Abstract
Introduction
Ixekizumab, a selective interleukin-17A antagonist, was compared with adalimumab in the SPIRIT-H2H study (NCT03151551) in patients with psoriatic arthritis (PsA) and concomitant psoriasis. This post hoc analysis reports outcomes to week 52 in patients from SPIRIT-H2H, stratified by baseline psoriasis severity.
Methods
SPIRIT-H2H was a 52-week, multicenter, randomized, open-label, rater-blinded, parallel-group study of biologic disease-modifying antirheumatic drug (DMARD)-naïve patients (N = 566) with PsA and active psoriasis (≥ 3% body surface area involvement). Patients were randomized to ixekizumab or adalimumab (1:1) with stratification by baseline concomitant use of conventional synthetic DMARDs and psoriasis severity (with/without moderate-to-severe psoriasis). Patients received on-label dosing according to psoriasis severity. The primary endpoint was the proportion of patients simultaneously achieving ≥ 50% improvement in American College of Rheumatology criteria (ACR50) and 100% improvement in Psoriasis Area Severity Index (PASI100) at week 24. Secondary endpoints included musculoskeletal, disease activity (defined by composite indices), skin and nail, quality of life and safety outcomes. In this post hoc analysis, primary and secondary endpoints of SPIRIT-H2H were analyzed by baseline psoriasis severity.
Results
A greater proportion of patients achieved the combined endpoint of ACR50 + PASI100 and PASI100 with ixekizumab compared with adalimumab at weeks 24 and 52, regardless of baseline psoriasis severity. ACR response rates were similar for ixekizumab and adalimumab across both patient subgroups. For musculoskeletal outcomes, similar efficacy was seen for ixekizumab and adalimumab, but ixekizumab showed greater responses for skin outcomes regardless of psoriasis severity. The safety profiles of ixekizumab and adalimumab were consistent between subgroups.
Conclusions
Regardless of baseline psoriasis severity, ixekizumab demonstrated greater efficacy than adalimumab with respect to simultaneous achievement of ACR50 + PASI100, and showed consistent and sustained efficacy across PsA-related domains. It also demonstrated higher response rates for skin outcomes. These subgroup analyses highlight the efficacy of ixekizumab in patients with PsA irrespective of the severity of concomitant psoriasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Psoriatic arthritis (PsA) is a chronic inflammatory disease commonly associated with psoriasis, and is treated using disease-modifying antirheumatic drugs (e.g., ixekizumab, an interleukin-17A antagonist, and adalimumab, a tumor necrosis factor-α inhibitor). |
The severity of concomitant psoriasis may influence treatment decision-making in PsA, as there is a belief among some clinicians that interleukin-17 inhibitors are best suited to treating patients with moderate-to-severe psoriasis, being less efficacious in treating mild psoriasis. |
The objective of this study was to establish whether the severity of concomitant psoriasis affects the response to interleukin-17A inhibitor treatment by using 52-week outcomes from the head-to-head SPIRIT-H2H study of ixekizumab versus adalimumab. |
What was learned from the study? |
In this post hoc subgroup analysis of SPIRIT-H2H, in which patients were stratified based on the severity of psoriasis at baseline, ixekizumab showed consistent efficacy compared with adalimumab, regardless of psoriasis severity. |
These findings may help inform clinicians when choosing the optimal treatment for their patients. |
Introduction
Psoriatic arthritis (PsA) is a heterogeneous inflammatory disease, characterized by a combination of musculoskeletal and non-musculoskeletal manifestations, including peripheral and axial arthritis, dactylitis and enthesitis, and skin and nail psoriasis [1, 2]. The detrimental impact of PsA on quality of life (QoL) is driven by the multifaceted nature of the disease [2]. Recent evidence suggests that improvements across both joint and skin manifestations are required to achieve optimal improvements in health-related QoL [3, 4].
According to recent European League Against Rheumatism (EULAR) recommendations, a treat-to-target management approach should be used for PsA, with the aim of achieving remission or low disease activity (LDA) [5]. In the event that these goals cannot be met with the use of conventional disease-modifying antirheumatic drugs (DMARDs), treatment with biologic DMARDs (bDMARDs), such as tumor necrosis factor (TNF)-α inhibitors or interleukin (IL) inhibitors, for example, those targeting IL-17 and IL-12/23, should be initiated [5]. EULAR recommendations have also recently adopted an individual domain-improvement approach to managing PsA, focusing on the treatment of specific disease manifestations [5]. Additionally, in patients with PsA, peripheral arthritis and an inadequate response to at least one conventional synthetic DMARD (csDMARD), the recommendations no longer distinguish between TNFα inhibitors and IL-17 or IL-12/23 inhibitors, although IL-17 or IL12/23 inhibitors may be preferred when there is ‘relevant’ skin involvement (i.e., moderate-to-severe psoriasis). Conversely, IL-12/23 inhibitors may not be as effective in patients with axial involvement, in which case treatment with another bDMARD is recommended [5].
