8 for both IXE doses [29]. In UNCOVER-2, both IXE doses had significant least square mean change from baseline in NAPSI scores versus placebo by week 4 [29, 30]. In IXORA-PEDS, mean change from baseline in NAPSI scores was significantly greater for those receiving IXE than those receiving placebo by week 12, irrespective of baseline severity of nail psoriasis [34] (Fig. 1). In IXORA-R, a significantly greater proportion of patients with moderate-to-severe nail psoriasis (PGA-F score ≥ 3) treated with IXE than those treated with GUS had reached clear nails or minimal nail psoriasis (PGA-F score of 0 or 1 with ≥ 2-point improvement) by week 24 (Table 3) [25].
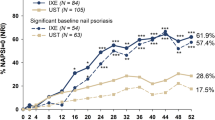
Summary of nail psoriasis data across IXE trials in patients with PsO with concomitant nail psoriasis at baseline. Note: Where available, additional IXE Q2W data are included in supplemental figure 1. aIn IXORA-S and IXORA-R, patients received IXE Q2W for the first 12 weeks of treatment before switching to IXE Q4W. IXE ixekizumab, IXE Q4W ixekizumab every 4 weeks, IXE Q2W ixekizumab every 2 weeks, PsO plaque psoriasis, NP nail psoriasis, ETN etanercept, PBO placebo, UST ustekinumab, GUS guselkumab, NAPSI Nail Psoriasis Severity Index. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 significant versus comparator
NAPSI scores continued to improve up to week 52 in IXORA-S and up to week 108 in IXORA-PEDS (Fig. 1) [22, 38]. In UNCOVER-3, further mean change from baseline improvement in NAPSI scores was reported for patients with baseline and significant baseline (85.3%) nail psoriasis by week 60 (Fig. 1) [24, 29]. Long-term extension demonstrated that these improvements were maintained up to 264 weeks for patients with baseline and significant baseline (87.6%) nail psoriasis (Fig. 1) [24].
Additionally, findings from IXORA-PEDS demonstrated improvements in nail psoriasis of both the nail bed and nail matrix regions with only marginally slower responses observed in the nail matrix compared to the nail bed [23]. In UNCOVER-3, similar findings were reported in adult patients with moderate-to-severe psoriasis [24].
PsA Trials
In SPIRIT-P1, significant improvement in fingernail NAPSI scores with IXE was noted as early as week 12 [35, 37] (Fig. 2). The primary endpoint [proportion of patients achieving American College of Rheumatology (ACR) 20 response] of SPIRIT-P1 and SPIRIT-P2 was evaluated at week 24, and fingernail NAPSI score change from baseline was significantly greater for IXE patients compared to placebo at this timepoint [35, 36]. Likewise, the primary endpoint (proportion of patients simultaneously achieving ACR50 and PASI 100) was measured at week 24 in SPIRIT-H2H, and fingernail NAPSI score change from baseline at this timepoint was significantly greater for IXE compared to ADA.
Summary of nail psoriasis data across IXE trials in patients with PsA with concomitant nail psoriasis at baseline (NAPSI > 0). Note: At 52 weeks, only extension period population nail psoriasis data has been reported, therefore only the extension period population data has been presented at this timepoint. Only IXE Q4W data have been included here. Where available, IXE Q2W data are included in supplemental figure 2. IXE ixekizumab, IXE Q4W ixekizumab every 2 weeks, IXE Q2W ixekizumab every 2 weeks, NP nail psoriasis, PsA psoriatic arthritis, PBO placebo, ADA adalimumab, NAPSI Nail Psoriasis Severity Index. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 significant versus comparator
Further improvement from baseline in nail psoriasis scores was reported at week 52 in SPIRIT-P1, SPIRIT-P2, and SPIRIT-H2H [37, 39, 40] (Fig. 2). Long-term extension of SPIRIT-P1 and SPIRIT-P2 studies demonstrated that improvement in fingernail NAPSI scores is maintained up to 156 weeks in patients with PsA on IXE treatment [41, 42] (Fig. 2). For those with concomitant moderate-to-severe PsO in SPIRIT-H2H, change from baseline was significantly greater at week 40 and similar at week 52 for IXE-treated patients compared to those treated with ADA (IXE: − 21.9 and ADA: − 20.9, p = 0.583) [43].
