Abstract
In recent years, single-cell protein (SCP) has been considered a promising aquaculture feed to cope with the growing issue of food security. SCP is derived from microbes including algae, yeasts, and bacteria. Algae and yeasts have been comprehensively studied as SCP sources in the last few years. However, their large-scale application is not yet economical. Recently, the use of purple non-sulfur bacteria (PNSB) has been realized as a sustainable source of SCP. PNSB display unique metabolic features that distinguish them from other SCP sources. They can grow under various light and electron donor/acceptor conditions, can use a variety of low-cost carbon sources, give high substrate yield under their preferred photoheterotrophic growth mode, and demonstrate anti-pathogenic properties. They also use the infrared region of light that enables their straightforward enrichment under non-axenic conditions. Despite the unique characteristics of PNSB, their use as SCP has not been widely reported. This review provides comprehensive knowledge about different factors that influence the quality and quantity of SCP produced from PNSB. The effects of key factors including light, redox conditions, trace metals, carbon substrate, and substrate availability are discussed. Special focus is given to the use of PNSB as SCP in aquaculture and PNSBs concomitant role in improving water quality. This information would expand knowledge and enhance understanding to utilize PNSB as an alternative SCP source for aquaculture feed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The global population is expected to exceed 11 billion by the end of the twenty-first century. Currently, 800 million people are undernourished and a dramatic increase in this number is expected as the population grows to a projected 11 billion by the end of the century. Even by 2050, a 25–70% increase in the food supply is required [1]. Protein is a major constituent of food necessary for effective tissue growth. The limited supply of high-quality protein and its uneven distribution in the world cause malnutrition [2]. Global protein production relies primarily on animal and to a lesser extent plant sources. Animal-based protein accounts for 40% of human protein consumption. Animal meat production has increased from 45 to 233 million metric tons per year between 1950 and 2000 [3]. Animal protein contains all essential nutrients required for the human diet with high digestibility and a balanced supply of essential amino acids [4]. However, animal protein shows low feed conversion ratios compared to other protein sources. For example, 2–15 kg of plant material is required for 1 kg of animal products [3]. Additionally, animal-derived protein sources contribute to many environmental issues such as pollution of waterways from manure and fertilizer runoff; and greenhouse gas (GHG) emissions from animal belching, manure degradation, and energy associated with fertilizer and water supply [5].
Proteins derived from plant sources including soybean meal, chickpea, and rapeseed have been widely used. It is estimated that 40–50% of global plant sources are being used for feed production [3]. Plant protein sources have several limitations such as the presence of anti-nutritional elements, an imbalanced composition of non-essential amino acids, the deficiency of essential amino acids such as lysine, cysteine, histidine, and methionine [4, 6], and low digestibility. Their production also puts additional stress on natural resources including land, water, and nutrients, although this is not as significant as for meat protein. The aforementioned characteristics of plant protein make them less favorable as a sustainable and primary source of protein.
Aquaculture is considered a sustainable, renewable, and alternative protein source and is traditionally a major protein source for human consumption. Aquaculture feed contains 35–60% crude protein compared to terrestrial livestock (12–26%) [7]. Fish are also a nutrient-rich food source containing vitamins A, B, and D, calcium, phosphorous, iodine, iron, and zinc [8]. Aquaculture is considered the fastest growing industry to cope with food security challenges [9]. An estimate shows an increase in world aquaculture from 32.4 million tons in 2000 to 52.5 million tons in 2008 [5]. Aquaculture production has reached a million tons per year in East Asia and the Pacific, 8.14 million tons per year in South Asia, and 1.89 million tons per year in the Middle East and North Africa. The largest aquaculture productivity is in China at 61 million tons per year [10] (Fig. 1). Aquaculture feed sales have increased from 12.2 million tons in 1995 to 50.7 million tons in 2015 [1].
Aquaculture is dependent on a high protein feed source. Aquaculture feed mainly relies on fishmeal, which is sourced from wild-captured forage fish. Often fishmeal is the preferred feed in aquaculture over animal and plant-based protein because of its high protein content and the presence of essential amino acids. Unfortunately, fishmeal has been overused to fulfill feed demand. It is estimated that a shortage of up to 1.3 million metric tons of fishmeal may occur by 2050, which would impair the aquaculture industry [7]. At present, soybean is being used as an alternative aquaculture feed, which presents nutritional and digestibility issues. This situation has put enormous pressure on forage fish supply and aquaculture sustainability. In this perspective, protein-rich biomass from single-cell organisms, termed single-cell protein (SCP), could be considered a suitable alternative to fishmeal, which does not compete directly with human feed.
SCP is less energy-intensive and requires less water and area than plant-based protein sources. It returns less GHG emissions than the other competing sources and can be produced using substrates that are inexpensive and possibly derived from waste streams [7, 8]. SCP can be produced in indoor facilities displacing or reducing the reliance on environmental and seasonal variations, particularly for non-phototrophic SCP sources. Finally, SCP does not require pesticides and herbicides in contrast to the production of plant-based protein [2].
Microorganisms used for SCP production vary in their productivity and cell composition due to their inherent metabolic features. Depending on the species, they can accumulate up to 80% of cell biomass as protein [7, 11]. Therefore, it is important to study the characteristics of SCP to determine its suitability as an aquaculture feed. Algae have been considered viable SCP and certain species such as Spirulina are even cultured for human consumption. However, low digestibility is an issue associated with the use of algae as aquaculture feed [3, 6]. Yeasts can produce 45–55% of their biomass as SCP and its amino acid profile is comparable with fishmeal. However, their large-scale application is still limited due to high production costs. Bacteria are also a promising source of SCP and they can accumulate protein up to 80% of dry cell biomass. Methane oxidizing bacteria and hydrogen oxidizing bacteria, in particular, have been studied and commercialized for SCP production [2]. However, their commercial application has been limited due to the requirement of the high-energy substrate and typically high DNA content [8].
PNSB have received growing interest as an emerging source of SCP as they contain essential amino acids, are relatively less toxic to the host [12, 13], can utilize inexpensive substrates from wastewater resources, do not require dissolved oxygen or carbon dioxide to grow, but can also survive under aerobic conditions, and present high biomass to substrate yield [14]. PNSB are also easily enriched in non-axenic conditions [15] due to their distinct combination of anoxygenic photoheterotrophy and photosynthesis utilizing the near-infrared region of the light spectrum. This allows them to outcompete other microorganisms that mostly grow to utilize the visible light region or chemoheterotrophically. Consequently, PNSB can maintain high abundance in the culture that leads to high purity of SCP. These features of PNSB, as summarized in Fig. 2, qualify them as promising feedstock for SCP production. Despite this, there are some technological and research barriers that impede the large-scale application of PNSB as an SCP source which are discussed in this article. The purpose of this study is to review the existing studies and expand knowledge on the potential of PNSB as a SCP source and aquaculture feed. The effect of key parameters such as light, oxygen, carbon source, and trace elements that regulate PNSB growth and SCP content are discussed. Methods and potential for integration of PNSB with wastewater treatment for economical SCP production are also presented, along with a discussion on the suitability of PNSB-based SCP as an aquaculture feed. Lastly, a perspective is provided on the existing challenges and emerging trends to utilize PNSB for the sustainable production of SCP.
2 Single-cell protein and sources
Single-cell protein is the form of protein produced from single-celled organisms [11], which includes microalgae, yeasts, and bacteria (Table 1). In addition to protein, SCP also contains carbohydrates, nucleic acids, minerals, omega-3 fatty acids, vitamins, enzymes, and carotenoids [16]. Unlike conventional protein sources, typically SCP is rich in essential amino acids including lysine and methionine.
Microorganisms display different biological characteristics, production potential, and SCP quality as an aquaculture feed [22], as elaborated in Table 2. Microalgae have been extensively studied as SCP sources. Several algae species have been exploited for the production of SCP [6]. The most prominent species used for SCP production are Chlorella sp., Dunaliella sp., and Spirulina sp. These species can grow in high salinity, alkalinity, and nutrient concentration [39]. They develop a natural protective system against light by producing pigments, chlorophylls, phycobiliproteins, and polyphenols, which provide health benefits. Digestibility is a major challenge to use algae as an SCP source. The presence of high fiber content and polysaccharides in algae biomass impact the digestibility [4, 6, 40]. Strict sterilization is also required to achieve a pure and high-quality algae biomass. Sterilization is an energy-intensive process posing an economical burden on algae biomass production. The current production of microalgae is only 9000 tons per year, which is much lower than other protein sources [39] and only contributes to 0.7% of total protein production globally. Current microalgae biomass production costs range $10–30 kg−1, which is much higher than soybean ($0.30 kg−1) [1].
Yeasts have been regarded as a promising source of SCP [1]. Yeasts generally grow heterotrophically under aerobic and anaerobic conditions. They can assimilate a wide range of organic carbon sources in pure form as well as those originating from waste streams. Yeasts are acidophiles in nature; therefore, they can grow at low pH and outcompete other microorganisms resulting in relatively pure biomass. They offer adequate nutritional quality as an SCP [16, 44]. The amino acid profile is comparable with conventional protein sources; however, they lack sulfur-containing proteins. Still, their feed trials have demonstrated that 60% substitution of yeasts with fishmeal showed similar aquaculture growth [45]. Yeasts have been reported as a suitable protein source for fishes such as salmon and shrimp too [7]. However, their biomass contains a relatively high amount of nucleic acid that causes allergy and may lead to the death of the aquaculture species. Currently, yeast-based SCP is being produced commercially, but its production is still limited due to high production costs [46].
Bacterial biomass can also be used as an SCP source. Bacteria can contain up to 80% of dry cell weight as a protein [7, 16]. They have a methionine content of more than 3% which is higher than other microbial sources. Several bacteria have been exploited as SCP sources such as hydrogen oxidizing bacteria (HOB), methane-oxidizing bacteria (MOB), and PNSB. Bacterial biomass requires much less energy to assimilate nitrogen into protein than other conventional protein sources. An estimate shows that MOB and HOB require assimilatory energy of 361 and 452 MJ·kg−1 N-protein, respectively. In comparison, 1000 MJ.kg−1 N-protein of energy is required for turkey poultry to assimilate nitrogen into protein. In a study, MOB showed higher protein productivity (2.8 kg protein m−3.day−1) than soybean (5.4 × 10–4 kg protein m−3·day−1). It should be noted that soybean production was determined using yield per area with the assumption that 1 m of vertical space is required (for plant height). Sustainable production of SCP using these bacteria is still challenging due to the requirement of a high-energy substrate [8]. Another disadvantage of most bacterial cells is their high content of nucleic acids and endotoxins.
PNSB are emerging source of SCP. They can contain crude protein up to 70% of their biomass. Their amino acid composition is similar to soybean. They present a relatively lower amount of nucleic acids than the other microorganisms [37]. PNSB biomass contains coenzyme Q10 (CoQ10) and carotenoids (natural pigments), both of which are antioxidants, as well as sulfur-containing amino acids such as methionine and cysteine, that are very beneficial for aquaculture growth. They are described further in the subsequent sections.