Ixekizumab (IXE), an IL-17A antagonist, is approved for the treatment of moderate-to-severe psoriasis in children (aged ≥ 6 years) and adults, and active PsA and radiographic/non-radiographic axial spondyloarthritis (axSpA) in adults [6]. The SPIRIT-H2H study (NCT03151551) directly compared the efficacy and safety of IXE with those of the TNFα inhibitor adalimumab (ADA) in patients with active PsA [7]. The primary endpoint in SPIRIT-H2H was the proportion of patients simultaneously achieving ≥ 50% improvement in American College of Rheumatology criteria (ACR50) and 100% improvement in Psoriasis Area and Severity Index (PASI100) at week 24; this endpoint was met, with IXE demonstrating superiority over ADA [7]. IXE also demonstrated superiority over ADA in PASI100 and non-inferiority in ACR50 at week 24.
Despite recent updates to treatment recommendations [5], IL-17 inhibitors are still considered by many clinicians to be most useful for treating patients with PsA and high levels of psoriasis involvement. The belief that high levels of skin involvement in PsA are primarily driven by IL-17 [8] may mean clinicians are more likely to recommend other biologics, such as TNFα inhibitors, for the treatment of PsA with mild skin involvement, where inhibition of IL-17 is thought to be less advantageous. Therefore, it is important to establish whether the severity of concomitant psoriasis can affect the response to IL-17A inhibitor treatment. The objective of this post hoc subgroup analysis was to evaluate the impact of baseline psoriasis severity on the relative efficacy and safety of IXE versus ADA through to week 52 in two subgroups of patients from SPIRIT-H2H, those with and those without moderate-to-severe psoriasis at baseline.
Methods
SPIRIT-H2H design
SPIRIT-H2H was a phase IIIb/IV, 52-week, multicenter, randomized, open-label, rater-blinded, parallel-group study conducted in 131 clinical centers in 22 countries (Europe, North and South America, and the rest of the world). The study evaluated the efficacy and safety of IXE versus ADA in adults with active PsA (fulfilling Classification for Psoriatic Arthritis [CASPAR] criteria, and with a tender joint count of ≥ 3 and a swollen joint count of ≥ 3) and concomitant psoriasis (body surface area [BSA] involvement of ≥ 3%), with an inadequate response to at least one csDMARD and who were naïve to bDMARDs [7]. Details of the study design have already been published [7, 9] and are therefore described only briefly here; further details are available online at http://www.clinicaltrials.gov/ct2/show/NCT03151551.
Randomization (1:1) was stratified by concomitant use of csDMARDs and the severity of psoriasis at baseline. In line with European Union labeling [6, 10], patients with moderate-to-severe psoriasis (defined as a PASI score of ≥ 12, a static Physician’s Global Assessment (sPGA) score of ≥ 3, and BSA involvement of ≥ 10%) received either IXE (160 mg loading dose at week 0, then 80 mg every 2 weeks to week 12 then every 4 weeks thereafter) or ADA (80 mg loading dose at week 0, then 40 mg every 2 weeks from week 1). Patients without moderate-to-severe psoriasis received either IXE (160 mg loading dose at week 0, then 80 mg every 4 weeks) or ADA (40 mg at week 0, then 40 mg every 2 weeks).
SPIRIT-H2H was conducted in accordance with ethical principles of the Declaration of Helsinki of 1964 and its later amendments. All patients provided written informed consent, and the study protocol was approved by the National Research Ethics Service Committee London (17/LO/0794) and the ethical review board of each investigative site prior to the start of study-related procedures.
Efficacy and Safety Analyses
The primary efficacy endpoint was superiority of IXE over ADA in the proportion of patients simultaneously achieving ACR50 and PASI100 responses (ACR50 + PASI100) at week 24. Key secondary endpoints were non-inferiority for ACR50 response and superiority for PASI100 response at week 24. Other secondary endpoints were the simultaneous achievement of ACR50 + PASI100, and ACR50 and PASI100 response rates at week 52 [7, 9].
After the week-24 database lock and initial analysis run, a medical inconsistency in baseline PASI data was identified: although nine patients were assessed as PASI = 0 at baseline, those patients fulfilled the criteria for having psoriasis as assessed by a BSA ≥ 3%. Therefore, these patients were judged as PASI100 responders if they achieved an absolute PASI = 0 and BSA = 0 at post-baseline visits.