Resolution of Nail Psoriasis with IXE in PsA and PsO Trials
Due to the significant quality of life burden and impairment of physical functioning associated with nail disease, complete clearance is arguably the most important treatment goal from both the patient’s and clinician’s perspective [4]. Additionally, it has been postulated that complete resolution of nail disease may be important in preventing the future development of PsA [14, 16]. Across all IXE clinical trials included in this review, a high proportion of patients with PsO and/or PsA achieved complete resolution of nail disease irrespective of severity at baseline (Figs. 1 and 2, Table 3).
PsO Trials
Integrated analysis from all three UNCOVER studies in PsO demonstrated rapid benefit of IXE treatment. A significantly greater proportion of patients treated with IXE achieved complete clearance of nail psoriasis compared to ETN at week 12 (IXE every four weeks: 14.8%, IXE every two weeks: 16.6%, ETN: 10.3%, and placebo [PBO]: 4.9% [p < 0.001 versus PBO, p ≤ 0.01 versus ETN]) [28]. In UNCOVER-3, the percentage of IXE-treated patients who achieved complete clearance of nail psoriasis increased from week 12 to week 60 and was maintained up to week 264 (Fig. 1)[44]. Similarly, 59.1 and 65.5% of patients with significant baseline nail psoriasis had achieved complete clearance of nail psoriasis at week 60 and week 264, respectively, demonstrating long-term efficacy of IXE in clearing nail psoriasis [24].
In IXORA-S, significantly more patients treated with IXE achieved complete nail clearance (IXE: 31.0% versus UST: 16.2%; p = 0.02) compared to UST by week 16 and by week 20 (IXE: 25.9% versus UST: 9.5%; p = 0.03) for patients with significant nail psoriasis [22]. By week 52, the majority of patients with baseline nail psoriasis and significant baseline nail psoriasis had achieved complete nail resolution [22] (Fig. 1, Table 3). In IXORA-R, a significantly greater proportion of patients with baseline nail psoriasis and significant baseline nail psoriasis achieved resolution at week 24 compared to GUS [25] (Fig. 1).
A numerically higher proportion of pediatric patients in IXORA-PEDS had achieved complete resolution of nail disease by week 12 with IXE treatment compared with placebo, regardless of baseline nail disease severity, and complete resolution of nail disease was maintained up to week 108 (Fig. 2) [23, 34, 38].
PsA Trials
In PsA trials SPIRIT-P2 and SPIRIT-H2H, a significantly greater proportion of patients had achieved complete resolution of nail disease with IXE treatment compared to placebo or active comparator at week 24 [35, 36, 43] (Fig. 2). In the SPIRIT-P1 study, at week 12, significantly more patients achieved complete nail clearance with IXE compared to placebo [35]. Analysis of SPIRIT-H2H reported that significantly greater numbers of patients with PsA and moderate-to-severe PsO treated with IXE (75.7%) achieved complete resolution of nail disease compared to comparator, ADA (51.2%, p = 0.035) at week 24 [26]. For those patients without moderate-to-severe PsO, the proportion of nail clearance was similar for both IXE- and ADA-treated patients at week 24 (53.9% versus 49.3%, p = 0.480) [26, 27].
By week 52, a similar number of patients across SPIRIT-P1 and SPIRIT-P2 achieved complete resolution of nail disease with IXE treatment (Fig. 2) [39, 40]. In SPIRIT-H2H, patients receiving IXE that achieved complete resolution of nail disease at 52 weeks was not statistically different from those receiving ADA [37] (Fig. 2). A higher percentage of patients with PsA and comorbid moderate-to-severe PsO treated with IXE achieved complete resolution of nail disease by week 52 than those treated with ADA (IXE: 78.4%, ADA: 68.3%; p = 0.444) [26]. Long-term extension studies demonstrated that a high proportion of patients maintain complete nail clearance up to 156 weeks, with continuous IXE treatment [41, 42] (Fig. 2).
