Abstract
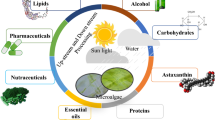
A microalgal biorefinery approach has been established through bioprospecting of an indigenous CO2-tolerant freshwater isolate Tetradesmus obliquus CT02 biomass in two sequential steps, resulting in alternate product cascades of bioactive molecules and biodiesel or biofertilizer. In the first step, crude microalgal extracts in five different solvents were screened for their antioxidant and anticancer activities. While acetone extract showed the highest antioxidant activity with an IC50 value of 137 µg mL−1, ethyl acetate extract exhibited maximum anticancer activity with an IC50 value of 306.67 µg mL−1. This antioxidant and anticancer activity may be attributed to the coordinated action of multiple bioactive molecules as detected by HR-LCMS analysis. In the next step, post extracted residual biomass of CT02 was evaluated as feedstock for biodiesel production. The lipid content was found to be 35.7–39.1% w/w, and further to that, FAME yield of 33.1–36.7% w/w was obtained via direct transesterification of both the acetone and ethyl acetate extracted biomass. Analysis of FAME composition revealed an abundance of palmitic acid (C16:0), stearic acid (C18:0), and elaidic acid (C18:1n9t) as the major constituents, making it suitable for use as biodiesel. The post extracted residual biomass was also evaluated for its application as biofertilizer through induced germination of Solanum lycopersicum seeds. The highest final germination percentage and germination index was estimated to be in the range of 75–80% and 117.5–118.5, respectively, comparable to that of commercial grade NPK (20:20:13).
Graphical abstract







Similar content being viewed by others
Availability of data
All data generated or analyzed during this study are included in this article and its supplementary material.
References
Santos FM, Gonçalves AL, Pires JCM (2019) Negative emission technologies. Bioenergy with Carbon Capture Storage Using Nat Resour Sustain Dev 1–13. https://doi.org/10.1016/B978-0-12-816229-3.00001-6
Ferreira A, Ribeiro B, Ferreira AF et al (2019) Scenedesmus obliquus microalga-based biorefinery – from brewery effluent to bioactive compounds, biofuels and biofertilizers – aiming at a circular bioeconomy. Biofuels Bioprod Biorefin 13:1169–1186. https://doi.org/10.1002/bbb.2032
Nobre BP, Villalobos F, Barragán BE et al (2013) A biorefinery from Nannochloropsis sp. microalga - extraction of oils and pigments. Production of biohydrogen from the leftover biomass. Bioresour Technol 135:128–136. https://doi.org/10.1016/j.biortech.2012.11.084
Kothari R, Pandey A, Ahmad S et al (2017) Microalgal cultivation for value-added products: a critical enviro-economical assessment. 3 Biotech 7:1–15. https://doi.org/10.1007/s13205-017-0812-8
Muthuraj M, Kumar V, Palabhanvi B, Das D (2014) Evaluation of indigenous microalgal isolate Chlorella sp. FC2 IITG as a cell factory for biodiesel production and scale up in outdoor conditions. J Ind Microbiol Biotechnol 41:499–511. https://doi.org/10.1007/s10295-013-1397-9
Sen S, De B, Devanna N, Chakraborty R (2013) Total phenolic, total flavonoid content, and antioxidant capacity of the leaves of Meyna spinosa Roxb., an Indian medicinal plant. Chin J Nat Med 11:149–157. https://doi.org/10.1016/S1875-5364(13)60042-4
Pal U, Ghosh S (2020) Limaye AM (2020) DNA methylation in the upstream CpG island of the GPER locus and its relationship with GPER expression in colon cancer cell lines. Mol Biol Reports 4710(47):7547–7555. https://doi.org/10.1007/S11033-020-05817-5
