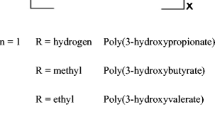Abstract
Polyhydroxyalkanoates (PHA) are a promising bio-based alternative to traditional plastics derived from petroleum. Cyanobacteria are photosynthetic organisms that produce PHA from CO2 and sunlight, which can potentially reduce production costs and environmental footprint in comparison to heterotrophic bacteria cultures because (1) they utilize inorganic carbon sources for growth and (2) they do not require intensive aeration for oxygenation. Moreover, supplementing precursors such as propionate, acetate, valerate, etc., can be used to obtain various copolymers with plastic customizable properties in comparison to the classical homopolymers, such as polyhydroxybutyrate, PHB. This critical review covers the latest advances in PHA production, including recent discoveries in the metabolism interplay between PHA and glycogen production, and new insights into cultivation strategies that enhance PHA accumulation, and purification processes. This review also addresses the challenges and suggests potential solutions for a viable industrial PHAs production process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nowadays plastics are an essential material for many manufacturing sectors, such as packaging, construction, automotive, electrical, electronics, household, medical, and agriculture. Unfortunately, nearly ~ 55% of plastic waste is disposed of in landfills or discarded in natural ecosystems (Hannah Ritchie and Max Roser 2019). Plastic waste accumulation in the ecosystems leads to adverse effects on human health and negative impacts on ecosystems, as well as disruption of economic activities, among others (Cook and Halden 2020). The current methods used to tackle plastic pollution, such as recycling and incineration, are inadequate in addressing the global scale of plastic usage and fall short of being ideal solutions. For example, plastic incineration can produce toxic components (e.g., Dioxins, Furans, Mercury, and Polychlorinated Biphenyls) (Castilho et al. 2009), while recycling can change the properties of the plastic, limiting their downstream applications (Castilho et al. 2009). Therefore, conventional petroleum-based plastics must be replaced with sustainable alternatives.
Bio-based polymers are a promising alternative to reduce the dependency on fossil resources and to address the accumulation and management challenges of plastic waste disposal (Tharanathan 2003; Abe et al. 2021). Out of these, PHAs are the only biopolymers directly synthesized by bacteria.
Polylactic acid (PLA), Polybutylene adipate terephthalate (PBAT) and Polybutylene succinate (PBS) are formed by the chemical polymerization of their monomers (Shaikh et al. 2021).
The conventional PHAs production process is sourced from heterotrophic microorganisms (e.g. Cupriavidus necator) (Chen 2009). Heterotroph PHAs commercialization is hampered by high costs, 2.4–7.5 € kg−1 (Manikandan et al. 2021; Rajendran and Han 2022) compared to 1.9 € kg−1 for Polylactic acid (PLA). The need for organic carbon sources accounts for 30–50% of the total production costs (Lee and Choi 1998; Lynd et al. 1999; Mudliar et al. 2008; Halami 2008; Singh et al. 2017; Troschl et al. 2017). In this context, cyanobacteria present a promising alternative to overcome these drawbacks as they can produce PHAs autotrophically, using inorganic C sources (CO2 or HCO3−) and sunlight without requiring intense aeration. However, compared to heterotrophic PHAs production, the process of PHAs production using cyanobacteria is not as well-developed. According to data from Web of Science© (consulted in November 2023), less than 5% of the scientific publications dealing with PHAs production were related to cyanobacteria. This means, that from the 8192 publications on PHAs production, only 208 were related to PHAs production with cyanobacteria.
Recently there have been many studies focused on improving the PHA yields in cyanobacteria in connection with the understanding of the effect of different cultivation factors in the process (Kamravamanesh et al. 2018b; Arias et al. 2020; Carpine et al. 2020; Koch et al. 2020a; Price et al. 2020; Afreen et al. 2021). However, an industrial PHAs production process with cyanobacteria will only be possible by optimizing the entire process, which includes cyanobacteria cultivation, PHAs production stimulation, PHAs extraction, and purification. This review analyses all aspects of PHAs production via cyanobacteria, from optimization of cultivation conditions to the PHAs purification process. To this aim, first, it describes the main characteristics and advantages of PHAs in front of other plastics. Later, the recently discovered genetic regulators of PHA and glycogen metabolism in cyanobacteria are discussed. As well as the strategies to enhance the PHAs accumulation in cyanobacteria and the recommendations to maintain a cyanobacteria-dominated culture when wastewater is used as cultivation broth. Finally, the recent advances and challenges in PHAs purification and recovery are presented, including prospects, challenges, and recommendations for the future development of biopolymer production with cyanobacteria.