Additional efficacy outcomes assessed at weeks 24 and 52 were the proportions of patients achieving ACR20, ACR70, and a minimal clinically important difference (MCID) of ≥ 0.35-point improvement from baseline in the Health Assessment Questionnaire-Disability Index (HAQ-DI). Other musculoskeletal outcomes were complete resolution in the Spondyloarthritis Research Consortium of Canada Enthesitis Index (SPARCC enthesitis) in patients with enthesitis at baseline, a Leeds Enthesitis Index score of 0 (LEI = 0) for patients who had enthesitis at baseline, and a Leeds Dactylitis Index–Basic score of 0 (LDI-B = 0) for patients who had dactylitis at baseline. Disease activity outcomes defined by composite indices included the achievement of PsA minimal disease activity (MDA, 18 entheseal points), very low disease activity (VLDA, 18 entheseal points), Disease Activity in Psoriatic Arthritis (DAPSA) LDA or remission, and DAPSA remission. Skin and nail outcomes included a Nail Psoriasis Severity Index score of 0 (NAPSI = 0), assessed in patients with nail psoriasis at baseline, and the proportions of patients achieving PASI75 and PASI90. QoL was assessed by a Dermatology Life Quality Index score of 0 or 1 (DLQI 0,1), and Short Form-36 Physical and Mental Component Summary (SF-36 PCS/MCS) scores. At week 24, all endpoints were prespecified with the exception of VLDA, DAPSA LDA or remission and DAPSA remission, which were post hoc; all endpoints were prespecified at week 52.
The safety of IXE and ADA were assessed as the incidences of treatment-emergent adverse events (TEAEs) and serious adverse events (SAEs). TEAEs were defined as AEs that first occurred or worsened following the first dose of study treatment and on/prior to the last visit within the treatment period. SAEs were defined as AEs resulting in death, initial or prolonged hospitalization, risk of fatality, persistent or significant disability, congenital anomaly or any event considered significant by the investigator.
In the current subgroup analysis, efficacy and safety endpoints at weeks 24 and 52 were assessed in two subgroups of patients, those presenting (subgroup A) or not presenting (subgroup B) with moderate-to-severe psoriasis at baseline.
Statistical Analysis
Continuous variables are presented as mean ± standard deviation, whereas qualitative variables are reported using absolute and relative numbers. The difference in proportion of responders between IXE and ADA was assessed using Fisher’s exact test; p values for treatment comparisons were evaluated at the 0.05 significance level. Logistic regression models with non-responder imputation were performed in the intent-to-treat population (i.e., all patients randomized to treatment at week 0), with treatment and severity of psoriasis at baseline as factors and treatment-by-baseline psoriasis severity as interaction term. Modified baseline-observation-carried-forward (mBOCF) was used for SF-36 PCS and MCS. A treatment-by-subgroup interaction test was used to determine whether there was a differential treatment effect for IXE versus ADA between the two patient subgroups. A significant treatment-by-subgroup interaction p value suggests that the difference in treatment effect between IXE and ADA is statistically significant between patient subgroups (i.e., in patients with moderate-to-severe psoriasis at baseline or patients without moderate-to-severe psoriasis at baseline). Interaction p values were considered statistically significant at the 0.10 level. Safety results were analyzed descriptively.
Results
Patient Characteristics
In total, 566 patients were included in the analysis, with 283 patients receiving IXE and 283 patients receiving ADA; one patient in the ADA treatment arm had a missing psoriasis diagnosis status at baseline. Of these, 49 (17.3%) IXE-treated patients and 51 (18.1%) ADA-treated patients had moderate-to-severe psoriasis at baseline (i.e., subgroup A).
Patient and disease characteristics were generally balanced between treatment arms within each patient subgroup based on baseline psoriasis severity (Table 1). However, there were a number of differences between the two subgroups. In subgroup A (i.e., patients with moderate-to-severe psoriasis), a higher proportion of patients were male compared with subgroup B (i.e., patients without moderate-to-severe psoriasis). Patients in subgroup A tended to have higher tender and swollen join counts, higher mean levels of C-reactive protein, higher HAQ-DI, PASI, NAPSI, sPGA, and DLQI mean scores, and greater BSA involvement at baseline than those from subgroup B. Additionally, more patients from subgroup A in the ADA treatment arm had dactylitis at baseline. A greater proportion of patients in subgroup B (vs. those in subgroup A) received concomitant csDMARDs (72.5 vs. 54.0%), with 60.6 and 53.0% of patients, respectively, receiving methotrexate; additional analyses of methotrexate use in subgroups A and B are provided in Supplementary Tables S1 and S2.