3 Metabolic features of purple non-sulfur bacteria
PNSB are versatile microorganisms characterized by a unique primary metabolism of anaerobic photoheterotrophy, with a broad range of other metabolic modes [47, 48]. They use light as an energy source, and organic carbon for biomass in an anoxygenic environment, fixing CO2 for additional redox balance depending on the substrate. They can consume a broad range of organics including environmental pollutants such as benzoic acid, nitrophenol, and halogenated aromatics to drive photosynthesis and energy generation. They can tolerate environmental stresses without pre-adaptation, such as high tolerance to salinity and heavy metals [49]. PNSB can switch between photolithoautotrophy, photo-organoheterotrophy, chemo-organoheterotrophy, and chemo-lithoautotrophy depending on the composition of electron donor and acceptor present in the growth medium. They are facultative anaerobes and can grow in both dark aerobic and dark anaerobic environments if light is not available [50, 51]. They can grow photoheterotrphically in the presence of light and limited oxygen. An intracytoplasmic membrane enables them to adapt to oxygen alterations. In dark conditions, when oxygen is available, they produce energy by oxidative as well as substrate-level phosphorylation [52, 53]. In the presence of light, they perform photosynthesis and respiration when oxygen is available. PNSB also achieve high biomass yield under photoheterotrophic growth as they directly use photon energy to activate electrons, received from the electron donor [54]. Table 3 summarizes the advantages and disadvantages of PNSB as a source of SCP.
4 Factors influencing SCP productivity
Figure 3 illustrates the production process of SCP using PNSB. Briefly, the substrates, which may include fermented solid wastes or wastewaters containing organic carbon and nutrients, are fed to the PNSB. Such waste streams should be devoid of heavy metal contamination or other harmful and persistent pollutants. Suitable streams include aquaculture water itself, as well as wastewaters from other agriculture activities and fermented food sources. PNSB assimilate the carbon and nutrients from them under different conditions of light/dark and anaerobic conditions, remove pollutants and develop their biomass during the cultivation process. After cultivation, biomass is separated from the aqueous medium and fed to aquaculture. To determine the suitability of biomass as an aquaculture feed, its biomass quality is evaluated based on certain factors mentioned in Fig. 3. The spent or treated water can be recycled to the cultivation tank or can be used for other purposes (agriculture/industrial processes). Several parameters are controlled during the cultivation to achieve a desired growth rate and composition of biomass. The most critical parameters include light, oxygen supply, carbon utility, and trace elements. The following sections highlight the impact of these factors on PNSB growth dynamics as well as SCP productivity, the latter of which is generally not well reported.
4.1 Light
Light plays a vital role in the growth of PNSB [55]. PNSB capture incident light through the light-harvesting complex (LHC) and photoreaction center (RC) in the photosynthetic unit (PSU), which converts light energy into chemical energy. In PNSB, photosynthetic pigments such as bacteriochlorophylls (BChl) and carotenoids absorb light at different wavelengths (450–1000 nm) [56, 57]. At first, the carotenoid absorbs light and transfers it to BChl, which is responsible for charge separation [58]. Electron transport in PNSB is carried out by the RC and cytochrome complex. Ubiquinone located in the cytoplasmic membrane is responsible for mediation between RC and cytochrome. Electron transfer is carried out due to proton motive force across the cytoplasmic membrane. Proton motive force is used to synthesize the intracellular energy carrier—adenosine triphosphate (ATP), transport the solute, and perform other metabolic reactions [59]. The cell’s response to the incident light depends on various characteristics of light including light intensity, photoperiod, and wavelength [60]. These characteristics of light have a profound effect on substrate conversion, microbial growth rate, biomass production, and bioproduct yield [61].
Light intensity
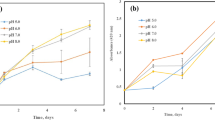
Light intensity significantly affects the growth and energy metabolism of PNSB. The appropriate light intensity can increase the growth rate and the bioproduct yield. Liu, Daigger, Kang, and Zhang [60] investigated the effect of light intensity on pigment formation of Rhodopseudomonas palustris. The highest combined carotenoids and chlorophyll concentrations were obtained at a light intensity of 150 μmol-photons·m−2·s−1, though higher carotenoids were observed at 240 μmol-photons·m−2·s−1 and high BChl at 3–50 μmol-photons·m−2·s−1. In a study, it found that a relatively high light intensity (8000 lx) was appropriate for the production of BChl and carotenoids [62]. Muzziotti, Adessi, Faraloni, Torzillo, and De Philippis [58] investigated the effect of light intensity on the carotenoid composition of Rhodopseudomonas palustris. It produced carotenoids such as spirilloxantin, rhodopin, anhydrorhodovibrin, lycopene, and rhodovibrin. The carotenoid composition changed with the light intensity, where lycopene was dominant at low light intensity and rhodovibrin at high light intensity. With an increase in light intensity (from 250 to 1500 μmol-photons·m−2·s−1), lycopene reduced from 46.6 to 3.0% only, and rhodovibrin increased from 12.7 to 42.2%. The total carotenoids decreased from 1.0 mg.g-CDW−1 under low light, to 0.26 mg.g-CDW−1 under high light conditions. They concluded that Rhodopseudomonas palustris presents a strategy to show photo-acclimation at a high light intensity. Furthermore, high light condition generates excess reducing power, which is dissipated by carotenoids through BChl. Light intensity also affects the biomass productivity of PNSB. Kim [14] found a decrease in the growth rate of Rhodopseudomonas palustris by 16% following a decrease in the light intensity from 350 to 87.5·W·m−2. Al-Azad, KarSoon, and Ransangan [63] determined the biomass productivity of Afifella marina at different light intensities (0–5000 lx) and found that the light intensity of 3000 lx governed the highest biomass productivity reaching a concentration of 4.97 g·L−1. A similar observation of 3000 lx was made by Siddique et al. [64] for a PNSB enriched mixed culture grown on fuel synthesis process water.
Photoperiod
Photoperiod affects the light photon absorption in the RC, electron transportation, and ATP synthesis [65]. Under a dark period, cell division and respiration occur that may lead to enhanced production of pigments by the expression of key genes in the PSU. Liu, Daigger, Kang, and Zhang [60] investigated the effect of photoperiod on the growth and pigments synthesis of Rhodopseudomonas palustris. The highest BChl (1.17 mg·g−1of biomass) production was observed under a light/dark cycle of 16/8 h. Zhi, Yang, Zhang, Zhu, Meng, and Li [66] found that a light/dark cycle of 24/24 h returned the highest biomass productivity (2.07 g·L−1) for a mixed culture of PNSB (80% dominated by Rhodopseudomonas). They further found that a light/dark cycle of 12/12 h gave the maximum carotenoid yield (1.3 mg·L−1of biomass), while 3/3 h cycle showed the highest protein productivity (826 mg·g−1 of biomass). ATP concentration was the highest (~ 1000 μmol·g−1 of protein) in the 12/12 h light/dark cycle. Light/dark cycle had no profound impact on RubisCo enzyme. Another study revealed that a light/dark cycle of 2/1 h was the most suitable to achieve high biomass productivity (2.06 g·L−1) of Rhodopseudomonas [67]. It should be noted that the disparity in the results might be due to the use of different strains, purity of the inoculum, and/or growth conditions. For instance, Cao, Zhi, and Zhang [68] used an inoculum containing 80% Rhodopseudomonas, whereas in other studies, the culture purity was not mentioned.
Light wavelength and source
Light wavelength can have a significant impact on PNSB growth and biomass composition. PNSB can absorb light in the near-infrared wavelength region (805–1035 nm) through BChl a and b. Depending on PNSB species, they can also absorb light in a wavelength range of 300–500 nm using carotenoids [13, 69]. They can produce more than 75 types of carotenoids, which provide them photo-protection [70].
The light source and its associated spectra are important in the photosynthetic process. Natural light is the most economical source but shows high variation in intensity diurnally, daily, and seasonally. Therefore, the effect of light on cell metabolism is best revealed under the provision of artificial light. Incandescent lamps have been widely used as a light source to carry out microbial cultivation in photobioreators, but they are expensive due to their wide spectra and high heat energy losses. Light emitting diodes (LEDs) are considered an economical alternative to conventional light sources with added advantages of low energy requirements and narrow selective wavelength band. Thus, LEDs can be useful to improve light conversion efficiency [56, 71].
Narrow wavelength infrared (IR) light at 860 nm has been successfully applied for the cultivation of Rhodobacter capsulatus. IR helps to restrict microalgae growth, maintain PNSB dominancy and improve wastewater treatment performance [50, 72]. Suwan, Chitapornpan, Honda, Wilai, and Chiemchaisri [73] demonstrated the effect of infrared LEDs and tungsten lamps (covered with infrared transmitting filter) on PNSB carotenoid formation and wastewater treatment performance. They found that infrared LED resulted in higher organic removal than the tungsten lamp, but carotenoid and BChl contents were less. High carotenoid and BChl formation under tungsten lamps was attributed to less light energy availability to the cells. Their study demonstrated that IR LED showed 12 times higher photon emission efficiency than the tungsten lamp. Studies also reveal that LEDs have higher energy yields (10–100 times per kWh) than the other light sources [13].
Hulsen, Hsieh, Tait, Barry, Puyol, and Batstone [74] employed IR and white light on a mixed culture enriched with PNSB. They tested IR irradiated at 18 W.m−2 and white light irradiated at 27–36 W.m−2 (1.5–2 times higher than IR). Despite less energy input, the cells in the presence of IR could remove 90% of COD, 90% nitrogen, and 45% of phosphorous from concentrated poultry processing wastewater. In comparison, the cells with white light removed 98% chemical oxygen demand (COD), 94% nitrogen, and 44% phosphorous. The culture with IR showed high relative abundance (~ 80%) of purple photosynthetic bacteria, compared with the white light (~ 35%). Moreover, IR showed higher amino acids content than white light. Hence, it could be concluded that IR provides comparable wastewater treatment performance and high-quality feed with relatively less energy input than white light.
Generally, PNSB prefer to grow under blue (435–480 nm) and red (605–700 nm) light [15, 65, 75], though reports of optimal wavelength differ between studies, possibly due to the species employed. Kuo, Chien, and Chen [56] investigated the effect of different light sources including incandescent lamp (IL), halogen lamp (HL), fluorescent lamp (FL), and LEDs with blue (B), white (W), yellow (Y), red (R), and green (G) at a fixed light intensity of 2000 lx with Rhodopseudomonas palustris. Energy efficiency for bacterial growth was in the order of B > W > Y > IL > G > HL > FL > R. Carotenoid was produced in the order of B > Y > W > G > IL > HL > R > FL. It was concluded that blue LED could save energy by 75% and increase carotenoid content by 348% compared to IL.
Hellingwerf, Vrij, and Konings [75] investigated the growth of Rhodobacter sphaeroides under a constant photon intensity of 1.25 × 1014 photons cm−2 s−1 and different wavelengths (400–950 nm). The specific growth rates (h−1) at 480 nm (blue color) and 580 nm (yellow color) were almost the same (12 h−1), whereas 660 nm (red) showed a lower specific growth rate (6.0 h−1). In contrast, Q Zhou, P Zhang, G Zhang, and M Peng [76] demonstrated the effect of yellow, white, blue, and red light on PNSB metabolism. They found that yellow light was the most suitable for pigments formation, while the red light was appropriate to achieve high biomass yield and pollutants removal from the wastewater.
It is important to mention that PNSB produce a variety of BChl and carotenoids. The most common method of pigments determination in relevant studies is based on spectrophotometric measurements. This method is problematic when several pigments are present in a sample having different absorption peaks and extinction coefficients. Absorption peak and extinction co-efficients for individual pigments are lacking in the literature. Therefore, this method provides only an approximate measurement of pigments. Accurate measurement of the pigments would require further investigations to determine absorption peaks for individual pigments and modifications in the existing formulae. Quantification and identification of pigments through HPLC–MS are an alternative method that is more accurate and reliable [13].