2 Polyhydroxyalkanoates (PHAs)
PHAs are a family of polyesters synthesized naturally by many different prokaryotic microorganisms. PHAs can be classified as medium-chain (mcl-PHAs) if their monomers contain six or more carbons (e.g. poly(3-hydroxyhexanoate)-P(3HHx), poly(3-hydroxyoctanoate)-P(3HO) or poly3-hydroxydecanoate-P(3HD)), or as short-chain (scl-PHAs) if they have 4 or 5 carbons (e.g. polyhydroxybutyrate-PHB and polyhydroxyvalerate-PHV) (Fig. 1) (Naser et al. 2021). Based on the types of microorganisms, carbon source, and the culture condition, PHAs of different chain lengths can be produced (Jiang et al. 2016) (Table 1).
Classification of the different types of PHA according to the number of carbons in their monomers (Wang and Chen 2017)
PHAs are a promising source of plastic (Naser et al. 2021). PHB shares similar properties with polypropylene (PP), except for its elongation at break, which can be enhanced by enriching it with HV to create the copolymer P(HB-co-HV). mcl-PHAs do not become brittle even at low temperatures, a property similar to that of rubber and latex (Muhr et al. 2013). Moreover, PHAs can be processed into final plastic products with the same techniques used for conventional polymers (Muthuraj et al. 2021). Moreover, differently than other biopolymers such as PLA, PHAs can be readily degraded in a variety of environments, such as marine, freshwater, landfill, soil, or home composting (Muthuraj et al. 2021). Additionally, PHA-based polymers have a low permeability to water vapour, CO2, and O2, comparable to those of synthetic plastics, and higher than those of other biodegradable polymers. These characteristic features are highly attractive to the food packaging industry (Byrom 1993; Naser et al. 2021). The major barrier to their further commercialization is production costs (Panuschka et al. 2019; Naser et al. 2021). Table 2 shows a comparison between PHAs and other plastics.
3 Cyanobacteria as PHAs producers
Most of the tested cyanobacteria can produce PHA. Indeed, Kaewbaingam et al. (2016) found that 134 strains from the 137 studied produced PHA. Synechocystis sp. PCC 6803 is the most studied cyanobacteria to produce PHA, as it is a well-studied model organism, easy to grow and genetically modify (Koch et al. 2020b). Nevertheless, other strains, such as Calothrix scytonemicola, Nostoc sp. or Oscillatoria subtilissima, may have higher PHA yields than Synechocystis sp. PCC 6803, but they are much less studied (Ansari and Fatma 2016; Kaewbaingam et al. 2016).
Cyanobacteria photosynthetically synthesize PHA (in the form of PHB) and can also produce other PHA co-polymers by supplementing organic carbon sources. PHAs production via cyanobacteria requires generally the cells to be in a state of nutrient starvation. First, cyanobacteria are grown in a medium rich in nitrogen and phosphorus. Once an adequate concentration of microorganisms is achieved, they are collected and placed in nutrient-limited media (two-step cultivation), or allowed to deplete the nitrogen or/and phosphorus (one-step cultivation). In one-step cultivation, initial N and P concentrations should be optimized to reach this depletion by the due time. Implementing a one-step strategy could significantly reduce production costs since less amount of nutrients are needed and energy consumption due to biomass harvesting is reduced (Drosg et al. 2015; Kamravamanesh et al. 2019). However, slightly higher biomass productivity is expected in the two-stage cultivation, due to the higher nutrient availability which implies a higher growth rate. For instance, Kamravamanesh et al. (2017) reported a biomass-specific growth rate of 0.67 d−1 using two-step cultivation, while using a one-step strategy and similar light conditions, Rueda et al. (2020), observed a specific growth rate of 0.08 d−1.
Once nutrients become limiting, biopolymers start to accumulate in cyanobacteria. Cells first accumulate glycogen and during prolonged chlorosis, a part of the accumulated glycogen is gradually catabolized and transformed into PHAs. Several strategies can be applied during the accumulation phase, also named the starvation phase to further enhance the PHAs accumulation such as the addition of organic carbon sources, inorganic carbon, salinity, or light intensity (Fig. 2) (Eberly and Ely 2012; Ansari and Fatma 2016; Gracioso et al. 2021; Rueda et al. 2022a, c).
Schematic representation of the PHA production process using cyanobacteria. First, cyanobacteria are grown with light and sufficient nutrients (growth phase). Nutrients are reduced with time. Next, the biomass is separated from the cultivation medium and placed in a new medium without N or/and P (two-step cultivation). It is possible to omit the separation step by optimizing the initial nutrient concentration so they are completely exhausted by the end of the growth phase. Without nutrients, the accumulation phase starts and other PHA-stimulating conditions can be applied (e.g., high salinity, acetate, light availability). During this phase, glycogen and PHAs accumulate. If the chlorosis persists for a long time, glycogen is degraded and converted into PHAs
In the next section, research advances on the physiological function of cyanobacterial PHAs and strategies to improve PHA production and reduce costs in an engineered environment are presented.