Efficacy
Simultaneous Achievement of ACR50 and PASI100
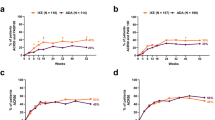
A significantly greater proportion of IXE-treated patients achieved ACR50 + PASI100 at week 24 (primary endpoint) in subgroup A (40.8% for IXE vs. 17.6% for ADA, p = 0.015) but not in subgroup B (35.0 vs. 30.3%, respectively; p = 0.279), while significant differences for IXE versus ADA were seen at week 52 (subgroup A: 38.8 vs. 17.6%, p = 0.026; subgroup B: 39.3 vs. 28.1%, p = 0.014) (Fig. 1). The efficacy of IXE was consistent from week 24 to week 52 within the two subgroups.
Musculoskeletal Outcomes
Similar proportions of patients achieved ACR20/ACR50/ACR70 over time with both IXE and ADA, regardless of psoriasis severity, and response rates were stable overtime (Fig. 2).
At week 24, a significantly greater proportion of IXE- than ADA-treated patients in subgroup B achieved SPARCC resolution of enthesitis and the response was maintained through to 52 weeks (Table 2). In patients in subgroup A, a numerically greater proportion of ADA- than IXE-treated patients achieved resolution of SPARCC enthesitis at weeks 24 and 52, but the difference between treatments was not significant. A similar pattern of response was seen for LEI (Table 2). Both IXE and ADA demonstrated high rates of dactylitis resolution (LDI-B = 0) in both patient subgroups, with no statistical differences between treatments or subgroups. The proportions of patients who achieved HAQ-DI MCID ≥ 0.35 were similar in patients treated with IXE or ADA at weeks 24 and 52.
Disease Activity Outcomes Defined by Composite Indices
At week 24 in subgroup A, a greater proportion of IXE- than ADA-treated patients achieved MDA, VLDA, DAPSA LDA/remission and DAPSA remission, and the difference between IXE and ADA for VLDA achievement was statistically significant (32.7 vs. 9.8%, p = 0.007) (Fig. 3).
Response rates were maintained at week 52, but there were no statistically significant differences between IXE and ADA. In subgroup B at week 24, similar proportions of patients achieved VLDA and DAPSA LDA/remission with IXE and ADA. Additionally, numerically more patients achieved MDA and DAPSA remission with IXE versus ADA, and the difference in MDA achievement was statistically significant (45.7 vs. 34.6%, p = 0.018). Response rates were maintained or improved at week 52, but no statistically significant differences between treatments were observed.
Skin and Nail Outcomes
A significantly greater proportion of patients achieved PASI100 with IXE compared with ADA as early as week 4, and the response was maintained through to week 52 in both subgroups (Fig. 4). For ADA, a lower PASI100 response rate was observed in subgroup A, with a response rate of 0–27.5% throughout the course of treatment. PASI90 response rates were significantly higher with IXE than with ADA in both subgroups at all timepoints, except at week 32 in subgroup A, and response rates were maintained through to week 52 (subgroup A: 81.6% for IXE vs. 60.8% for ADA; p = 0.028; subgroup B: 70.9 vs. 52.8%, respectively; p < 0.001). A significantly greater proportion of IXE-treated patients achieved PASI75 compared to ADA in subgroup B at all timepoints (week 52: 76.9 vs. 66.2%, respectively; p = 0.013), while similar PASI75 responses were seen for IXE and ADA from weeks 24 to 52 in subgroup A (week 52: 85.7 vs. 80.4%, respectively; p = 0.597). Similar to PASI100 response, significant improvements in PASI75 and PASI90 response rates were seen as early as week 4 with IXE compared with ADA, regardless of baseline psoriasis severity.
A significantly greater proportion of IXE- than ADA-treated patients achieved complete resolution of nail psoriasis in subgroup A at week 24 (75.7 vs. 51.2%, p = 0.035), and the response with IXE was maintained at week 52 (78.4 vs. 68.3%, p = 0.444) (Table 2). In subgroup B, similar proportions of IXE- and ADA-treated patients showed resolution of nail psoriasis at week 24 (53.9 vs. 49.3%, p = 0.480), and these responses were maintained at week 52 (64.9 vs. 55.9%, p = 0.119).