Gabrielyan, Sargsyan, and Trchounian [77] demonstrated the effect of electromagnetic irradiation (EMI) on the growth of Rhodobacter sphaeroides. EMI is electromagnetic waves with a wavelength of 0.1–10 mm and 30–300 GHz frequency. EMI is commonly produced by communication devices and is known to affect the cytoplasmic membrane, genome, water splitting, and other enzyme activities. They found an illumination with EMI for only 15 min increased the specific growth rate by 1.2-fold compared to cells without EMI exposure. They concluded that EMI could alter membrane-associated metabolic activities of the cells. EMI can modify the cell’s structure, hydrogen flux, and ATP synthesis capability [77].
4.2 Oxygen supply
Oxygen influences the electron transport chain, respiration, and the composition of the RC. Oxygen is an important factor to regulate the proliferation of PNSB in a microbial community matrix [65]. PNSB are facultative anaerobes so they can survive under aerobic conditions and show tolerance to oxygen supply, but prefer anaerobic conditions. Generally, under aerobic conditions, ATP is produced by oxidative phosphorylation, whereas substrate-level phosphorylation or photo-phosphorylation are involved under anaerobic conditions to produce ATP. The provision of oxygen to PNSB in the presence of light allows them to shift their ATP synthesis mechanism between oxidative or photo-phosphorylation [78]. In PNSB, a competition exists between oxidative phosphorylation and the photosynthetic pathway. The selection of a final metabolism depends on the ratio of oxygen and light [15].
In oxidative phosphorylation, the respiration chain is the most important to synthesize ATP. In this, organic material is converted into intermediate byproducts and then moved to the tricarboxylic acid (TCA) cycle [51, 79]. Oxygen is used as a final terminal electron acceptor that warrants further degradation of the organic material into the final product (Fig. 4a,b). In photo-phosphorylation, the photosynthetic electron chain is important. In this process, light is first absorbed by light pigments, which are then transferred to BChl. Thereby, BChl moves from ground state to high-energy state after absorbing light and releases an electron that transports through the electron chain and ATP is produced [78]. In a study of the effect of oxygen and light in different treatments; namely light-anaerobic, natural light-microaerobic, and dark-aerobic conditions, it was found that Rhodobacter sphaeroides strain could remove > 90% COD, TN, TP in all these treatments [79]. The biomass yield, macromolecules degradation, and pollutant removal were higher under aerobic (dark or light) conditions in comparison to light-anaerobic conditions [51]. They argue that under aerobic conditions the cells carried out oxidative phosphorylation to remove pollutants while photo-phosphorylation was observed under anaerobic conditions. Meng, Yang, Zhang, and Wang [80] investigated the effect of DO on artificial sugar wastewater treatment, pigment removal, and biomass production using Rhodopseudomonas as an inoculant. It found that biomass production increased with an increase in DO and reached the maximum (1645 mg·L−1) at 4–5 mg·L−1 of DO. The highest COD (93%) and ammonia (83%) were also found at this DO level. A DO of 2–4 mg·L−1 was suitable for pollutants removal and 1–2 mg·L−1 DO for pigments production. They were of the view that at DO level 0–4 mg·L−1 oxidative phosphorylation was the dominant energy metabolism, whereas a DO < 0.5 mg·L−1 photophosphorylation was the possible metabolic pathway. In another study, while using Rhodobacter sphaeroides, the same group concluded that aerobic condition was preferred for COD removal from the wastewater but detrimental for PNSB growth [81].
a Oxidative phosphorylation in PNSB and its features. b Photo-phosphorylation in PNSB and its features. Composed from Lu, Zhang, and Dong [51]
Most of the aforementioned studies do not demonstrate a complete behavior of PNSB to oxygen supply. A major limitation in the studies [51, 79,80,81] was that they did not carry out any microbial test to identify microbial community during or after the treatment process. Though the cultures started out using a single strain of PNSB no details of filtered air supply were provided. In aerobic treatment, there is a possibility that aerobic bacteria might contaminate the system and contribute to the treatment process. However, a number of studies have demonstrated the ability of Rhodobacter and Rhodopseduomonas palustris to compete or grow in aerobic conditions. Izu, Nakajima, Yamamoto, and Kurisu [50] investigated the changes in bacterial community and proliferation of PNSB during the treatment of organic wastewater under aerobic and anaerobic conditions using Rhodobacter sphaeroides as an initial inoculant. Bacterial community changes were analyzed by fluorescence in situ hybridization (FISH) and gradient gel electrophoresis (DGGE). Under anaerobic conditions, the proportion of PNSB was up to 80% of the community and the dominant species were Rhodobacter sphaeroides and Rhodopseudomonas palustris. Under the aerobic condition, the presence of Aquaspirillum delicatum, Xenococcus sp., and Cytophaga sp. was observed. They recommended that to keep the dominancy of PNSB in a microbial culture, oxidation–reduction potential (ORP) should be kept low (− 200 to − 300 mV).
Madigan and Gest [82] showed the growth of Rhodopseudomonas capsulata (now Rhodobacter capsulatus) chemoautotrophically by supplying a mixture of gases, H2 as energy, CO2 as a carbon, and O2 as an electron acceptor. They indicated that 5% O2 permits cell growth, 10% increased the growth rate, but at 20% growth was halted during the early-log phase (low cell density). However, 10–20% oxygen supply during the log phase (high cell density) stimulated the cell growth and prevented the cells entering stationary phase. Exceeding 20% completely inhibited the growth. They argued that growth inhibition at high O2 concentration was attributed to the inhibition of hydrogenase enzyme. Different species of PNSB display different levels of oxygen tolerance; therefore, the determination of accurate level of oxygen supply requires a dedicated study for each PNSB species.
H S a P L Rogers [83] used mixed culture of Rhodobacter capsulatus and Klebsiella sp. for the treatment of simulated agricultural wastewater. They found that 1% DO increased the cell biomass and the population of Rhodobactere capsulatus increased (60%) with a significant increase in the level of BChl. Further increase in DO to 15% resulted in depletion of BChl; however, 50% of the microbial population was still composed of Rhodobacter capsulatus. COD removal with 15% DO was higher (1305 mg.L−1) than at 1% DO (1107 mg.L−1). It validates the facultative nature of Rhodobacter capsulatus. H S a P L Rogers [84] also utilized mono-culture of Rhodobacter capsulatus to treat wastewater under anaerobic and aerobic (1% DO level) conditions. It found COD removal of aerobic culture was higher than the anaerobic. Studies revealed that Rhodobacter capsulatus could utilize the photosynthetic chain and/or the respiration chain [7, 15]. In the presence of excess oxygen, the respiration electron chain was active while in the presence of light photosynthetic electron chain was active. There is, however, a need to confirm the role of aerobic respiration with other PNSB species.
4.3 Carbon utilization and wastewater treatment
Carbon is an essential component of microbial growth. PNSB can use a wide variety of carbon sources such as organic acids, short-chain alcohols, hydrocarbons, sugars, yeast extract, amino acids, and inorganic carbon sources including CO2 [22]. Under photoheterotrophic metabolism, they utilize light as an energy source and organic material as a carbon source, which provides higher biomass yields than photoautotrophic and chemoheterotrophic bacteria [14]. The utilization of carbon sources is inter-dependent on the presence of other macro- and micro-nutrients. Thus, maintaining a certain C:N:P ratio is critical to drive PNSB growth and wastewater treatment. Nitrogen is less critical than other nutrients since N-fixation is common among PNSB; however, N-fixation comes at an energy cost and will therefore result in lower yields [85].
For aerobic heterotrophic bacterial cultures, a typical COD:N:P ratio is up to 100:5:1, while for anaerobic heterotrophic cultures, the ratio is up to 250:5:1. In contrast, for PNSB, the optimum COD:N:P ratio is 100:9:1.5 under anaerobic conditions due to the higher proportion of carbon directed to biomass [39]. However, this higher efficiency of biomass generation also means that if a natural complex wastewater substrate is being used, there is a higher likelihood that nutrient addition will be required [86]. PNSB do not necessarily require an electron donor or acceptor to generate ATP; therefore, their substrate yield can reach 1.0 g COD·g COD removed−1 [13]. It was reported that PNSB could use a variety of pure and waste carbon sources including acetate, butyrate, propionate, citric acid, mixed organic carbons, amylose, lactate, domestic wastewater, food processing wastewater, and anaerobic digestate. In general, pure substrates showed high biomass yield (0.5–1.0 g COD·g COD removed−1) than waste carbon substrates (0.2–0.7 COD·g COD removed−1). Acetate is reported to be the best-suited carbon source giving a biomass yield up to 1.0 g COD·g COD removed−1.
Carbon supply in pure form shows good growth performance; however, it is typically expensive making SCP production cost-prohibitive. Wastewater or effluent-derived substrates provide a combined benefit of low-cost substrate and recuperation of costs associated with wastewater treatment and management. Wastewater-derived carbon sources must be compatible with the end-use of SCP and contain no residual harmful compounds that could affect the quality or safety of SCP as a feed. Compounds present in the wastewater stream that may be detrimental in their original state must be able to be fully converted to non-harmful compounds and cell biomass in the culture process. Substrates containing heavy metal pollution are not suitable for use with PNSB to produce SCP as the negatively charged surface of PNSB leads to biosorption of positively charged heavy metals. This process is further enhanced by the excretion of extracellular polysaccharides that show a strong affinity for heavy metals. SCP production from safe and abundantly available wastewaters with consistent nutrients concentration is a challenge. Public perception and acceptability to use wastewater-derived SCP as aquaculture/animal feed are also important to select a suitable substrate.
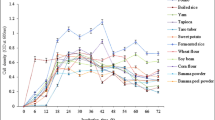
Substrates can be composed of complex organic material that may not be degraded by a single type of microbe. In this case, a mixed microbial culture is employed to degrade organic materials. Initially, it was thought that PNSB could not degrade macromolecules without pretreatment to solubilize them. However, recent research provides evidence of macromolecules degradation by PNSB under microaerobic conditions [87]. The organic strength of the waste substrate is also important for growth. Dilute wastewater from the aquaculture system is reported to be suitable for SCP production and its use as a fish feed [22, 68, 88]. High-strength wastewater such as meat processing may cause ammonia inhibition and less light permeation due to high turbidity. To overcome this issue, the wastewater may need several orders of dilution to grow PNSB. Petrochemical wastewater containing a high concentration of alkane and hydrocarbon can be a potential substrate for PNSB. Only a few studies have demonstrated the growth potential of PNSB in high-strength wastewaters and the effect of dilution. PNSB have been reported to grow in pharmaceutical wastewater containing recalcitrant organic compounds and toxic substances [39]. A number of substrates have been reported for the production of SCP through PNSB (Table 4). The basic selection criteria for any substrate would be its organic carbon content, pre-treatment requirements, easy availability, and cost. Food wastewater originating from the processing of potato, fruit, vegetable, and oil have been a promising feedstock for SCP production. Similarly, wastewater originating from the juice and beverage industries can be a potential substrate for PNSB [89]. Wastewater produced from these industries contains high COD concentrations but low concentrations of nitrogen and phosphorous. Thus, the addition of nitrogen and phosphorous would be required to utilize it as a substrate. Rhodopseudomonas palustris has also been cultivated on palm and soybean cooking oils which could provide an effective way to valorize waste streams of used cooking oil [90]. However, its impact on SCP, like various other substrates tested for PNSB degradation and culturing, was not demonstrated. This is a necessary connect that should be focused on in future.