3.1 PHA metabolism in cyanobacteria
The synthesis of PHA in cyanobacteria is a complex process that involves several metabolic pathways and has many regulators which are not completely known. The exact function of many of these regulators of the PHA metabolism is still unclear (Koch and Forchhammer 2021).
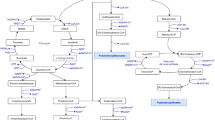
Cyanobacteria produce two main interrelated carbon storage compounds: glycogen and PHA. PHA biosynthesis is strongly related to glycogen synthesis, and both molecules compete for the metabolic intermediate 3-phosphoglycerate (3-PGA) as shown in Fig. 3. This intermediate is produced in the Calvin cycle during CO2 fixation (Zhang and Bryant 2011; Singh and Mallick 2017). Recently, many attempts have been made to explain the interaction between the two polymers and how they are regulated. Dutt and Srivastava, (2018) observed that only 26% of the carbon used to synthesize PHA came from the fixation of inorganic carbon, while the rest came from internal carbon recycling. Koch et al., (2019), discovered that PHA was produced mainly by glycogen catabolism through the Emden–Meyerhof–Parnas (EMP) pathway (Fig. 3, the green pathway), rather than the Entner–Doudoroff (ED) or the oxidative pentose phosphate (OPP) routes. Rueda et al., (2022b) also observed a positive correlation between the expression of genes related to glycogen catabolism with the ones of the PHA metabolism. Considering altogether these evidences, it is hypothesized that the synthesis of PHA from Acetyl-CoA consumes one mol of NADPH per mol of HB, and is used as a sink of electrons to rebalance the ATP/NADPH ratio (Hauf et al. 2013). This fact means that PHA production, rather than other fermentative products is advantageous for the cell, as the intracellular carbon is conserved while ATP is recovered, suggesting that PHA will be produced in those cultivation conditions that favor high reduction equivalents (high content of NAD(P)H or FADH2) (Koch and Forchhammer 2021). PHA is, therefore, an efficient way to recover the reducing power needed in the cell metabolism while keeping the stored carbon.
Simplified representation of the glycogen/PHA metabolism pathways and regulators in the cyanobacteria taking research mostly conducted in Synechocystis sp. The metabolic pathways and enzymes highlighted in bold correspond to the routes upregulated during nutrient starvation. Red lines indicate molecules that cause the up or downregulation of different enzymes. The pathway highlighted in green corresponds to part of the EMP pathway, which is the main route used in cyanobacteria to transform glycogen into PHA (Koch et al. 2019). Pathways highlighted in blue light correspond to the day-night regulation of the GlgB enzyme, by the formation or disappearance of the SbtB:c-di-AMP complex as described by (Selim et al. 2021). The pathways highlighted in yellow correspond to the effect of phosphate inside the cell, that stimulates the synthesis of acetyl phosphate and this one activates the PHA synthase (Krasaesueb et al. 2021). Pathways in light grey correspond to the inhibition of the transformation of the 3-PGA to 2-PGA due to the bounding of the PirC to the PGAM. PirC is released from the PII (and free to be bound to the PGAM) due to the accumulation of the 2-OG. The accumulation of 3-PGA, due to the inhibition of the 3-PGA to 2-PGA transformation, stimulates the activity of the GlgC (Orthwein et al. 2021). Pathways in dark grey are glycolytic pathways present in cyanobacteria but do not play an important role in forming PHA. Abbreviations: ADP-glucose: Adenosine diphosphoglucose; 3-PGA: glycerate-3P; 2-PGA: glycerate-2P; PEP: Phenolpyruvate; ATP: Adenosine triphosphate; ADP: Adenosine diphosphate; PP: Polyphosphate; PGAM: 2,3-phosphoglycerate-independent phosphoglycerate mutase; TCA: tricarboxylic acid cycle; 2-OG: 2-oxoglutarate; CBC: Calvin–Benson cycle; c-di-AMP: second messenger nucleotide; EMP: Emden–Meyerhof–Parnas; ED: Entner–Doudoroff; OPP: oxidative pentose phosphate; GlgA: glycogen synthase, GlgC: Glucose-1-phosphate adenylyltransferase, GlgP1 and GlgP2: glycogen phosphorylase, GlgX: glycogen debranching enzyme, GltA: Citrate synthase, Thl/PhaA: Acetyl-CoA acetyltransferase, PhaB: Acetyl-CoA reductase, PhaC/PhaE: poly(3-hydroxyalkanoate) synthase; PhaP: poly(3-hydroxyalkanoate) inclusion protein; Slr0058: Phasin family protein
PHA may also serve as a valuable structural component for the cell to survive stressful situations and changing environments, such as nutrient depletion and osmotic shock (Damrow et al. 2016; Obruca et al. 2017). In Synechocysts sp. PCC 6803, during resuscitation from chlorosis, it was observed that PHAs are not degraded, but they are equally distributed among daughter cells (Koch et al. 2020c). Indicating that PHAs may not serve as a carbon source used during chlorosis recovery, but as a structural component related to the nucleotide and essential to restart cell proliferation.