Indirect Comparative Data on Nail Psoriasis
Three recent network meta-analyses (NMA) have evaluated the comparative efficacy of approved biologics and small molecules in treating nail psoriasis [19,20,21]. Using a Bayesian NMA, Szebényi et al. reported that in the short-term (10–16 weeks) IXE every four weeks ranked highest for NAPSI percentage improvement, followed by IXE every two weeks [20]. Reich et al. reported the probability for complete resolution of nail disease at week 24–26 in patients with moderate-to-severe PsO and concomitant baseline nail psoriasis, which was highest for IXE (46.5%), followed by brodalumab (37.0%), ADA (28.3%), GUS (27.7%), UST (20.8%), and infliximab (0.8%) [21]. Similarly, Huang et al. reported from an NMA that IXE presented the greatest improvement in nail psoriasis at 24–26 weeks. Additionally, IXE ranked the best amongst five treatments (IXE, GUS, ADA, PBO, and UST) for complete resolution of nail psoriasis [19].
Conclusions
Numerous trials in both PsO and PsA have highlighted the efficacy of IXE in clearing nail PsO, which often precedes the development of PsA and may be the earliest visible sign of psoriatic joint involvement [25, 46, 47]. This review highlights the rapid and sustained efficacy of IXE in the treatment of nail psoriasis in patients with PsO and/or PsA.
References
Mease PJ, Gladman DD, Papp KA, et al. Prevalence of rheumatologist-diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol. 2013;69(5):729–35.
Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80(1):251-65.e19.
Augustin M, Reich K, Blome C, et al. Nail psoriasis in Germany: epidemiology and burden of disease. Br J Dermatol. 2010;163(3):580–5.
Klaassen KM, van de Kerkhof PC, Pasch MC. Nail psoriasis: a questionnaire-based survey. Br J Dermatol. 2013;169(2):314–9.
Sobolewski P, Walecka I, Dopytalska K. Nail involvement in psoriatic arthritis. Reumatologia. 2017;55(3):131–5.
van der Velden HMJ, Klaassen KMG, van de Kerkhof PCM, Pasch MC. The impact of fingernail psoriasis on patients’ health-related and disease-specific quality of life. Dermatology (Basel, Switzerland). 2014;229(2):76–82.
Jiaravuthisan MM, Sasseville D, Vender RB, Murphy F, Muhn CY. Psoriasis of the nail: anatomy, pathology, clinical presentation, and a review of the literature on therapy. J Am Acad Dermatol. 2007;57(1):1–27.
McGonagle D. Enthesitis: an autoinflammatory lesion linking nail and joint involvement in psoriatic disease. J Eur Acad Dermatol Venereol. 2009;23(Suppl 1):9–13.
Scarpa R, Soscia E, Peluso R, et al. Nail and distal interphalangeal joint in psoriatic arthritis. J Rheumatol. 2006;33(7):1315–9.
Ash ZR, Tinazzi I, Gallego CC, et al. Psoriasis patients with nail disease have a greater magnitude of underlying systemic subclinical enthesopathy than those with normal nails. Ann Rheum Dis. 2012;71(4):553–6.
Pennington SR, FitzGerald O. Early origins of psoriatic arthritis: clinical, genetic and molecular biomarkers of progression from psoriasis to psoriatic arthritis. Front Med (Lausanne). 2021;8: 723944.
Zabotti A, Tinazzi I, Aydin SZ, McGonagle D. From psoriasis to psoriatic arthritis: insights from imaging on the transition to psoriatic arthritis and implications for arthritis prevention. Curr Rheumatol Rep. 2020;22(6):24.
Lee DK, Lipner SR. Optimal diagnosis and management of common nail disorders. Ann Med. 2022;54(1):694–712.
Raposo I, Torres T. Nail psoriasis as a predictor of the development of psoriatic arthritis. Actas Dermosifiliogr. 2015;106(6):452–7.
Busard CI, Nolte JYC, Pasch MC, Spuls PI. Reporting of outcomes in randomized controlled trials on nail psoriasis: a systematic review. Br J Dermatol. 2018;178(3):640–9.
Kaeley GS, Eder L, Aydin SZ, Rich P, Bakewell CJ. Nail psoriasis: diagnosis, assessment, treatment options, and unmet clinical needs. J Rheumatol. 2021;48(8):1208–20.
Pasch MC. Nail psoriasis: a review of treatment options. Drugs. 2016;76(6):675–705.
Rigopoulos D, Baran R, Chiheb S, et al. Recommendations for the definition, evaluation, and treatment of nail psoriasis in adult patients with no or mild skin psoriasis: a dermatologist and nail expert group consensus. J Am Acad Dermatol. 2019;81(1):228–40.