Feng W, Zhu Y, Wu F et al (2015) (2015) Characterization of phosphorus forms in lake macrophytes and algae by solution 31P nuclear magnetic resonance spectroscopy. Environ Sci Pollut Res 238(23):7288–7297. https://doi.org/10.1007/S11356-015-5913-5
Parsons TR, Maita Y, Lalli CM et al (1984) Determination of phosphate. A Man Chem Biol Methods Seawater Anal 22–25. https://doi.org/10.1016/B978-0-08-030287-4.50015-3
Jain MS, Jambhulkar R, Kalamdhad AS (2018) Biochar amendment for batch composting of nitrogen rich organic waste: effect on degradation kinetics, composting physics and nutritional properties. Bioresour Technol 253:204–213. https://doi.org/10.1016/J.BIORTECH.2018.01.038
Cataldo DA, Haroon MH, Schrader LE, Youngs VL (1975) Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun Soil Sci Plant Anal 6:71–80. https://doi.org/10.1080/00103627509366547
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Strickland JDH, Parsons TR (1968) A practical handbook of sea water analysis. Bull Fish Res Board Can (Ottawa) 167:311
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917. https://doi.org/10.1139/O59-099
Dubois M, Gilles KA, Hamilton JK et al (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Bhola V, Swalaha F, Ranjith Kumar R et al (2014) Overview of the potential of microalgae for CO2 sequestration. Int J Environ Sci Technol 11:2103–2118. https://doi.org/10.1007/s13762-013-0487-6
Solovchenko A, Khozin-Goldberg I (2013) High-CO2 tolerance in microalgae: possible mechanisms and implications for biotechnology and bioremediation. Biotechnol Lett 35:1745–1752. https://doi.org/10.1007/s10529-013-1274-7
Uggetti E, Sialve B, Hamelin J, et al (2018) CO2 addition to increase biomass production and control microalgae species in high rate algal ponds treating wastewater. J CO2 Util 28:292–298. https://doi.org/10.1016/j.jcou.2018.10.009
Wang H, Nche-Fambo FA, Yu Z, Chen F (2018) Using microalgal communities for high CO2-tolerant strain selection. Algal Res 35:253–261. https://doi.org/10.1016/j.algal.2018.08.038
Ren H-Y, Liu B-F, Ma C, et al (2013) A new lipid-rich microalga Scenedesmus sp. strain R-16 isolated using Nile red staining: effects of carbon and nitrogen sources and initial pH on the biomass and lipid production. Biotechnol Biofuels 61 6:1–10. https://doi.org/10.1186/1754-6834-6-143
Xin L, Hong-ying H, Ke G, Jia Y (2010) Growth and nutrient removal properties of a freshwater microalga Scenedesmus sp. LX1 under different kinds of nitrogen sources. Ecol Eng 36:379–381. https://doi.org/10.1016/j.ecoleng.2009.11.003
Wu YH, Yu Y, Hu HY, Zhuang LL (2016) Effects of cultivation conditions on the production of soluble algal products (SAPs) of Scenedesmus sp. LX1. Algal Res 16:376–382. https://doi.org/10.1016/j.algal.2016.04.006
Arumugam M, Agarwal A, Arya MC, Ahmed Z (2013) Influence of nitrogen sources on biomass productivity of microalgae Scenedesmus bijugatus. Bioresour Technol 131:246–249. https://doi.org/10.1016/j.biortech.2012.12.159
Shen Y, Yuan W, Pei Z, Mao E (2010) Heterotrophic culture of Chlorella protothecoides in various nitrogen sources for lipid production. Appl Biochem Biotechnol 160:1674–1684. https://doi.org/10.1007/s12010-009-8659-z
Goswami G, Sinha A, Kumar R, Dutta BC, Singh H, Das D (2019) Process engineering strategy for cultivation of high density microalgal biomass with improved productivity as a feedstock for production of bio-crude oil via hydrothermal liquefaction. Energy 189:116–136. https://doi.org/10.1016/j.energy.2019.116136