4.4 Trace elements
Metal ions play an important role in cell metabolism [98, 99]. Trace metals serve as cofactors for various enzymes to catalyze biochemical reactions. Trace metals include Ca, Cu, Fe, K, Mg, Mn, Mo, Na, Zn, Ba, Co, Ni, Sr, and V. An appropriate concentration of trace metals is required to maintain cell growth. Low concentrations can prevent key metabolic processes while high concentrations can result in binding with the cellular surface, internal cell accumulation, and inhibition. The binding and transportation of metals are a complex phenomenon. It is affected by several parameters including salinity, the concentration of monovalent and divalent ions, pH, and competition between metals ions, and other nutrients. An imbalance in the supply of metal ions can change the cell structure, morphology, and functionality of key biomolecules including proteins, nucleic acids, and the activity of the RC. Each metal ion plays a unique role in microbial metabolism [98].
Fe is a common metal and is involved in respiration, photosynthesis, and nitrogen fixation. It helps to detoxify reactive oxygen and electron transport in the cell. Fe limitation can lead to an arrested yield of phycobilisomes, light, and oxidative stress. The valence state of Fe impacts its bioavailability and transportation in the cell [70]. It can regulate the genes in the Fe-S cluster, which is the center of many biomolecules including cytochrome, nicotinamide adenine dinucleotide (NADH), and succinate. It helps to improve pigment yield and BChl. Fe can improve the synthesis of ATP. Wu, Zhang, Li, Lu, and Zhao [100] observed a 42% higher ATP yield in a medium amended with 20 mg·L−1 of Fe than in the control. However, Fe dose higher than 20 mg·L−1 would oxidize intracellular oxygen which damages the hydrogenase activity, and eventually, growth was halted. They stressed that an optimized concentration of Fe should be supplied to the cells to achieve high biomass yield and substrate conversion.
Cu plays an important role to improve microbial growth and biomass production [98]. Cu is an important component of plastocyanin and the electron transport chain; it helps to turn light energy into chemical energy. Cu exists in free ions, inorganic complexes, and chelates, but it is mostly bio-available to the organisms in free ionic form. Panwichian, Kantachote, Wittayaweerasak, and Mallavarapu [101] indicated that an inappropriate concentration of Cu2+ (0.57 mM) can alter the shape of PNSB from rod-like structure to filamentous that impacts pollutants removal potential of PNSB such as heavy metals.
Co is an effective element to promote microbial growth and is a component of vitamin B12 and succinyl-coenzyme, which plays a leading role in substrate-level phosphorylation. It is also important to promote ATP production [98, 102, 103]. Co can substitute other trace nutrients including Zn and Cd. It accelerates the generation of ATP, amino acids, protein, and substrate removal. A typical optimal dose of Co ranges from 5 to 140 mg·L−1 [70].
Zn is an important element of microbial growth, DNA transcription, protein structure, and gas transfer [104]. A study found that 130 mg·L−1 of Zn was an appropriate dose to achieve high substrate conversion [105]. Na and K are essential for the maintenance of osmotic pressure in the cells [70], but requirements differ significantly based on genus and strain. Mo is important to regulate electron transfer and energy metabolism. Mo is used for the assimilation of nitrogen and regulation of the nitrogenase enzyme [70, 98, 106]. Ni improves anaerobiosis and the pollutants removal efficiency of PNSB [107]. Mn is essential for photosynthesis and it plays a central role in the electron transport chain. Mn serves as a cofactor for several enzymes. The addition of Mn also increases COD removal and biomass yield. Like other trace metals, its high concentration (194 mg.L−1) also inhibits treatment efficiency and biomass yield [70].
Mg is an essential element to activate several enzymes in the cell. Mg concentration affects carotenoids yield and BChl functioning [108]. An appropriate concentration of Mg can help to increase the production of ATP, BChl, and cell growth, but is largely dependent on the substrate and other operational conditions. Mg shows high efficacy as an additive when used in light anaerobic conditions. Wu, Zhang, and Li [109] investigated the effect of Mg on biomass development of PNSB. It was found that with the addition of the optimal dose (10 mg·L−1) of Mg, the biomass was improved by 70%. They concluded that Mg improved BChl and ATP production by 60% and 33%, respectively. Synthesis of intracellular biomolecules such as protein, lipids, and nucleic acids involves the consumption of ATP molecules generated from the PSU. Thus, increased photosynthetic activity and high ATP yields increased biomass yield.
Despite the studies described, the effect of trace metals on PNSB growth and subsequently on SCP production has not been adequately studied, particularly with relation to cell and amino acid composition and with different genera of PNSB. The available studies show that trace metals catalyze various metabolic functions in the cell and have a significant impact on microbial growth, biomass yield, ATP synthesis, pollutant removal, and pigment formation. Thus, manipulating the concentrations of trace metals provides a potential means to improve SCP productivity and enhance its quality.
5 SCP as an aquaculture feed
SCP can be derived from different microbial sources and used as aquaculture feed. It should ideally contain good ingredient characteristics and have a high digestibility, palatability, nutrient utilization, and ingredient functionality [110, 111]. Ingredient characterization involves the biochemical composition of the SCP, its source, and processing. Key composition parameters include protein content, amino acid profile, nucleic acid, lipids, dry matter, moisture content, fats, toxins, and vitamins and vary depending on the aquaculture species grown. Digestibility is characterized by energy contents and nutrients excreted by aquaculture organisms relative to what was fed and is one of the key issues using SCP as an aquaculture feed as low digestabtility leads to increased feed requirements and increased water treatment costs. The digestibility of SCP varies from 65 to 96% [7]. SCP digestibility can be improved by controlling microbial cultivation conditions, pre-treatment of microbial biomass, and the addition of methionine and cysteine in the feed [39]. A study reported that a pre-treated SCP showed the same nutrient and protein quality as a control (without pre-treatment), but the former showed high digestibility for amino acids, fats, and carbohydrates. Ingredient palatability refers to the taste or flavor of the diet, which is important to ensure the feed is consumed by the aquaculture organism. Nutrient utilization is a measure of SCP contribution to growth measured in terms of weight gain, feed conversion ratio (FCR), blood glucose, and thyroid hormone level. Ingredient functionality demonstrates physical properties of feed including sink rates, pellets durability, porosity, viscosity, adsorption capacity, and gelatinization property [63, 112]
5.1 PNSB as an SCP aquaculture feed
PNSB biomass can be used as an alternative to fishmeal. It is characterized by high crude protein content, amino acids including those that contain sulfur such as methionine, and cysteine, and other nutrients including CoQ10, and carotenoids [13]. Depending on PNSB species, they can produce a crude protein content up to 0.3–0.6 g-protein·g-dry biomass−1. Recent investigations have shown that the amino acid profile of PNSB-based SCP is comparable with commercial fishmeal. PNSB biomass contains carotenoids that are used as supplements in aquaculture feed. Carotenoids are anti-oxidants and protect against diseases. Carotenoids enhance salmonid health and improve their immune system.
A typical value of carotenoids in PNSB biomass ranges 0.5–13 mg·g dry biomass−1 [73, 113, 114]. Although carotenoids can be added to aquaculture as a supplement, direct use of carotenoids containing biomass is recommended for efficiency. CoQ10 is an immune-stimulant and anti-oxidant compound that improves feed efficiency, digestive enzyme activity, and the growth of aquaculture species [115]. Depending on PNSB species and growth conditions, they can produce CoQ10 ranging from 2.5 9.3 mg·g dry biomass−1. In comparison, carotenoids and CoQ10 in microalgae range 1.0–70 mg·g dry biomass−1 [116, 117] and 0.094–0.141 mg·g dry biomass−1, [118, 119], respectively. Large variation in pigments yield has been reported in the literature among different species of microalgae. It was noted that the pigment yield has been determined in different experimental settings across the studies; therefore, any comparison based on these studies would not provide an accurate estimation. It is also important to note that the dominant carotenoids differ between microalgae and PNSB. Schmidt [120] investigated pigments in eleven strains of PNSB. They found that rhodopin and rhodopinal were the major pigments, whereas spirilloxanthin and β-Carotene were also present in those strains. Silva, Ferreira, Dias, and Barreiro [121] reported on the basis of 10-year bibliometric study on microalgae-derived pigments that the most dominant pigments in microalgae were chlorophylls, phycocyanin, astaxanthin, and β-carotene.
Delamare-Deboutteville, Batstone, Kawasaki, Stegman, Salini, Tabrett, Smullen, Barnes and T Hülsen [122] used a mixed culture of PNSB as an aquaculture feed under different replacement ratios (33%, 66%, and 100%) and evaluated its impact on Barramundi (Lates calcarifer) growth, mortality, and FCR. A fishmeal replacement of 33% and 66% had little or no detrimental impact on fish growth; however, 100% replacement reduced the growth and increased the FCR. Saejung, Chaiyarat, and Sanoamuang [113] investigated the effect of feeding three different PNSB species: Rhodobacter sphaeroides, Rhodopseudomonas palustris, and Rhodopseudomonas faecalis PA2, to shrimp. The shrimp fed with Rhodopseudomonas faecalis PA2 and Rhodopseudomonas palustris showed 82% and 46% survival rates, respectively. Shrimp using Rhodobacter sphaeroides completely died within 12 days of cultivation. These negative results are in contrast to most reported literature that shows a positive impact of PNSB in aquaculture feed. For instance, in a similar study [47] utilizing Rhodopseudomonas palustris and Rhodobacter capsulatus as a shrimp feed observed an increase of shrimp weight by 25% compared to a commercial diet. The use of PNSB in the diet showed better-feed conversion rate than the commercial diet. Moreover, the use of PNSB in feed suppressed growth of Vibrio pathogens by 80%. Chumpol, Kantachote, Rattanachuay, Torpee, Nitoda, and Kanzaki [123] also showed that PNSB produce antivibrio compounds that inhibit shrimp pathogenic Vibrio sp. and safeguard them against luminous vibriosis and hepatopancreatic disease, which are common issues in shrimp farming. They also found that PNSB improve the survival rate and growth rate of shrimp [124]. Seangtumnor, Kantachote, Nookongbut, and Sukhoom [125] found that PNSB produce proteolytic enzymes and antivibiro compounds, identifying ten PNSB strains that were able to avert the pathogenic effect of Vibrio sp. and support shrimp cultivation. Until now, the use of PNSB as aquaculture feed has not been widely explored, and thus, its large-scale application is not demonstrated yet. Future studies should be carried out to establish the suitability of PNSB biomass as aquaculture feed, its limitations, and strategies to overcome them. However, initial testing indicates a highly beneficial feed for the aquaculture industry.
5.2 Integration of PNSB for aquaculture wastewater purification and SCP production
Disease outbreaks, especially in shrimp farming, is a primary constraint in the aquaculture system. Poor water quality is a primary cause leading to the growth of pathogenic bacteria and the production or accumulation of toxins [9]. Pathogenic infection is controlled by the use of antibiotics and chemical additives [126]. The use of antibiotics in large quantities leads to the presence of antimicrobial residues in the aquaculture system and is a threat to human and animal health. Also, in shrimp aquaculture, only 20–30% of nitrogen supplied turns into shrimp biomass, the rest deposits at the bottom of the shrimp pond. This causes the generation of ammonia and hydrogen sulfide which are toxic to the shrimp's health [89, 127]. It affects oxygen concentration, pH, and contamination by supporting the growth of unwanted phytoplankton and microorganisms [9, 128]. The use of chemicals to control water quality has adverse environmental and ecological effects, and thus, impairs the sustainable aquaculture industry. Therefore, an alternative, safe, and environmentally friendly approach is required to maintain aquaculture water quality.