Huang IH, Wu PC, Yang TH, et al. Small molecule inhibitors and biologics in treating nail psoriasis: a systematic review and network meta-analysis. J Am Acad Dermatol. 2021;85(1):135–43.
Szebényi J, Gede N, Hegyi PJ, et al. Efficacy of biologics targeting tumour necrosis factor-alpha, interleukin-17 -12/23, -23 and small molecules targeting JAK and PDE4 in the treatment of nail psoriasis: a network meta-analysis. Acta Derm Venereol. 2020;100(18):adv00318.
Reich K, Conrad C, Kristensen LE, et al. Network meta-analysis comparing the efficacy of biologic treatments for achieving complete resolution of nail psoriasis. J Dermatol Treat. 2021;33(3):1652–60.
Wasel N, Thaçi D, French LE, et al. Ixekizumab and ustekinumab efficacy in nail psoriasis in patients with moderate-to-severe psoriasis: 52-week results from a phase 3, head-to-head study (IXORA-S). Dermatol Ther (Heidelb). 2020;10(4):663–70.
Seyger MMB, Reich A, Baou CE, et al. Efficacy of ixekizumab on nail psoriasis in paediatric patients with moderate-to-severe psoriasis: a post hoc analysis from IXORA-PEDS. JEADV. 2021;35:e911–3.
Egeberg A, Kristensen LE, Vender R, et al. Sustained resolution of nail psoriasis through 5 years with ixekizumab: a post hoc analysis from UNCOVER-3. Acta Derm Venereol. 2022;102:adv00787.
Blauvelt A, Leonardi C, Elewski B, et al. A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 24-week efficacy and safety results from a randomized, double-blinded trial. Br J Dermatol. 2021;184(6):1047–58.
Kristensen LE, Okada M, Tillett W, et al. Ixekizumab demonstrates consistent efficacy versus adalimumab in biologic disease-modifying anti-rheumatic drug-naïve psoriatic arthritis patients regardless of psoriasis severity: 52-week post hoc results from SPIRIT-H2H. Rheumatol Ther. 2022;9(1):109–25.
Reich K, Kristensen LE, Smith SD, et al. Efficacy and safety of ixekizumab versus adalimumab in biologic-naïve patients with active psoriatic arthritis and moderate-to-severe psoriasis: 52-week results from the randomized SPIRIT-H2H Trial. Dermatol Pract Concept. 2022;12(2): e2022104.
Papp KA, Leonardi CL, Blauvelt A, et al. Ixekizumab treatment for psoriasis: integrated efficacy analysis of three double-blinded, controlled studies (UNCOVER-1, UNCOVER-2, UNCOVER-3). Br J Dermatol. 2018;178(3):674–81.
van de Kerkhof P, Guenther L, Gottlieb AB, et al. Ixekizumab treatment improves fingernail psoriasis in patients with moderate-to-severe psoriasis: results from the randomized, controlled and open-label phases of UNCOVER-3. J Eur Acad Dermatol Venereol. 2017;31(3):477–82.
van de Kerkhof P, Guenther L, Gottlieb AB, et al. Improvements in fingernail lesions in patients with moderate-to-severe psoriasis treated with ixekizumab versus placebo and etanercept: results from UNCOVER-2. J Am Acad Dermatol. 2016; 74(5): AB255
Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386(9993):541–51.
Ghislain P-D, Conrad C, Dutronc Y, et al. Comparison of ixekizumab and ustekinumab efficacy in the treatment of nail lesions of patients with moderate-to-severe plaque psoriasis: 24-week data from a phase 3 trial [abstract]. Arthritis Rheumatol. 2017;69(suppl 10). https://acrabstracts.org/abstract/comparison-of-ixekizumab-and-ustekinumab-efficacy-in-the-treatment-of-nail-lesions-of-patients-with-moderate-to-severe-plaque-psoriasis-24-week-data-from-a-phase-3-trial/
Blauvelt A, Papp K, Gottlieb A, et al. A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 12-week efficacy, safety and speed of response from a randomized, double-blinded trial. Br J Dermatol. 2020;182(6):1348–58.