Muthuraj M, Chandra N, Palabhanvi B, Kumar V, Das D (2015) Process engineering for high-cell-density cultivation of lipid rich microalgal biomass of Chlorella sp FC2 IITG. Bioenergy Res 8(2):726–739. https://doi.org/10.1007/s12155-014-9552-3
Palabhanvi B, Muthuraj M, Mukherjee M, Kumar V, Das D (2016) Process engineering strategy for high cell density-lipid rich cultivation of Chlorella sp. FC2 IITG via model guided feeding recipe and substrate driven pH control. Algal Res 16:317–329. https://doi.org/10.1016/j.algal.2016.03.024
Surkatti R, Al-Zuhair S (2018) Effect of cresols treatment by microalgae on the cells’ composition. J Water Process Eng 26:250–256. https://doi.org/10.1016/j.jwpe.2018.10.022
Sánchez Mirón A, Cerón García MC, García Camacho F et al (2002) Growth and biochemical characterization of microalgal biomass produced in bubble column and airlift photobioreactors: studies in fed-batch culture. Enzyme Microb Technol 31:1015–1023. https://doi.org/10.1016/S0141-0229(02)00229-6
Wang L, Wang L, Manzi HP et al (2020) Isolation and screening of Tetradesmus dimorphus and Desmodesmus asymmetricus from natural habitats in Northwestern China for clean fuel production and N, P removal. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-01034-z
Mishra S, Mohanty K (2019) Comprehensive characterization of microalgal isolates and lipid-extracted biomass as zero-waste bioenergy feedstock: an integrated bioremediation and biorefinery approach. Bioresour Technol 273:177–184. https://doi.org/10.1016/j.biortech.2018.11.012
Banskota AH, Sperker S, Stefanova R et al (2019) Antioxidant properties and lipid composition of selected microalgae. J Appl Phycol 31:309–318. https://doi.org/10.1007/s10811-018-1523-1
Lourenço SC, Moldão-Martins M, Alves VD (2019) Antioxidants of natural plant origins: from sources to food industry applications. Molecules 24:14–16. https://doi.org/10.3390/molecules24224132
Sathisha AD, Lingaraju HB, Prasad KS (2011) Evaluation of antioxidant activity of medicinal plant extracts produced for commercial purpose. E-Journal Chem 8:882–886. https://doi.org/10.1155/2011/693417
Dimova D, Dobreva D, Panayotova V, Makedonski L (2021) DPPH antiradical activity and total phenolic content of methanol and ethanol extracts from macroalgae (Ulva rigida) and microalgae (Chlorella). Scr Sci Pharm 6:37. https://doi.org/10.14748/ssp.v7i2.7369
Cengiz Sahin S (2019) Scenedesmus obliquus: a potential natural source for cosmetic industry. Int J Second Metab 6:129–136. https://doi.org/10.21448/ijsm.545771
Assunção MFG, Amaral R, Martins CB et al (2017) Screening microalgae as potential sources of antioxidants. J Appl Phycol 29:865–877. https://doi.org/10.1007/s10811-016-0980-7
Ambi A, Bauchi FP, Bashir M (2021) Yellow and red varieties of Hibiscus sabdariffa calyces contain novel antioxidants as analysed by liquid chromatography-electrospray ionization tandem mass spectrometry (LC-ESI) Acta Scientific NUTRITIONAL HEALTH ( ISSN : 2582–1423 ) Yellow and Red Varie
Mène-Saffrané L, Jones AD, DellaPenna D (2010) Plastochromanol-8 and tocopherols are essential lipid-soluble antioxidants during seed desiccation and quiescence in Arabidopsis. Proc Natl Acad Sci U S A 107:17815–17820. https://doi.org/10.1073/pnas.1006971107
Kim BR, Kim HM, Jin CH et al (2020) Composition and antioxidant activities of volatile organic compounds in radiation-bred coreopsis cultivars. Plants 9. https://doi.org/10.3390/plants9060717
Jamil MDHM, Taher M, Susanti D et al (2020) Phytochemistry, traditional use and pharmacological activity of Picrasma quassioides: a critical reviews. Nutrition 12:2584. https://doi.org/10.3390/NU12092584