PNSB can remove toxic metabolites including nitrogen, phosphorous, and metals. PNSB can degrade organic materials and help the recycling of nutrients, thus, providing ecological benefits for sustainable aquaculture production. In a study, it found the use of the mixed culture of PNSB containing Rhodobacter sphaeroides and Afifella marina STW18 reduced the ammonia, nitrate, nitrite, and COD of aquaculture water. In a similar study, Zhang, Shu, Wang, Fu, Li, Deng, Liang, and Shen [88] found that the addition of Rhodopseudamonas palustris improved the quality of aquaculture water by removing nitrogen. Sujjat and Julian [129] found that Afifella marina was useful to control dissolved inorganic carbon in aquaculture tank and it was comparable with commercially established probiotics Bacillus sp. Moreover, PNSB serve as biocontrol agents and can displace the need for the use of antibiotics or other chemicals to suppress the growth of pathogens and increase aquaculture resistance against the diseases [126].
6 Challenges and prospects
PNSB are promising feedstocks for SCP production; however, their large-scale application is still not economical. SCP cost largely depends on the substrate yield and therefore selection of suitable wastewater can greatly reduce this cost. Identification of an appropriate wastewater stream requiring minimum or no modifications.i.e. dilution, nutrients addition, and pre-treatment is of utmost importance for the economical production of SCP. Safety is a primary concern in this regard; this can be overcome by using clean wastewater sources including dairy, beverage, juice, and sugar industry wastewaters. Additional resource recovery from wastewater and re-utilization of material (water/residual nutrients) could also potentially reduce SCP production costs.
A further challenge is consistent SCP composition, particularly due to the limited control over wastewater composition. In this context, only those wastewaters should be selected which show relatively consistent composition. PNSB provide some advantage in this regard as they are easily enriched and can therefore provide a dominant component of the SCP that remains stable when MMC cultured SCP is used. Howeer, the presence of RNA and toxins produced by different microorganisms in MMC remains a key concern. The toxins can cause an allergic reaction and can also lead to aquaculture death. The presence of toxins in SCP can be minimized by selecting a suitable production microorganism that does not produce toxins. However, risks are much reduced with PNSB as they contain lipopolysaccharides that are not harmful, and have even been demonstrated to reduce the effects of harmful lipopolysaccharides from other gram-negative bacteria [130,131,132]. Nevertheless, more study is required in this area to understand the antagonistic effects between PNSB lipopolysaccharides and the lipopolysaccharides from other organisms, as well as their overall risk.
PNSB biomass productivity is crucial to demonstrate the economical production of SCP. Until now, PNSB cultivation has been mainly carried out in photo-bioreactors (PBRs). The biomass productivity in PBRs is limited mainly due to improper mixing, in-efficient light permeation, nutrients gradient, and mass transfer issues. The most commonly used PBRs for PNSB are flat panel, tubular, bubble column, and upflow anaerobic sludge blanket (UASB). A detailed description of the PBRs can be found elsewhere in the literature [133, 134]. The existing design of these PBRs is actually derived from microalgae cultivation systems, which are generally suitable for aerobic growth. PNSB, being anaerobes/facultative anaerobes demand different design configurations. Their mixing requirements can be much cheaper than algae as they would not require mixing of air and CO2 in the culture. Therefore, purpose-built PBRs should be designed for PNSB cultivation. Recently, the use of biofilm reactor for PNSB growth has received widespread attention as it provides high-density culture with a minimum or no dewatering requirements for biomass harvest [135, 136]. Still, its use is challenging due to the light, nutrients, and pH gradient formation across the biofilm support.
Another critical issue of PNSB SCP which has received minimal research attention is harvesting [39]. Harvesting is very challenging due to dilute and stable cell suspension. Harvesting accounts for the major cost (20–30% of total) of biomass production with most organisms [137] due to the energy-intensive nature of most separation technologies such as centrifugation, filtration, chemical flocculation, and dissolved air flotation. Recent research have demonstrated that harvesting through bio-flocculation can substantially reduce the harvesting cost as in this technique cell aggregation is carried out merely by manipulating cultivation conditions. Until now bio-flocculation has been rarely studied in the perspective of PNSB harvest. Another method of cost-effective harvest could be the proliferation of PNSB growth conditions to develop a biofilm. Bio-film growth can give almost 100 times more concentrated biomass than the traditional suspended culture growth system, and thus, reduces the dewatering and harvesting costs [138]. Moreover, it returns high SCP yield and better quality than the suspended culture. T Hulsen, E M Sander, P D Jensen and D J Batstone [135] revealed that the biofilm-based biomass of PNSB showed significantly higher crude protein, amino acids, BChl, and total carotenoids than the suspended culture biomass in a culture treating a red meat processing water. In addition, PNSB abundance in the biofilm was higher (57%) than in the suspended culture (43%). Nevertheless, further studies are required to assess the reliability of biofilm technology across different species of PNSB and the optimum culture conditions to induce biofilm.
Finally, public awareness and acceptability are important to enter SCP in commercial markets. Thus, quality and regulatory issues should be addressed and communicated to the public. The probiotic demonstrations of PNSB to date with shrimp farming studies provide an area of great commercial and environmental value that could be beneficial to persuade the public and industry, due to the reduction in chemical usage and mortality. Further research attention is required to fully demonstrate the role of PNSB as probiotics with different aquaculture species and further validate which PNSB provide these benefits and under what conditions. Moreover, most studies to date that have included feed trials have done so at a small scale. Larger scale, longer duration feed testing is required to fully demonstrate the value or risks associated with this source of SCP, including a comprehensive evaluation of the SCP quality by the various aspects that comprise a good feed [111, 112].
7 Conclusions
PNSB are interesting mediators to synthesize SCP. They present unique metabolic characteristics that can promise an economical and sustainable production of SCP and produce biomass of suitable composition, with various other beneficial biomolecules that enhance their value as an SCP source. However, their real potential as SCP has only been evaluated with a handful of aquaculture species and requires more in-depth benchmarking against other microbial SCP and commercial aquaculture feeds.
While various culturing conditions have been studied with PNSB, the majority of studies have focused on biohydrogen production and to a lesser degree polyhydroxyalkanoates and CoQ10. Limited studies have focused on how culturing conditions such as lighting intensity, lighting wavelength, lighting frequency, pH, mixing, and oxygen influence the cellular composition of PNSB, and thus, the quality of SCP.
The integration of the resource recovery approach with SCP production would offer numerous benefits. However, there is a need to identify suitable wastewater streams, PNSB species which can grow in these substrates that will return high substrate yield and evaluate micronutrient requirements that may be necessary to add. So far, little attention has been paid to determining the role of trace elements but they play a vital role in light absorption, catalyzing photosynthetic reaction, and many other metabolic functions. Exploring the effect of trace metals can govern an improvement in SCP yield, composition, and cell morphology (to achieve easy harvest) and must be assessed against the economic of productivity gains.
SCP digestibility, nutrient utilization, and the feed conversion efficiency can help to determine the suitability of PNSB SCP for aquaculture and these values are barely reported. The presence of toxins and other harmful agents in SCP and their impact on aquaculture health should also be explored in addition to further identifying the role of PNSB as a probiotic. These factors should be assessed both at a laboratory scale and with further large-scale long-term feed trials.
Data availability
Not applicable.
Abbreviations
- SCP:
-
Single-cell protein
- PNSB:
-
Purple non-sulfur bacteria
- GHG:
-
Greenhouse gas
- HOB:
-
Hydrogen oxidizing bacteria
- MOB:
-
Methane-oxidizing bacteria
- CoQ10:
-
Coenzyme Q10
- MMC:
-
Mixed microbial culture
- LEDs:
-
Light-emitting diodes
- IL:
-
Incandescent lamp
- FL:
-
Fluorescent lamp
- W:
-
White
- R:
-
Red
- EMI:
-
Electromagnetic irradiation
- TP:
-
Total phosphorous
- FISH:
-
Fluorescence in situ hybridization
- ORP:
-
Oxidation-reduction potential
- NADH:
-
Nicotinamide adenine dinucleotide
- PBRs:
-
Photo-bioreactors
- LHC:
-
Light-harvesting complex
- RC:
-
Photoreaction center
- PSU:
-
Photosynthetic unit
- BChl:
-
Bacteriochlorophylls
- ATP:
-
Adenosine triphosphate
- CDW:
-
Cell dry weight
- IR:
-
Infrared
- COD:
-
Chemical oxygen demand
- HL:
-
Halogen lamp
- B:
-
Blue
- Y:
-
Yellow
- G:
-
Green
- TN:
-
Total nitrogen
- DO:
-
Dissolved oxygen
- DGGE:
-
Gradient gel electrophoresis
- TCA:
-
Tricarboxylic acid
- FCR:
-
Feed conversion ratio
- UASB:
-
Upflow anaerobic sludge blanket
References
Hua K, Cobcroft JM, Cole A, Condon K, Jerry DR, Mangott A, Praeger C, Vucko MJ, Zeng C, Zenger K, Strugnell JM (2019) The future of aquatic protein: implications for protein sources in aquaculture diets. One Earth 1:316–329. https://doi.org/10.1016/j.oneear.2019.10.018
Sillman J, Nygren L, Kahiluoto H, Ruuskanen V, Tamminen A, Bajamundi C, Nappa M, Wuokko M, Lindh T, Vainikka P, Pitkänen J-P, Ahola J (2019) Bacterial protein for food and feed generated via renewable energy and direct air capture of CO2: can it reduce land and water use? Glob Food Secur 22:25–32. https://doi.org/10.1016/j.gfs.2019.09.007
Spiegel MN, van der Fels-Klerx MYHJ (2013) Safety of novel protein sources (insects, microalgae, seaweed, duckweed, and rapeseed) and legislative aspects for their application in food and feed production. Compr Rev Food Sci Food Saf 12:662–678. https://doi.org/10.1111/1541-4337.12032
Sanchez-Muros MJ, Renteria P, Vizcaino A, Barroso FG (2018) Innovative protein sources in shrimp ( Litopenaeus vannamei) feeding. Rev Aquac 12:186–203. https://doi.org/10.1111/raq.12312
Boland MJ, Rae AN, Vereijken JM, Meuwissen MPM, Fischer ARH, van Boekel MAJS, Rutherfurd SM, Gruppen H, Moughan PJ, Hendriks WH (2013) The future supply of animal-derived protein for human consumption. Trends Food Sci Technol 29:62–73. https://doi.org/10.1016/j.tifs.2012.07.002
Bleakley S, Hayes M (2017) Algal proteins: extraction, application, and challenges concerning production. Foods 6:33. https://doi.org/10.3390/foods6050033
Jones SW, Karpol A, Friedman S, Maru BT, Tracy BP (2020) Recent advances in single cell protein use as a feed ingredient in aquaculture. Curr Opin Biotechnol 61:189–197. https://doi.org/10.1016/j.copbio.2019.12.026
El Abbadi SH, Criddle CS (2019) Engineering the dark food chain. Environ Sci Technol 53:2273–2287. https://doi.org/10.1021/acs.est.8b04038
Mukkata K, Kantachote D, Wittayaweerasak B, Techkarnjanaruk S, Boonapatcharoen N (2016) Diversity of purple nonsulfur bacteria in shrimp ponds with varying mercury levels. Saudi J oBiol Sci 23:478–487. https://doi.org/10.1016/j.sjbs.2015.05.014
(FAO) U, Aqua culture production UN Food and Agriculture Organization (FAO) database, https://ourworldindata.org/seafood-production#how-is-our-seafood-produced, 2021.