Paller AS, Seyger MMB, Alejandro Magariños G, et al. Efficacy and safety of ixekizumab in a phase III, randomized, double-blind, placebo-controlled study in paediatric patients with moderate-to-severe plaque psoriasis (IXORA-PEDS). Br J Dermatol. 2020;183(2):231–41.
Mease PJ, van der Heijde D, Ritchlin CT, et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis. 2017;76(1):79–87.
Nash P, Kirkham B, Okada M, et al. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet. 2017;389(10086):2317–27.
Smolen JS, Mease P, Tahir H, et al. Multicentre, randomised, open-label, parallel-group study evaluating the efficacy and safety of ixekizumab versus adalimumab in patients with psoriatic arthritis naïve to biological disease-modifying antirheumatic drug: final results by week 52. Ann Rheum Dis. 2020;79(10):1310–9.
Paller AS, Seyger MMB, Magariños GA, et al. Long-term efficacy and safety of up to 108 weeks of Ixekizumab in pediatric patients with moderate to severe plaque psoriasis: the IXORA-PEDS randomized clinical trial. JAMA Dermatol. 2022. https://doi.org/10.1001/jamadermatol.2022.0655.
van der Heijde D, Gladman DD, Kishimoto M, et al. Efficacy and safety of ixekizumab in patients with active psoriatic arthritis: 52-week results from a phase III study (SPIRIT-P1). J Rheumatol. 2018;45(3):367–77.
Genovese MC, Combe B, Kremer JM, et al. Safety and efficacy of ixekizumab in patients with PsA and previous inadequate response to TNF inhibitors: week 52 results from SPIRIT-P2. Rheumatology (Oxford). 2018;57(11):2001–11.
Chandran V, van der Heijde D, Fleischmann RM, et al. Ixekizumab treatment of biologic-naïve patients with active psoriatic arthritis: 3-year results from a phase III clinical trial (SPIRIT-P1). Rheumatology (Oxford). 2020;59(10):2774–84.
Orbai AM, Gratacós J, Turkiewicz A, et al. Efficacy and safety of ixekizumab in patients with psoriatic arthritis and inadequate response to TNF inhibitors: 3-year follow-up (SPIRIT-P2). Rheumatol Ther. 2021;8(1):199–217.
Reich K, Kristensen LE, Smith SD, et al. Ixekizumab shows early and sustained resolution of nail psoriasis in patients with psoriatic arthritis and moderate-to-severe psoriasis: 52-week results from a multicentre, randomised, open-label, rater-blinded study (SPIRIT-H2H). European Academy of Dermatology and Venereology Congress. (2020).
Blauvelt A, Lebwohl MG, Mabuchi T, et al. Long-term efficacy and safety of ixekizumab: a 5-year analysis of the UNCOVER-3 randomized controlled trial. J Am Acad Dermatol. 2021;85(2):360–8.
Dennehy EB, Zhang L, Amato D, Goldblum O, Rich P. Ixekizumab is effective in subjects with moderate to severe plaque psoriasis with significant nail involvement: results from UNCOVER 3. J Drugs Dermatol. 2016;15(8):958–61.
Elewski BE, Blauvelt A, Gallo G, et al. Simultaneous nail and skin clearance in ixekizumab head-to-head trials for moderate-to-severe psoriasis and psoriatic arthritis. Dermatol Ther (Heidelb). 2022;12(4):911–20.
Paul C, Griffiths CEM, van de Kerkhof PCM, et al. Ixekizumab provides superior efficacy compared with ustekinumab over 52 weeks of treatment: results from IXORA-S, a phase 3 study. J Am Acad Dermatol. 2019;80(1):70-9.e3.
Acknowledgements
Funding
This study is sponsored by Eli Lilly and Company, Indianapolis, IN, USA. Eli Lilly and Company funded the journal’s Rapid Service Fee.
Medical Writing, Editorial, and Other Assistance
Medical writing assistance in the preparation of this article was provided by Nicola Roe, PhD of Eli Lilly and Company. Support for this assistance was funded by Eli Lilly and Company.