Quality of Life Outcomes
In subgroup A, the proportion of patients achieving DLQI (0,1) was significantly higher for IXE than for ADA in subgroup A at week 24 (59.2 vs. 33.3%, p = 0.016) and the response rate was maintained at week 52 (Table 2). In subgroup A, a numerically greater mean change from baseline in SF-36 MCS was seen with IXE than with ADA at weeks 24 and maintained at week 52, although the difference was not significant at either timepoint (Table 2). In the same patient subgroup, SF-36 PCS mean change from baseline was similar between IXE and ADA at both times. In subgroup B, mean changes from baseline in SF-36 PCS and MCS were similar between IXE and ADA at weeks 24 and maintained at week 52.
Differential Treatment Effects Between Subgroups A and B
Differential treatment effects between subgroups A and B were statistically significant at week 24 for ACR50 + PASI100, resolution of SPARCC enthesitis, VLDA, PASI100, and DLQI (0,1) (treatment-by-subgroup interaction p ≤ 0.1 for all). For example, at week 24, the difference between IXE and ADA in ACR50 + PASI100 response rates was 23.2% in subgroup A, while the difference between IXE and ADA in subgroup B was 4.7%; the different treatment effect between subgroups A and B was significant (treatment-by-subgroup interaction p = 0.061; statistical significance for interaction p values was set at 0.10). At week 52, no statistically significant differential treatment effects were observed for any outcome.
Safety
The incidence of TEAEs was similar for IXE and ADA in subgroup A (59 vs. 59%) and subgroup B (77 vs. 71%). In subgroup A, fewer SAEs were observed with IXE than with ADA (0 vs. 10%) and fewer IXE- than ADA-treated patients discontinued the study because of AEs (2 vs. 8%). Similar trends were observed in subgroup B with regards to both the incidence of SAEs (5 vs. 13%) and discontinuations due to AEs (5 vs. 7%).
Discussion
It has previously been shown that optimal health-related QoL as an ultimate treatment goal in PsA requires improvements in both joint and skin manifestations [3]. Although IXE demonstrated significantly higher ACR50 + PASI100 response rates than ADA and similar ACR50 response rates from week 8 to 52 of the SPIRIT-H2H trial [7, 9], the simultaneous achievement of ACR50 + PASI100 signaled additional benefits beyond skin improvements alone. In a post hoc analysis of SPIRIT-H2H, patients who simultaneously achieved ACR50 + PASI100 were more likely to achieve ACR70, MDA or DAPSA remission, enthesitis and dactylitis resolution, and greater improvements in health-related QoL, than those who did not achieve this combined endpoint [11].
This analysis shows that responses to IXE vs. ADA were consistent through to 52 weeks in both subgroups of patients with PsA when considering the combined endpoint of ACR50 + PASI100, ACR response rates, composite outcomes (e.g., MDA, VLDA, and DAPSA), resolution of enthesitis or dactylitis, and PASI response rates. In these patients with polyarthritis, the efficacies of IXE and ADA with respect to joint outcomes, as assessed by ACR 20/50/70 responses, were similar in both patient subgroups and showed consistent improvement through to week 52. Remission status, reflected by DAPSA remission, was also similar between IXE and ADA through to week 52 in both patient subgroups. A statistically significant difference in VLDA response between IXE and ADA at week 24 was seen in subgroup A that may be explained by the greater efficacy of IXE in the treatment of psoriasis at week 24.
At week 24, IXE demonstrated a significantly greater enthesitis resolution rate than ADA as assessed by SPARCC enthesitis score in subgroup B; this difference in response was maintained at week 52. Resolution of enthesitis is an important treatment target in patients with PsA, since enthesitis may contribute to worsening of physical function and QoL [12].
The PASI100 response and nail resolution patterns were generally consistent with DLQI (0,1) findings in subgroup A. Improvements in skin and nail outcomes translated into DLQI improvements, with the difference between treatments more apparent in patients with moderate-to-severe psoriasis (i.e., subgroup A, in whom nail psoriasis affected 75.5–80.4% of patients across both treatments at baseline). Note that the definition for moderate-to-severe psoriasis (PASI ≥ 12, sPGA ≥ 3 and BSA ≥ 10%) used in this study is more stringent than that currently recommended in updated EULAR 2019 guidelines (BSA > 10% or important to the patient) [5].
IXE and ADA performed similarly in the two subgroups over time with respect to QoL and functional improvements, assessed with the SF-36 PCS and HAQ-DI, respectively. This comparable performance was likely driven by similar improvements in musculoskeletal disease activity. Physical function and HR-QoL improvements are an important treatment target in patients with PsA [13].
Apart from selected endpoints at week 24 (ACR50 + PASI100, resolution of SPARCC enthesitis, VLDA, PASI100, NAPSI = 0 and DLQI (0,1)), the results of this analysis support the hypothesis that there is little to no differential treatment effect for IXE versus ADA between patients with and without moderate-to-severe psoriasis at baseline.