Jamal P, Alam MZ, Salleh NU (2008) Media optimization for bioproteins production from cheaper carbon source. J Eng Sci Technol 3:124–130
Chumpol S, Kantachote D, Nitoda T, Kanzaki H (2018) Administration of purple nonsulfur bacteria as single cell protein by mixing with shrimp feed to enhance growth, immune response and survival in white shrimp (Litopenaeus vannamei) cultivation. Aquaculture 489:85–95. https://doi.org/10.1016/j.aquaculture.2018.02.009
Capson-Tojo G, Batstone DJ, Grassino M, Vlaeminck SE, Puyol D, Verstraete W, Kleerebezem R, Oehmen A, Ghimire A, Pikaar I, Lema JM, Hulsen T (2020) Purple phototrophic bacteria for resource recovery: challenges and opportunities. Biotechnol Adv 43:107567. https://doi.org/10.1016/j.biotechadv.2020.107567
Kim JH (2018) Effect of light intensity on nutrient removal and pigment production by purple non-sulfur bacteria. https://repository.tudelft.nl/islandora/object/uuid%3Afe864307-f531-49e6-af36-41a466a592ed
Chen J, Wei J, Ma C, Yang Z, Li Z, Yang X, Wang M, Zhang H, Hu J, Zhang C (2020) Photosynthetic bacteria-based technology is a potential alternative to meet sustainable wastewater treatment requirement? Environ Int 137:105417. https://doi.org/10.1016/j.envint.2019.105417
Sharif M, Zafar MH, Aqib AI, Saeed M, Farag MR, Alagawany M (2021) Single cell protein: sources mechanism of production nutritional value and its uses in aquaculture nutrition. Aquaculture 531:735885. https://doi.org/10.1016/j.aquaculture.2020.735885
Liu B, Song J, Li Y, Niu J, Wang Z, Yang Q (2013) Towards industrially feasible treatment of potato starch processing waste by mixed cultures. Appl Biochem Biotechnol 171:1001–1010. https://doi.org/10.1007/s12010-013-0401-1
Yadav J S S, Yan, S, Ajila, C, Bezawada, J, Tyagi, R D, Surampalli, R Y (2016) Food-grade single-cell protein production, characterization and ultrafiltration recovery of residual fermented whey proteins from whey. Food Bioprods Proceses 99. https://doi.org/10.1016/j.fbp.2016.04.012
Gao Y, Li D, Liu Y (2012) Production of single cell protein from soy molasses using Candida tropicalis. Annals Microbiol 62:1165–1172. https://doi.org/10.1007/s13213-011-0356-9
Wiebe M (2002) Myco-protein from fusarium venenatum: a well-established product for human consumption. App Microbiol Biotechnol 58:421–427. https://doi.org/10.1007/s00253-002-0931-x
Paraskevopoulou A, Athanasiadis I, Kanellaki M, Bekatorou A, Blekas G, Kiosseoglou V (2003) Functional properties of single cell protein produced by kefir microflora. Food Res Int 36:431–438. https://doi.org/10.1016/S0963-9969(02)00176-X
Ritala A, Häkkinen, S T, Toivari, M, Wiebe, M G (2017) Single cell protein—state-of-the-art, industrial landscape and patents 2001–2016. Front Microbiol 8. https://doi.org/10.3389/fmicb.2017.02009
De Gregorio A, Mandalari G, Arena N, Nucita F, Tripodo MM, Lo Curto RB (2002) SCP and crude pectinase production by slurry-state fermentation of lemon pulps. Bioresour Technol 83:89–94. https://doi.org/10.1016/S0960-8524(01)00209-7
Becker EW (2007) Micro-algae as a source of protein. Biotechnol Adv 25:207–210. https://doi.org/10.1016/j.biotechadv.2006.11.002
Pelizer LH, Danesi EDG, Rangel C, d O, Sassano, C E N, Carvalho, J C M, Sato, S, Moraes, I O, (2003) Influence of inoculum age and concentration in Spirulina platensis cultivation. J Food Eng 56:371–375. https://doi.org/10.1016/S0260-8774(02)00209-1
Safafar H, Nørregaard, P, Ljubic, A, Møller, P, Holdt, S, Jacobsen, C (2016) Enhancement of protein and pigment content in two Chlorella species cultivated on industrial processwater. J Mar Sci Eng 4. https://doi.org/10.3390/jmse4040084
Zepka L, Jacob-Lopes E, Goldbeck R, Souza-Soares L, Queiroz M (2010) Nutritonal evaluation of single-cell protein produced by Aphanothece microscopica Nägeli. Bioresour Technol 101:7118–7122. https://doi.org/10.1016/j.biortech.2010.04.001
Duong VT, Ahmed F, Thomas-Hall SR, Quigley S, Nowak E, Schenk PM (2015) High protein- and high lipid-producing microalgae from northern australia as potential feedstock for animal feed and biodiesel. Front Bioeng Biotechnol 3:53. https://doi.org/10.3389/fbioe.2015.00053
Rodríguez-Zavala J, Ortiz-Cruz MA, Mendoza-Hernández G, Moreno-Sanchez R (2010) Increased synthesis of α-tocopherol, paramylon and tyrosine by Euglena gracilis under conditions of high biomass production. J Appl Microbiol 109:2160–2172. https://doi.org/10.1111/j.1365-2672.2010.04848.x
Kurbanoglu EB, Algur OF (2002) Single-cell protein production from ram horn hydrolysate by bacteria. Bioresour Technol 85:125–129. https://doi.org/10.1016/S0960-8524(02)00094-9
Liu B, Li Y, Song J, Zhang L, Dong J, Yang Q (2014) Production of single-cell protein with two-step fermentation for treatment of potato starch processing waste. Cellulose 21:3637–3645. https://doi.org/10.1007/s10570-014-0400-6
Wang JP, Kim JD, Kim JE, Kim I-S (2013) Amino acid digestibility of single cell protein from Corynebacterium ammoniagenes in growing pigs. Anim Feed Sci Technol 180:111–114. https://doi.org/10.1016/j.anifeedsci.2012.12.006
Taran M, Asadi N (2014) A novel approach for environmentally friendly production of single cell protein from petrochemical wastewater using a halophilic microorganism in different conditions. Pet Sci Technol 32:625–630. https://doi.org/10.1080/10916466.2011.596888
Overland M, Tauson AH, Shearer K, Skrede A (2010) Evaluation of methane-utilising bacteria products as feed ingredients for monogastric animals. Arch Anim Nutr 64:171–189. https://doi.org/10.1080/17450391003691534
Lee JZ, Logan A, Terry S, Spear JR (2015) Microbial response to single-cell protein production and brewery wastewater treatment. Microbial Biotechnol 8:65–76. https://doi.org/10.1111/1751-7915.12128
Getha K, Vikineswary S, Chong VC (1998) Isolation and growth of the phototrophic bacterium Rhodopseudomonas palustris strain B1 in sago-starch-processing wastewater. World J Microbiol Biotechnol 14:505–511. https://doi.org/10.1023/A:1008855125634
Garimella S, Kudle K, Kasoju A, Merugu R (2017) Current status on single cell protein (scp) production from photosynthetic purple non sulphur bacteria. J Chem Pharm Sci 2:915–922. https://www.ipindexing.com/article/875
Poulain AJ, Newman D (2009) Rhodobacter capsulatus catalyzes light-dependent Fe(II) oxidation under anaerobic conditions as a potential detoxification mechanism. Appl Environ Microbiol 75:6639–6646
Hulsen T, Carvalho G, Egger F, Cruz H, Vertstraete W, Batstone DJ, Pikaar I (2020) Production of single-cell proteins from organic matter and residual nitrogen. Wastewater Treat Residues Resour Biorefinery Prod Biofuels Book Chapter 16:355–389. https://doi.org/10.1016/b978-0-12-816204-0.00016-3
Joubert Y, Fleurence J (2008) Simultaneous extraction of proteins and DNA by an enzymatic treatment of the cell wall of Palmaria palmata (Rhodophyta). J ApplPhycol 20:55–61. https://doi.org/10.1007/s10811-007-9180-9
Anupama R, P, (2000) Value-added food: single cell protein. Biotechnol Adv 18:459–479. https://doi.org/10.1016/S0734-9750(00)00045-8
Singh A, Abidi AB, Agrawal AK, Darmwal NS (1991) Single cell protein production by Aspergillus niger and its evaluation. Zentralbl Mikrobiol 146:181–184. https://doi.org/10.1016/S0232-4393(11)80178-2
Shipman RH, Fan LT, Kao IC (1977) Single-cell protein production by photosynthetic bacteria. Adv Appl Microbiol 21:161–183. https://doi.org/10.1016/s0065-2164(08)70041-8
Matassa S, Boon N, Pikaar I, Verstraete W (2016) Microbial protein: future sustainable food supply route with low environmental footprint. Microbial Biotechnol 9:568–575. https://doi.org/10.1111/1751-7915.12369
Gamboa-Delgado J, Fernández-Díaz B, Nieto-López M, Cruz-Suárez LE (2016) Nutritional contribution of torula yeast and fish meal to the growth of shrimp Litopenaeus vannamei as indicated by natural nitrogen stable isotopes. Aquaculture 453:116–121. https://doi.org/10.1016/j.aquaculture.2015.11.026
Overland M, Skrede A (2017) Yeast derived from lignocellulosic biomass as a sustainable feed resource for use in aquaculture. J Sci Food Agric 97:733–742. https://doi.org/10.1002/jsfa.8007
Alloul A, Wille M, Lucenti P, Bossier P, Van Stappen G, Vlaeminck SE (2021) Purple bacteria as added-value protein ingredient in shrimp feed: Penaeus vannamei growth performance, and tolerance against Vibrio and ammonia stress. Aquaculture 530:735788. https://doi.org/10.1016/j.aquaculture.2020.735788
Hulsen T, Batstone DJ, Keller J (2014) Phototrophic bacteria for nutrient recovery from domestic wastewater. Water Res 50:18–26. https://doi.org/10.1016/j.watres.2013.10.051
Grattieri, (2020) Purple bacteria photo-bioelectrochemistry: enthralling challenges and opportunities. Photochem Photobiol Sci 19:424–435. https://doi.org/10.1039/c9pp00470j
Izu K, Nakajima F, Yamamoto K, Kurisu F (2001) Aeration conditions affecting growth of purple nonsulfur bacteria in an organic wastewater treatment process. Syst Appl Microbiol 24:294–302. https://doi.org/10.1078/0723-2020-00027
Lu H, Zhang G, Dong S (2011) Quantitative study of PNSB energy metabolism in degrading pollutants under weak light-micro oxygen condition. Bioresour Technol 102:4968–4973. https://doi.org/10.1016/j.biortech.2011.01.027
Alsiyabi A, Immethun CM, Saha R (2019) Modeling the interplay between photosynthesis, CO2 fixation, and the quinone pool in a purple non-sulfur bacterium. Sci Rep 9:12638. https://doi.org/10.1038/s41598-019-49079-z
Klamt S, Grammel H, Straube R, Ghosh R, Gilles ED (2008) Modeling the electron transport chain of purple non-sulfur bacteria. Mol Syst Biol 4:156
Cerruti M, Stevens B, Ebrahimi S, Alloul A, Vlaeminck SE, Weissbrodt DG (2020) Enrichment and aggregation of purple non-sulfur bacteria in a mixed-culture sequencing-batch photobioreactor for biological nutrient removal from wastewater. Front Bioeng Biotechnol 8:557234. https://doi.org/10.3389/fbioe.2020.557234
Brotosudarmo THP, Limantara L, Heriyanto, Prihastyanti MNU (2015) Adaptation of the photosynthetic unit of purple bacteria to changes of light illumination intensities. Procedia Chem 14:414–421. https://doi.org/10.1016/j.proche.2015.03.056
Kuo F-S, Chien Y-H, Chen C-J (2012) Effects of light sources on growth and carotenoid content of photosynthetic bacteria Rhodopseudomonas palustris. Bioresour Technol 113:315–318. https://doi.org/10.1016/j.biortech.2012.01.087