Dubuisson M, Marchand C, Rees JF (2004) Firefly luciferin as antioxidant and light emitter: the evolution of insect bioluminescence. Luminescence 19:339–344. https://doi.org/10.1002/bio.789
Salehi B, Azzini E, Zucca P et al (2020) Plant-derived bioactives and oxidative stress-related disorders: a key trend towards healthy aging and longevity promotion. Appl Sci 10. https://doi.org/10.3390/app10030947
Puente-Garza CA, Meza-Miranda C, Ochoa-Martínez D, García-Lara S (2017) Effect of in vitro drought stress on phenolic acids, flavonols, saponins, and antioxidant activity in Agave salmiana. Plant Physiol Biochem 115:400–407. https://doi.org/10.1016/j.plaphy.2017.04.012
Bajguz A (2010) An enhancing effect of exogenous brassinolide on the growth and antioxidant activity in Chlorella vulgaris cultures under heavy metals stress. Environ Exp Bot 68:175–179. https://doi.org/10.1016/j.envexpbot.2009.11.003
Dawood Shah M, Seelan Sathiya Seelan J, Iqbal M (2020) Phytochemical investigation and antioxidant activities of methanol extract, methanol fractions and essential oil of Dillenia suffruticosa leaves. Arab J Chem 13:7170–7182. https://doi.org/10.1016/j.arabjc.2020.07.022
Shah MD, Yong YS, Iqbal M (2014) Phytochemical investigation and free radical scavenging activities of essential oil, methanol extract and methanol fractions of Nephrolepis biserrata. Int J Pharm Pharm Sci 6:269–277
Aumsuwan P, Khan SI, Khan IA et al (2016) The anticancer potential of steroidal saponin, dioscin, isolated from wild yam (Dioscorea villosa) root extract in invasive human breast cancer cell line MDA-MB-231 in vitro. Arch Biochem Biophys 591:98–110. https://doi.org/10.1016/j.abb.2015.12.001
Agarwal DS, Anantaraju HS, Sriram D et al (2016) Synthesis, characterization and biological evaluation of bile acid-aromatic/heteroaromatic amides linked via amino acids as anti-cancer agents. Steroids 107:87–97. https://doi.org/10.1016/j.steroids.2015.12.022
Kaskova ZM, Tsarkova AS, Yampolsky IV (2016) 1001 lights: Luciferins, luciferases, their mechanisms of action and applications in chemical analysis, biology and medicine. Chem Soc Rev 45:6048–6077. https://doi.org/10.1039/c6cs00296j
Min HY, Jang HJ, Park KH et al (2019) The natural compound gracillin exerts potent antitumor activity by targeting mitochondrial complex II. Cell Death Dis 10. https://doi.org/10.1038/s41419-019-2041-z
Kang HM, Son HS, Cui YH, et al (2017) Phytosphingosine exhibits an anti-epithelial-mesenchymal transition function by the inhibition of EGFR signaling in human breast cancer cells. Oncotarget 8:77794–77808. https://doi.org/10.18632/oncotarget.20783
Mahdavian E, Palyok P, Adelmund S et al (2014) Biological activities of fusarochromanone: a potent anti-cancer agent. BMC Res Notes 7:1–11. https://doi.org/10.1186/1756-0500-7-601
Habila MM, Festus EA, Morumda D, Joseph I (2021) FTIR and GC-MS analysis of the aqueous and ethanolic extracts of Jatropha tanjorensis leaves
Han HS, Kwon YJ, Park SH et al (2004) Potent effect of 5-HPBR, a butanoate derivative of 4-HPR, on cell growth and apoptosis in cancer cells. Int J Cancer 109:58–64. https://doi.org/10.1002/ijc.11643
Aminzadeh-Gohari S, Feichtinger RG, Vidali S et al (2017) A ketogenic diet supplemented with medium-chain triglycerides enhances the anti-tumor and anti-angiogenic efficacy of chemotherapy on neuroblastoma xenografts in a CD1-nu mouse model. Oncotarget 8:64728–64744. https://doi.org/10.18632/oncotarget.20041
Marrez DA, Naguib MM, Sultan YY, Higazy AM (2019) Antimicrobial and anticancer activities of Scenedesmus obliquus metabolites. Heliyon 5:e01404. https://doi.org/10.1016/j.heliyon.2019.e01404