Analyses showed that the safety of IXE was consistent across subgroups, and the by-subgroup analyses revealed no new safety signals, confirming the safety profile already established with this agent [7, 9].
SPIRIT-H2H is the first study to compare the efficacy of two bDMARDs in patients with PsA and active psoriasis, with randomization stratified by baseline psoriasis severity. Another strength of the SPIRIT-H2H study was the use of optimal dosing for IXE and ADA based on the severity of concomitant psoriasis. Per protocol and label, patients with moderate-to-severe psoriasis initially received the optimum dosing regimen (IXE: 160 mg loading dose at week 0, then 80 mg every 2 weeks to week 12 then every 4 weeks thereafter; ADA: 80 mg loading dose at week 0, then 40 mg every 2 weeks from week 1), while patients without moderate-to-severe psoriasis received the doses approved for PsA (IXE: 160 mg loading dose at week 0, then 80 mg every 4 weeks; ADA: 40 mg at week 0, then 40 mg every 2 weeks). Despite this dosing difference, the efficacy of IXE with respect to disease activity and QoL was similar in the two psoriasis severity subgroups. Findings were not as consistent between subgroups for ADA. In addition, patients in SPIRIT-H2H were permitted to continue with concomitant csDMARDs if necessary. This may reflect a real-life clinical setting more closely than some other clinical trials.
The open-label study design of SPIRIT-H2H could be considered a limitation, as it may contribute to assessment bias. To minimize bias, key outcomes were measured by blinded assessors. A further limitation could be the stringent definition used for moderate-to-severe psoriasis. In addition, the current analysis is an exploratory analysis, and some of the results may warrant further investigation.
Conclusions
IXE demonstrated greater efficacy than ADA with respect to simultaneous achievement of ACR50 + PASI100 in patients with and without moderate-to-severe psoriasis. IXE also showed consistent and sustained efficacy across both subgroups, whereas ADA showed less consistent efficacy between the two patient subgroups, with efficacy appearing greater in those without moderate-to-severe psoriasis. IXE also performed as well as or better than ADA across multiple disease activity, musculoskeletal and non-musculoskeletal outcome measures, regardless of baseline psoriasis severity. The efficacy of IXE with respect to all PsA manifestations was consistent over time and TEAEs were similar in terms of nature and frequency in both patient subgroups. The efficacy and safety results from this post hoc analysis were in line with findings from the overall SPIRIT-H2H population and highlight the efficacy of IXE in improving all PsA-related manifestations in patients with PsA and concomitant psoriasis, regardless of concomitant psoriasis severity.
References
McArdle A, Pennington S, FitzGerald O. Clinical features of psoriatic arthritis: a comprehensive review of unmet clinical needs. Clin Rev Allergy Immunol. 2018;55(3):271–94.
Holland R, Højgaard P, Tillett W, et al. Evidence for Psoriatic Arthritis Impact of Disease (PsAID12) as core instrument to measure health-related quality of life in psoriatic arthritis: a systematic review of psychometric properties. J Psoriasis Psoriatic Arthritis. 2020;5(1):12–22.
Kavanaugh A, Gottlieb A, Morita A, et al. The contribution of joint and skin improvements to the health-related quality of life of patients with psoriatic arthritis: a post hoc analysis of two randomised controlled studies. Ann Rheum Dis. 2019;78(9):1215–9.
Tillett W, Merola JF, Thaçi D, et al. Disease characteristics and the burden of joint and skin involvement amongst people with psoriatic arthritis: a population survey. Rheumatol Ther. 2020;7(3):617–37. https://doi.org/10.1007/s40744-020-00221-8.
Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis. 2020;79(6):700–12.
European Medicines Agency. Ixekizumab (Taltz): summary of product characteristics 2020. https://www.ema.europa.eu/en/documents/product-information/taltz-epar-product-information_en.pdf. Accessed 29 July 2020.
Mease P, Smolen J, Behrens F, et al. A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naïve patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Ann Rheum Dis. 2020;79(1):123–31.
Hawkes JE, Yan BY, Chan TC, et al. Discovery of the IL-23/IL-17 signaling pathway and the treatment of psoriasis. J Immunol. 2018;201(6):1605–13.
Smolen JS, Mease P, Tahir H, et al. Multicentre, randomised, open-label, parallel-group study evaluating the efficacy and safety of ixekizumab versus adalimumab in patients with psoriatic arthritis naïve to biological disease-modifying antirheumatic drug: final results by week 52. Ann Rheum Dis. 2020;79(10):1310–9. https://doi.org/10.1136/annrheumdis-2020-217372.