George DM, Vincent AS, Mackey HR (2020) An overview of anoxygenic phototrophic bacteria and their applications in environmental biotechnology for sustainable Resource recovery. Biotechnol Rep 28:e00563. https://doi.org/10.1016/j.btre.2020.e00563
Muzziotti D, Adessi A, Faraloni C, Torzillo G, De Philippis R (2017) Acclimation strategy of Rhodopseudomonas palustris to high light irradiance. Microbiol Res 197:49–55. https://doi.org/10.1016/j.micres.2017.01.007
McEwan AG (1994) Photosynthetic electron transport and anaerobic metabolism in purple non-sulfur phototrophic bacteria. Antonie Van Leeuwenhoek 66:151–164. https://doi.org/10.1007/BF00871637
Liu S, Daigger GT, Kang J, Zhang G (2019) Effects of light intensity and photoperiod on pigments production and corresponding key gene expression of Rhodopseudomonas palustris in a photobioreactor system. Bioresour Technol 294:122172. https://doi.org/10.1016/j.biortech.2019.122172
Cheirsilp B, Torpee S (2012) Enhanced growth and lipid production of microalgae under mixotrophic culture condition: effect of light intensity, glucose concentration and fed-batch cultivation. Bioresour Technol 110:510–516. https://doi.org/10.1016/j.biortech.2012.01.125
Zhou Q, Zhang P, Zhang G (2014) Enhancement of cell production in photosynthetic bacteria wastewater treatment by low-strength ultrasound. Bioresour Technol 161:451–454. https://doi.org/10.1016/j.biortech.2014.03.106
Al-Azad S, KarSoon T, Ransangan J (2013) Effects of light intensities and photoperiods on growth and proteolytic activity in purple non-sulfur marine bacterium, Afifella marina strain ME (KC205142). Adv Biosci Biotechnol 04:919–924. https://doi.org/10.4236/abb.2013.410120
Siddique A, Vincent AS, Hamish RM (2021) Effect of pH and light intensity on the growth, protein content and PHA formation of purple non‐sulphur bacteria treating fuel synthesis wastewater. International Water Association 4th Resource Recovery conference, Istanbul, Turkey (A conference proceeding)
He S, Lu H, Zhang G, Ren Z (2021) Production of coenzyme Q10 by purple non-sulfur bacteria: current development and future prospect. J Clean Prod 307:127326. https://doi.org/10.1016/j.jclepro.2021.127326
Zhi R, Yang A, Zhang G, Zhu Y, Meng F, Li X (2019) Effects of light-dark cycles on photosynthetic bacteria wastewater treatment and valuable substances production. Bioresour Technol 274:496–501. https://doi.org/10.1016/j.biortech.2018.12.021
Zhou Q, Zhang P, Zhang G (2015) Biomass and pigments production in photosynthetic bacteria wastewater treatment: effects of light sources. Bioresour Technol 179:505–509. https://doi.org/10.1016/j.biortech.2014.12.077
Cao K, Zhi R, Zhang G (2020) Photosynthetic bacteria wastewater treatment with the production of value-added products: a review. Bioresour Technol 299:122648. https://doi.org/10.1016/j.biortech.2019.122648
Craven J, Sultan MA, Sarma R, Wilson S, Meeks N, Kim DY, Hastings JT, Bhattacharyya D (2019) Rhodopseudomonas palustris-based conversion of organic acids to hydrogen using plasmonic nanoparticles and near-infrared light. RSC Adv 9:41218–41227. https://doi.org/10.1039/C9RA08747H
Chen Y, Yang A, Meng F, Zhang G (2019) Additives for photosynthetic bacteria wastewater treatment: latest developments and future prospects. Bioresour Technol Rep 7:100229. https://doi.org/10.1016/j.biteb.2019.100229
Bhavya ML, Umesh Hebbar H (2019) Efficacy of blue LED in microbial inactivation: effect of photosensitization and process parameters. Int J Food Microbiol 290:296–304. https://doi.org/10.1016/j.ijfoodmicro.2018.10.021
Bertling K, Hurse TJ, Kappler U, Rakić AD (2006) Lasers—an effective artificial source of radiation for the cultivation of anoxygenic photosynthetic bacteria. Biotechnol Bioengg 94:337–345. https://doi.org/10.1002/bit.20881
Suwan D, Chitapornpan S, Honda RC, Wilai C, C, (2014) Conversion of organic carbon in food processing wastewater to photosynthetic biomass in photo-bioreactors using different light sources. Environ Eng Res 19:293–298. https://doi.org/10.4491/eer.2014.S1.009
Hulsen T, Hsieh K, Tait S, Barry EM, Puyol D, Batstone DJ (2018) White and infrared light continuous photobioreactors for resource recovery from poultry processing wastewater - a comparison. Water Res 144:665–676. https://doi.org/10.1016/j.watres.2018.07.040
Hellingwerf K, Vrij W, Konings W (1982) Wavelength dependence of energy transduction in Rhodopseudomonas sphaeroides: action spectrum of growth. J Bacteriol 151:534–541. https://doi.org/10.1128/JB.151.2.534-541.1982
Zhou Q, Zhang P, Zhang G, Peng M (2015) Biomass and pigments production in photosynthetic bacteria wastewater treatment: effects of photoperiod. Bioresour Technol 190:196–200. https://doi.org/10.1016/j.biortech.2015.04.092
Gabrielyan L, Sargsyan H, Trchounian A (2016) Biohydrogen production by purple non-sulfur bacteria Rhodobacter sphaeroides: effect of low-intensity electromagnetic irradiation. J Photochem Photobiol 162:592–596. https://doi.org/10.1016/j.jphotobiol.2016.07.039
Jones MR, McEwan AG, Jackson JB (1990) The role of c-type cytochromes in the photosynthetic electron transport pathway of Rhodobacter capsulatus. Biochimica et Biophysica Acta (BBA) Bioenerg 1019:59–66. https://doi.org/10.1016/0005-2728(90)90124-M
Lu H, Zhang G, Wan T, Lu Y (2011) Influences of light and oxygen conditions on photosynthetic bacteria macromolecule degradation: different metabolic pathways. Bioresour Technol 102:9503–9508. https://doi.org/10.1016/j.biortech.2011.07.114
Meng F, Yang A, Zhang G, Wang H (2017) Effects of dissolved oxygen concentration on photosynthetic bacteria wastewater treatment: pollutants removal, cell growth and pigments production. Bioresour Technol 241:993–997. https://doi.org/10.1016/j.biortech.2017.05.183
Meng F, Yang A, Zhang G, Li J, Li X, Ma X, Peng M (2019) Effects of dissolved oxygen on key enzyme activities during photosynthetic bacteria wastewater treatment. Process Biochem 76:165–170. https://doi.org/10.1016/j.procbio.2018.09.025
Madigan MT, Gest H (1979) Growth of the photosynthetic bacterium Rhodopseudomonas capsulata chemoautotrophically in darkness with H2 as the energy source. J Bacteriol 137:524–530. https://doi.org/10.1128/jb.137.1.524-530.1979
Rogers PL (1977) Photosynthetic bacteria in waste treatment -mixed culture studies with RhodoPsezadbmonas copsulala-. J Ferment Technol 55:311–325
Rogers PL (1977) Photosynthetic bacteria in waste treatment -pure culture studies with Rhodepsez lemonas ccipsulala-. J Ferment Technol 55:297–310
Madigan M, Jung D (2009) An overview of purple bacteria: systematics, physiology, and habitats. The Purple Phototrophic Bacteria. Dordrecht: Springer Netherlands 2009:1–15
Hulsen T, Barry EM, Lu Y, Puyol D, Keller J, Batstone DJ (2016) Domestic wastewater treatment with purple phototrophic bacteria using a novel continuous photo anaerobic membrane bioreactor. Water Res 100:486–495. https://doi.org/10.1016/j.watres.2016.04.061
Basak N, Das D (2006) The prospect of purple non-sulfur (pns) photosynthetic bacteria for hydrogen production: The present state of the art. World J Microbiol Biotechnol 23:31–42. https://doi.org/10.1007/s11274-006-9190-9
Zhang X, Shu M, Wang Y, Fu L, Li W, Deng B, Liang Q, Shen W (2014) Effect of photosynthetic bacteria on water quality and microbiota in grass carp culture. World J Microbiol Biotechnol 30:2523–2531. https://doi.org/10.1007/s11274-014-1677-1
Azad S, Wong M, Mohamad Lal MT (2018) Utilization of vegetable waste Juice by purple non-sulfur bacterium ( Afifella marina Strain ME) for biomass production. J Geosci Environ Prot 06:210–219. https://doi.org/10.4236/gep.2018.65017
Phongjarus N, Suvaphat C, Srichai N, Ritchie RJ (2018) Photoheterotrophy of photosynthetic bacteria ( Rhodopseudomonas palustris ) growing on oil palm and soybean cooking oils. Environl Technol Innov 10:290–304. https://doi.org/10.1016/j.eti.2018.03.002
Zheng G, Kang Z, Qian Y, Wang L (2009) Enhanced biohydrogen generation from organic wastewater containing NH4+by phototrophic bacteria Rhodobacter sphaeroides AR-3. Front Environ Sci Eng China 3:387. https://doi.org/10.1007/s11783-009-0154-9
Seifert K, Waligorska M, Laniecki M (2010) Brewery wastewaters in photobiological hydrogen generation in presence of Rhodobacter sphaeroides O.U. 001. Int J Hydrogen Energ 35:4085–4091. https://doi.org/10.1016/j.ijhydene.2010.01.126
Seifert K, Waligorska M, Laniecki M (2010) Hydrogen generation in photobiological process from dairy wastewater. Int J Hydrogen Energ 35:9624–9629. https://doi.org/10.1016/j.ijhydene.2010.07.015
Singh SP, Srivastava SC, Pandey KD (1994) Hydrogen production by Rhodopseudomonas at the expense of vegetable starch, sugarcane juice and whey. Int J Hydrogen Energ 19:437–440. https://doi.org/10.1016/0360-3199(94)90020-5
Bunraksa T, Kantachote D, Chaiprapat S (2020) The potential use of purple nonsulfur bacteria to simultaneously treat chicken slaughterhouse wastewater and obtain valuable plant growth promoting effluent and their biomass for agricultural application. Biocatal Agric Biotechnol 28:101721. https://doi.org/10.1016/j.bcab.2020.101721
Hulsen T, Hsieh K, Lu Y, Tait S, Batstone DJ (2018) Simultaneous treatment and single cell protein production from agri-industrial wastewaters using purple phototrophic bacteria or microalgae – a comparison. Bioresour Technol 254:214–223. https://doi.org/10.1016/j.biortech.2018.01.032
Kim MK, Choi K-M, Yin C-R, Lee K-Y, Im W-T, Lim JH, Lee S-T (2004) Odorous swine wastewater treatment by purple non-sulfur bacteria, Rhodopseudomonas palustris, isolated from eutrophicated ponds. Biotechnol Lett 26:819–822. https://doi.org/10.1023/B:BILE.0000025884.50198.67
Facey JA, Apte SC, Mitrovic SM (2019) A review of the effect of trace metals on freshwater cyanobacterial growth and toxin production Toxins (Basel) 11. https://doi.org/10.3390/toxins11110643
Kis M, Sipka G, Asztalos E, Rázga Z, Maróti P (2015) Purple non-sulfur photosynthetic bacteria monitor environmental stresses. J Photochem Photobiol B: Biol 151:110–117. https://doi.org/10.1016/j.jphotobiol.2015.07.017