Authorship
All named authors met the International Committee of Medical Journal Editors criteria for authorship, approved the version to be published, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Author Contribution
All authors met the authorship criteria and contributed substantially to the conception and design (Joseph F. Merola, Khai Jing Ng), acquisition of data (Andreas Pinter, Bruce W. Kirkham, Thorsten Holzkaemper), analysis and interpretation of data (Alexander Egeberg, Andreas Pinter, Bruce W. Kirkham, Christopher Schuster, Gaia Gallo, Khai Jing Ng, Luis Puig, Peter Nash, Rebecca Bolce, Thorsten Holzkaemper, Frank Behrens) and drafting and critical revision of the manuscript for intellectual content (Alexander Egeberg, Andreas Pinter, Bruce W. Kirkham, Christopher Schuster, Gaia Gallo, Joseph F. Merola, Khai Jing Ng, Luis Puig, Peter Nash, Rebecca Bolce, Thorsten Holzkaemper, Frank Behrens).
Disclosures
Andreas Pinter has been an investigator and/or speaker and/or advisor for AbbVie, Almirall-Hermal, Amgen, Biogen Idec, Biontec, Boehringer-Ingelheim, Celgene, GSK, Eli Lilly and Company, Galderma, Hexal, Janssen, LEO-Pharma, MC2, Medac, Merck Serono, Mitsubishi, MSD, Novartis, Pascoe, Pfizer, Tigercat Pharma, Regeneron, Roche, Sandoz Biopharmaceuticals, Sanofi-Genzyme, Schering-Plough und UCB Pharma. Frank Behrens has been an adviser and/or received speakers’ honoraria and/or received grants for AbbVie, Pfizer, Roche, Chugai, Prophylix, Novartis, Iron4U, UCB, BMS, Celgene, MSD, Novartis, Biotest, Janssen, Genzyme, Sanofi, Eli Lilly and Company, Sandoz, Boehringer Ingelheim. Alexander Egeberg has received research funding from Pfizer, Eli Lilly and Company, Novartis, AbbVie, Janssen Pharmaceuticals, the Danish National Psoriasis Foundation, and the Kgl Hofbundtmager Aage Bang Foundation, and honoraria as consultant and/or speaker from AbbVie, Almirall, Leo Pharma, Samsung Bioepis Co., Ltd., Pfizer, Eli Lilly and Company, Novartis, Galderma, Dermavant, UCB, Mylan, Bristol-Myers Squibb, Sun Therapeutics, Galápagos NV, Union Therapeutics, and Janssen Pharmaceuticals. Joseph F. Merola is a consultant and/or investigator for Amgen, Bristol-Myers Squibb, AbbVie, Dermavant, Eli Lilly and Company, Novartis, Janssen, UCB, Sanofi-Regeneron, Sun Pharma, Biogen, Pfizer and Leo Pharma. Peter Nash is a consultant for: AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly and Company, Janssen, Novartis, Pfizer, Roche, Sanofi, and UCB, is on the speakers bureau of: AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly and Company, Janssen, Novartis, Pfizer, Roche, Sanofi, and UCB, and has received research funding from: AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly and Company, Janssen, Novartis, Pfizer, Roche, Sanofi, and UCB. Thorsten Holzkämper, Gaia Gallo, Khai Jing Ng, Rebecca Bolce, and Christopher Schuster are employees and shareholders of Eli Lilly and Company. Luis Puig has received consulting fees and/or grants and/or speakers’ Honoraria from AbbVie, Almirall, Amgen, Baxalta, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Fresenius-Kabi, Janssen, JS BIOCAD, Leo-Pharma, Eli Lilly and Company, Mylan, Novartis, Pfizer, Regeneron, Roche, Sandoz, Samsung-Bioepis, Sanofi, UCB. Bruce W. Kirkham. has received speaker’s payments from AbbVie, Eli Lilly and Company, Galapagos, Janssen, Novartis, Pfizer, and UCB. B. W. has received research support from AbbVie, Eli Lilly and Company, and Novartis. B. W. has been an adviser for AbbVie, Eli Lilly and Company, Galapagos, Janssen, Novartis, Pfizer, and UCB.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kirkham, B.W., Egeberg, A., Behrens, F. et al. A Comprehensive Review of Ixekizumab Efficacy in Nail Psoriasis from Clinical Trials for Moderate-to-Severe Psoriasis and Psoriatic Arthritis. Rheumatol Ther 10, 1127–1146 (2023). https://doi.org/10.1007/s40744-023-00553-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-023-00553-1