European Medicines Agency. Adalimumab (Humira): summary of product characteristics 2020. https://www.ema.europa.eu/en/documents/product-information/humira-epar-product-information_en.pdf. Accessed 29 July 2020.
Behrens F, Liu Leage S, Sapin C, et al. Measuring treatment effect on psoriatic arthritis-related domains: insights from the SPIRIT-H2H study at weeks 24 and 52. Clin Rheumatol. 2021. https://doi.org/10.1007/s10067-021-05891-5 ((Epub ahead of print)).
Mease P. Enthesitis in psoriatic arthritis (Part 3): clinical assessment and management. Rheumatology (Oxford). 2020;59(Suppl 1):i21–8.
Haugeberg G, Michelsen B, Kavanaugh A. Impact of skin, musculoskeletal and psychosocial aspects on quality of life in psoriatic arthritis patients: a cross-sectional study of outpatient clinic patients in the biologic treatment era. RMD Open. 2020;6(1):e001223.
Acknowledgements
None
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Eli Lilly and Company, Indianapolis, IN, USA.
Medical Writing
The authors would like to acknowledge Dr. Elinor Wylde and Dr. Sue Chambers (Rx Communications, Mold, UK) for medical writing assistance with the preparation of this manuscript, funded by Eli Lilly and Company.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Lars-Erik Kristensen made substantial contributions to the acquisition and interpretation of data, and critical revision of the manuscript. Masato Okada made substantial contributions to the interpretation of data and critical revision of the manuscript. William Tillett made substantial contributions to the interpretation of data and critical revision of the manuscript. Soyi Liu Leage made substantial contributions to conception and design of the work, the analysis and interpretation of data, and drafting and critical revision of the manuscript. Celine El Baou made substantial contributions to the design of the work, the analysis and interpretation of data, and critical revision of the manuscript. Christophe Sapin made substantial contributions to the design of the work, the analysis and interpretation of data, and critical revision of the manuscript. Andrew Bradley made substantial contributions to the analysis and interpretation of data, and critical revision of the manuscript. Gabriella Meszaros made substantial contributions to conception and design of the work, the analysis and interpretation of data, and drafting and critical revision of the manuscript. Jan Dutz made substantial contributions to the interpretation of data and critical revision of the manuscript. Kurt de Vlam made substantial contributions to the acquisition and interpretation of data, and critical revision of the manuscript.
List of Investigators
The authors would like to acknowledge the investigators and participants in the SPIRIT-H2H study. The SPIRIT-H2H investigators were: Leonardo Naftal, Rodolfo Ariel Pardo Hidalgo, Eduardo Mario Kerzberg, Veronica Gabriela Savio, Alicia Lazaro, Benito Jorge Velasco, Norma Beatriz Verzero, Cecilia Adma Asnal, Eduardo Fabian Mysler, Alberto Berman, Federico Javier Ariel, Maureen Rischmueller, Jane Margaret Zochling, Paul A Bird, Stephen Hall, Andrew Ostor, Evange Romas, Georg Stummvoll, Klaus Machold, Peter Spellitz, Ursula Hanusch, Marc Vanden Berghe, Marc Leon, Johan Louis Magda Vanhoof, Filip Eduard Jeanne Van den Bosch, Kurt Leo Francois De Vlam, Frederic Morin, Louis Bessette, Derek A Haaland, Annette Margrethe Schlemmer, Lars Erik Kristensen, Pentti Jarvinen, Ritva Liisa Peltomaa, Laura Pirila, Jorma Vuotila, Eric Lespessailles, Philippe Goupille, Bernard Combe, Arnaud Constantin, Gregoire Cormier, Celine Cozic, Cyrille B Confavreux, Hendrik Schulze-Koops, Andrea Everding, Micheala Kohm, Siegfried Wassenberg, Magdolna Nagy, Noemi Bakos, Eva Melegh, Edit Drescher, Lalit Duggal, Piyush Narayanbhai Joshi, Srabani Ghosh Zoha, A Ramakrishnam Naidu, Puja Pushkarkumar Srivastava, Vishnu Devkinandan Sharma, Ajit Bapurao Nalawadae, Jyotsna Laxmikant Oak, Sarath Chandra Mouli Veeravalli, Jugal Kishore Kadel, Manisha Ashwin Daware, Yogesh S Marfatia, Sumeet Agarwal, Yolanda