Reyna-Martinez R, Gomez-Flores R, López-Chuken U et al (2018) Antitumor activity of Chlorella sorokiniana and Scenedesmus sp. microalgae native of Nuevo León State. México PeerJ 2018:1–15. https://doi.org/10.7717/peerj.4358
Tavares-Carreón F, De La Torre-Zavala S, Arocha-Garza HF et al (2020) In vitro anticancer activity of methanolic extract of Granulocystopsis sp., a microalgae from an oligotrophic oasis in the Chihuahuan desert. PeerJ 2020:1–21. https://doi.org/10.7717/peerj.8686
Cha TS, Chen JW, Goh EG et al (2011) Differential regulation of fatty acid biosynthesis in two Chlorella species in response to nitrate treatments and the potential of binary blending microalgae oils for biodiesel application. Bioresour Technol 102:10633–10640. https://doi.org/10.1016/j.biortech.2011.09.042
Singh N, Goyal A, Moholkar VS (2020) Microalgal bio-refinery approach for utilization of Tetradesmus obliquus biomass for biodiesel production. Mater Today Proc 32:760–763. https://doi.org/10.1016/j.matpr.2020.03.541
Kumar V, Muthuraj M, Palabhanvi B et al (2014) High cell density lipid rich cultivation of a novel microalgal isolate Chlorella sorokiniana FC6 IITG in a single-stage fed-batch mode under mixotrophic condition. Bioresour Technol 170:115–124. https://doi.org/10.1016/j.biortech.2014.07.066
Gouveia L, Oliveira AC (2009) Microalgae as a raw material for biofuels production. J Ind Microbiol Biotechnol 36:269–274. https://doi.org/10.1007/s10295-008-0495-6
Aslam A, Thomas-Hall SR, Manzoor M et al (2018) Mixed microalgae consortia growth under higher concentration of CO2 from unfiltered coal fired flue gas: fatty acid profiling and biodiesel production. J Photochem Photobiol B Biol 179:126–133. https://doi.org/10.1016/j.jphotobiol.2018.01.003
Mukherjee C, Chowdhury R, Ray K (2015) Phosphorus recycling from an unexplored source by polyphosphate accumulating microalgae and cyanobacteria-a step to phosphorus security in agriculture. Front Microbiol 6:1–7. https://doi.org/10.3389/fmicb.2015.01421
Nayak M, Swain DK, Sen R (2019) Strategic valorization of de-oiled microalgal biomass waste as biofertilizer for sustainable and improved agriculture of rice (Oryza sativa L.) crop. Sci Total Environ 682:475–484. https://doi.org/10.1016/j.scitotenv.2019.05.123
Acknowledgements
The current study was supported by the MHRD, Govt. of India. Authors are thankful to SAIF, IIT Bombay, and CIF, IIT Guwahati for providing HR-LCMS-QTOF and FESEM facilities. Our sincere thanks to Dr. Anil Mukund Limaye, Associate Professor, Department of Biosciences and Bioengineering, IIT Guwahati, for providing mammalian cell culture facility and Mr. Uttariya Pal, PhD Scholar, Department of Biosciences and Bioengineering, IIT Guwahati, for performing anticancer activity assays.
Funding
Partial financial support was received from MHRD, Govt. of India.
Author information
Authors and Affiliations
Contributions
Ankan Sinha: Planning and execution of experiments, analysis and interpretation of data, and manuscript preparation. Gargi Goswami: Conceptualization and manuscript writing. Ratan Kumar: Experimentation and manuscript preparation. Debasish Das: Conceptualization, overall supervision of the work, and manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sinha, A., Goswami, G., Kumar, R. et al. A microalgal biorefinery approach for bioactive molecules, biofuel, and biofertilizer using a novel carbon dioxide-tolerant strain Tetradesmus obliquus CT02. Biomass Conv. Bioref. 13, 12605–12618 (2023). https://doi.org/10.1007/s13399-021-02098-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-02098-1