Wu P, Zhang G, Li J, Lu H, Zhao W (2012) Effects of Fe2+ concentration on biomass accumulation and energy metabolism in photosynthetic bacteria wastewater treatment. Bioresour Technol 119:55–59. https://doi.org/10.1016/j.biortech.2012.05.133
Panwichian S, Kantachote, D, Wittayaweerasak, B, Mallavarapu, M (2010) Isolation of purple nonsulfur bacteria for the removal of heavy metals and sodium from contaminated shrimp ponds Electron J Biotechnol 13. https://doi.org/10.2225/vol13-issue4-fulltext-8
Helliwell KE, Lawrence AD, Holzer A, Kudahl UJ, Sasso S, Kräutler B, Scanlan DJ, Warren MJ, Smith AG (2016) Cyanobacteria and eukaryotic algae ese different chemical variants of vitamin B12. Curr Biol 26:999–1008. https://doi.org/10.1016/j.cub.2016.02.041
Raimunda D, Long JE, Padilla-Benavides T, Sassetti CM, Argüello JM (2014) Differential roles for the Co2+/Ni2+ transporting ATPases, CtpD and CtpJ, in Mycobacterium tuberculosis virulence. Mol Microbiol 91:185–197. https://doi.org/10.1111/mmi.12454
Andreini C, Banci L, Bertini I, Rosato A (2006) Zinc through the three domains of Life. J Proteome Res 5:3173–3178. https://doi.org/10.1021/pr0603699
Zhao W, Zhang G (2014) Optimization of photosynthetic bacteria wastewater treatment and study of microbial species diversity. Desalt Water Treat 52:5357–5365. https://doi.org/10.1080/19443994.2013.815688
Paudyal SP, Aryal RR, Chauhan SVS, Maheshwari DK (2010) Effect of heavy metals on growth of rhizobium strains and symbiotic efficiency of two species of tropical legumes. Sci World 5:27–32. https://doi.org/10.3126/sw.v5i5.2652
Liu S, Zhang G, Li X, Wu P, Zhang J (2015) Enhancement of Rhodobacter sphaeroides growth and carotenoid production through biostimulation. J Environ Sci 33:21–28. https://doi.org/10.1016/j.jes.2015.01.005
Solovev AA, Erokhin YE (2013) Role of bacteriochlorophyll in stabilization of the structure of the core and peripheral light-harvesting complexes from purple photosynthetic bacteria. Microbiol 82:554–561. https://doi.org/10.1134/S0026261713050123
Wu P, Zhang G, Li J (2015) Mg2+ improves biomass production from soybean wastewater using purple non-sulfur bacteria. J Environ Sci 28:43–46. https://doi.org/10.1016/j.jes.2014.05.040
Glencross B, Curnow J, Hawkins W, Kissil GWM, Peterson D (2003) Evaluation of the feed value of a transgenic strain of the narrow-leaf lupin (Lupinus angustifolius) in the diet of the marine fish, Pagrus auratus. Aquacult Nutr 9:197–206. https://doi.org/10.1046/j.1365-2095.2003.00247.x
Glencross B, Curnow J, Hawkins W, Kissil GWM, Peterson D (2007) A feed is only as good as its ingredients – a review of ingredient evaluation strategies for aquaculture feeds. Aquacult Nutr 13:17–34
Glencross BD, Huyben D, Schrama JW (2020) The application of single-cell ingredients in aquaculture feeds—A review. Fishes 5:22. https://doi.org/10.3390/fishes5030022
Saejung C, Chaiyarat A, Sanoamuang L-o (2021) Optimization of three anoxygenic photosynthetic bacteria as feed to enhance growth, survival, and water quality in fairy shrimp (Streptocephalus sirindhornae) cultivation. Aquaculture 534:736288. https://doi.org/10.1016/j.aquaculture.2020.736288
Azad SA, Vikineswary S, Ramachandran KB, Chong VC (2001) Growth and production of biomass of Rhodovulum sulfidophilum in sardine processing wastewater. Lett Appl Microbiol 33:264–268. https://doi.org/10.1046/j.1472-765X.2001.00993.x
El Basuini MF, Shahin SA, Teiba II, Zaki MAA, El-Hais AM, Sewilam H, Almeer R, Abdelkhalek N, Dawood MAO (2021) The influence of dietary coenzyme Q10 and vitamin C on the growth rate, immunity, oxidative-related genes, and the resistance against Streptococcus agalactiae of Nile tilapia (Oreochromis niloticus). Aquaculture 531:735862. https://doi.org/10.1016/j.aquaculture.2020.735862
Raposo MFdJ, de Morais AMMB, de Morais RMSC (2015) Carotenoids from marine microalgae: a valuable natural source for the prevention of chronic diseases. Mar Drugs 13:5128–5155. https://doi.org/10.3390/md13085128
Ambati RR, Gogisetty D, Aswathanarayana RG, Ravi S, Bikkina PN, Bo L, Yuepeng S (2019) Industrial potential of carotenoid pigments from microalgae: current trends and future prospects. Crit Rev Food Sci Nutr 59:1880–1902. https://doi.org/10.1080/10408398.2018.1432561
Abu-Ghosh S, Dubinsky Z, Verdelho V, Iluz D (2021) Unconventional high-value products from microalgae: a review. Bioresour Technol 329:124895. https://doi.org/10.1016/j.biortech.2021.124895
Klein BC, Walter C, Lange HA, Buchholz R (2012) Microalgae as natural sources for antioxidative compounds. J Appl Phycol 24:1133–1139. https://doi.org/10.1007/s10811-011-9743-7
Schmidt K (1971) Carotenoids of purple nonsulfur bacteria. Arch Mikrobiol 77:231–238. https://doi.org/10.1007/BF00408115
Silva SC, Ferreira ICFR, Dias MM, Barreiro MF (2020) Microalgae-derived pigments: a 10-year bibliometric review and industry and market trend analysis. Molecules 25:3406. https://doi.org/10.3390/molecules25153406
Delamare-Deboutteville J, Batstone DJ, Kawasaki M, Stegman S, Salini M, Tabrett S, Smullen R, Barnes AC, Hülsen T (2019) Mixed culture purple phototrophic bacteria is an effective fishmeal replacement in aquaculture. Water Res X 4:100031. https://doi.org/10.1016/j.wroa.2019.100031
Chumpol S, Kantachote D, Rattanachuay P, Torpee S, Nitoda T, Kanzaki H (2019) Optimization of culture conditions for production of antivibrio compounds from probiotic purple nonsulfur bacteria against acute hepatopancreatic necrosis disease-causing Vibrio parahaemolyticus and Vibrio spp. Aquaculture 505:72–83. https://doi.org/10.1016/j.aquaculture.2019.02.040
Chumpol S, Kantachote D, Nitoda T, Kanzaki H (2017) The roles of probiotic purple nonsulfur bacteria to control water quality and prevent acute hepatopancreatic necrosis disease (AHPND) for enhancement growth with higher survival in white shrimp (Litopenaeus vannamei) during cultivation. Aquaculture 473:327–336. https://doi.org/10.1016/j.aquaculture.2017.02.033
Seangtumnor N, Kantachote D, Nookongbut P, Sukhoom A (2018) The potential of selected purple nonsulfur bacteria with ability to produce proteolytic enzymes and antivibrio compounds for using in shrimp cultivation. Biocatal Agric Biotechnol 14:138–144. https://doi.org/10.1016/j.bcab.2018.02.013
Jamal M, Chithambaran S, Abdulrahman I, Harbi M (2019) Probiotics as alternative control measures in shrimp aquaculture: a review. J Appl Biol Biotechnol 69–77. https://doi.org/10.7324/jabb.2019.70313
Azad S, Chin F, Mohamad Lal MT (2019) Efficacy of purple non sulphur bacterium Rhodobacter sphaeroides strain UMSFW1 in the utilization of palm oil mill effluent. J Geosci Environ Prot 07:1–12. https://doi.org/10.4236/gep.2019.710001
Barman D (2020) Bioremediation of waste waters and application in Aaquaculture - a mini review. Research Biotica 2:20–25
Sujjat A-A, Julian R (2016) Efficacy of purple non-sulfur bacterium Afifella marina strain ME to control dissolved inorganic nutrients in aquaculture system. Trans Sci Technol 3(2–2):407–412
Weckesser J, Mayer H (1988) Different lipid A types in lipopolysaccharides of phototrophic and related non-phototrophic bacteria. FEMS Microbiol Rev 4:143–153. https://doi.org/10.1111/j.1574-6968.1988.tb02740.x
Henricson BE, Perera PY, Qureshi N, Takayama K, Vogel SN (1992) Rhodopseudomonas sphaeroides lipid A derivatives block in vitro induction of tumor necrosis factor and endotoxin tolerance by smooth lipopolysaccharide and monophosphoryl lipid A. Infect Immun 60:4285–4290. https://doi.org/10.1128/iai.60.10.4285-4290.1992
Staubach K, Weber H, Song L, Sabranski M, Brade H, Bruch HP (1997) Induction of endotoxin (ET) tolerance by atoxic endotoxin - a new prophylactic concept to the septic syndrome. Crit Care 1:P024. https://doi.org/10.1186/cc30
Uyar B (2016) Bioreactor design for photofermentative hydrogen production. Bioprocess Biosyst Eng 39:1331–1340. https://doi.org/10.1007/s00449-016-1614-9
Posten C (2009) Design principles of photo-bioreactors for cultivation of microalgae. Eng Life Sci 9:165–177. https://doi.org/10.1002/elsc.200900003
Hulsen T, Sander EM, Jensen PD, Batstone DJ (2020) Application of purple phototrophic bacteria in a biofilm photobioreactor for single cell protein production: biofilm vs suspended growth. Water Res 181:115909. https://doi.org/10.1016/j.watres.2020.115909
Sarti A, Pozzi E, Chinalia FA, Zaiat M, Foresti E (2006) The performance of an anaerobic sequencing batch biofilm reactor treating domestic sewage colonized by anoxygenic phototrophic bacteria. Chemosphere 62:1437–1443. https://doi.org/10.1016/j.chemosphere.2005.06.007
Rawat I, Ranjith Kumar R, Mutanda T, Bux F (2011) Dual role of microalgae: phycoremediation of domestic wastewater and biomass production for sustainable biofuels production. Appl Energ 88:3411–3424. https://doi.org/10.1016/j.apenergy.2010.11.025
Ozkan A, Kinney K, Katz L, Berberoglu H (2012) Reduction of water and energy requirement of algae cultivation using an algae biofilm photobioreactor. Bioresour Technol 114:542–548. https://doi.org/10.1016/j.biortech.2012.03.055
Funding
Open Access funding provided by the Qatar National Library. The authors would like to acknowledge the support of the Qatar National Research Fund and Ministry of Municipality and Environment in funding this research under grant MME01-0910–190029, Qatar National Library for open access funding, as well as the support of Qatar Shell Research and Technology Centre.
Author information
Authors and Affiliations
Contributions
Naim Rashid: conceptualization, writing—original draft, writing—review and editing. Hamish R. Mackey: writing—review and editing, funding acquisition, supervision. Udeogu Onwusogh: writing—review and editing.
Corresponding author
Ethics declarations
Consent to participate
All the authors voluntarily participated in this research study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors declare that the manuscript has not been published previously.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rashid, N., Onwusogh, U. & Mackey, H.R. Exploring the metabolic features of purple non-sulfur bacteria for waste carbon utilization and single-cell protein synthesis. Biomass Conv. Bioref. 14, 12653–12672 (2024). https://doi.org/10.1007/s13399-022-03273-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-03273-8