Braun Moscovici, Ori Elkayam, Yair Molad, Tatiana Reitblat, Moshe Tishler, Yair Levy, Merav Lidar, Devy Zisman, Antonio Costanzo, Paolo Amerio, Riccardo Meliconi, Rosario Foti, Roberto Perricone, Maurizio Rossini, Elisa Gremese, Beatriz Elena Zazueta Montiel, Juan Manuel Miranda Limon, Marco Antonio Maradiaga Cecena, Sandra Araceli Sicsik Ayala, Miguel Angel Alvarez Guerrero, Jorge Rojas Serrano, Efren Antonio Canul Novelo, Francisco Fidencio Cons-Molina, Ed N Griep, Andrzej Kaszuba, Jolanta Bogna Węgłowska, Barbara Bazela, Maria Rell-Bakalarska, Malgorzata Miakisz, Catherine Elizabeth Spargo, Elsa Margaretha Van Duuren, Asokan Naidoo, Nomawethu Cleopatra Matsiliza, Johannes Breedt, Gareth Scott Tarr, Jenny Potts, Savithree Nayiager, Mahmood Moosa Tar Mahomed Ally, Francisco Javier Blanco Garcia, Antonio Mera Varela, Jose Rosas, Juan Miguel Sanchez Burson, Javier Garcia Miguel, Jon Lampa, Milad Rizk, Giovanni Cagnotto, Per Larsson, Guozhong Fei, Ruediger Mueller-Mar, Andrea Rubbert-Roth, Michael John Nissen, Oleh Iaremenko, Svitlana Anatoliivna Trypilka, Dmytro Rekalov, Mykola Stanislavchuk, Viktoriia Viktorivna Vasylets, Olena Garmish, Svitlana Smiyan, David Walker, Deepak Jadon, Stuart Ralston, Stephen Boyle, Pippa Anne Watson, James E Dale, Sara Carty, Karen Douglas, Christopher J Edwards, David Hutchinson, and Nick Barkham.
Prior Presentation
This manuscript is based on work that has been previously published [7, 9] and on an abstract presented on 8 November at ACR Convergence 2020 (virtual meeting): Kristensen L, et al. Efficacy of ixekizumab versus adalimumab in psoriatic arthritis (PsA) patients with and without moderate-to-severe psoriasis: 52-week results from a multicentre, randomised open-label study [abstract]. Arthritis Rheumatol. 2020;72(Suppl 10). https://acrabstracts.org/abstract/efficacy-of-ixekizumab-versus-adalimumab-in-psoriatic-arthritis-psa-patients-with-and-without-moderate-to-severe-psoriasis-52-week-results-from-a-multicentre-randomised-open-label-study/.
Disclosures
LEK has received consultancy fees and speaker’s bureau fees from AbbVie, Amgen, Biogen, Bristol Myers Squibb, Celgene, Eli Lilly and Company, Janssen Pharmaceuticals, Merck, Sharp & Dohme (MSD), Novartis, Pfizer, Roche, Sanofi, and UCB Pharma (unrelated to the present work). MO and KdV have nothing to disclose. SLL, CS, AB and GM are employees and stockholders of Eli Lilly and Company. CEB is an independent consultant contracted by Eli Lilly and Company. WT has received research grants, consulting fees or speaker fees from AbbVie, Amgen, Celgene, Eli Lilly and Company, Janssen, MSD, Novartis, Pfizer and UCB. JD has performed clinical trials for Corbus, Eli Lilly and Company and Janssen; has received honoraria from AbbVie, Amgen, Bausch, Celgene, Leo, Janssen, Novartis and Sanofi; and has worked on the speaker bureau for Celgene and Eli Lilly and Company.
Compliance with Ethics Guidelines
SPIRIT-H2H was conducted in accordance with the ethical principles of the Helsinki Declaration of 1964 and its later amendments. All patients involved provided written informed consent, and the study protocol was approved by the ethical review board of each investigative site (Supplementary Table S3) and the National Research Ethics Service Committee London (17/LO/0794) prior to the start of study-related procedures. SPIRIT-H2H is registered at ClinicalTrials.gov (NCT03151551).
Data Availability
Eli Lilly and Company provides access to all individual participant data collected during the trial, after anonymization. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at http://www.vivli.org.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kristensen, LE., Okada, M., Tillett, W. et al. Ixekizumab Demonstrates Consistent Efficacy Versus Adalimumab in Biologic Disease-Modifying Anti-rheumatic Drug-Naïve Psoriatic Arthritis Patients Regardless of Psoriasis Severity: 52-Week Post Hoc Results from SPIRIT-H2H. Rheumatol Ther 9, 109–125 (2022). https://doi.org/10.1007/s40744-021-00388-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-021-00388-8