Many PHA and glycogen metabolism regulators control the production and transformation of these two polymers (Fig. 3). For instance, P transport and content in the cell play an important role in PHA and glycogen metabolism (Kamravamanesh et al. 2018a; Krasaesueb et al. 2021). Kamravamanesh et al., (2018a) produced a random UV mutated Synechocystis sp. PCC 6714 strain with 2.5-fold higher PHA production, increasing from 14%dcw in the wild-type strain to 37%dcw. This strain had a mutation in the pstA gene, which is a membrane protein related to the phosphate transport system. This mutation derived from a complex change of the cell metabolism which included the downregulation of the sphU gene, which is a regulator of the P transport that prevents the assimilation of additional phosphate by closing the transporter when inorganic phosphorus is abundant in the culture medium. The authors could not completely link the metabolic changes to the new genotype, but it was hypothesized that imbalanced P transport caused the increased PHA production (Kamravamanesh et al. 2018a). Subsequently, Krasaesueb et al., (2021) further studied the effect of this SphU regulator on PHA production. Under normal photosynthetic conditions (wild-type) the SphU regulon is activated in response to phosphate-limiting conditions leading to the upregulation of genes involved in the phosphate transportation and to the accumulation of polyphosphate and acetyl phosphate in the cell (Summers et al. 1999). Silencing the sphU gene in Synechocystis sp. PCC 6803 allowed the incorporation of P inside the cell even when P was abundant in the cultivation media, which translated in a 15-fold increase in PHA production, which means a 14.5%dcw compared to a 1%dcw in the wild type under non-producing conditions. This increased phosphate inside of the cell activated the synthesis of acetyl phosphate, which is an intermediate for the synthesis of acetyl-CoA and intracellular acetate (Fig. 3, yellow pathway). This discovery may be of great interest to produce PHA under non-limited nutrient conditions, which means that there would be no need to disconnect the growth and accumulation phase. PHA would be produced directly during growth, which would increase productivity.
Metabolic regulations directly impacting glycogen synthesis and catabolism have been also studied. Cyanobacteria coordinate the glycogen metabolic switch to survive the day-night cycles by implication of the second messenger nucleotide (c-di-AMP) (Selim et al. 2021). During the day, SbtA (HCO3− transporter) interacts with the c-di-AMP to form SbtB:c-di-AMP complex. This SbtB:c-di-AMP complex further stimulates the glycogen-branching enzyme (GlgB). During the night, glycogen synthesis is stopped due to the reduction of the c-di-AMP and the subsequent absence of the SbtB:c-di-AMP complex. The energy required to survive the night is produced through the catabolism of the glycogen stored during the day (Fig. 3, blue pathway) (Selim et al. 2021). When there is a nitrogen shortage, cyanobacteria switch their metabolism to produce glycogen and PHA (Orthwein et al. 2021). Under these circumstances, a small inhibitory protein known as PirC, a regulator of carbon storage, inhibits phosphoglycerate mutase (PGAM), slowing the flow of carbon toward lower glycolysis through PGAM. Upon N-starvation, the levels of the Tricarboxylic Acid Cycle (TCA) intermediate, 2-oxoglutarate (2-OG), increase, which is sensed by the signalling protein PII (Forchhammer et al. 2022). The PII-PirC protein complex, which prevails under normal growth conditions, dissociates at increased 2-OG levels, releasing the PGAM inhibitor PirC. The binding of PirC to PGAM inhibits the conversion of 3-phosphoglycerate (3-PGA) into 2-phosphoglycerate (2-PGA). Consequently, the increase in the concentration of 3-PGA stimulates glycogen synthesis. The metabolic disruption of PirC, allows more carbon to flow towards 2-PGA which finally leads to PHA levels that are increased up to 49%dcw from a 29%dcw in the wild-type, during prolonged nitrogen starvation (Orthwein et al. 2021) (Fig. 3, grey pathway).
The formation of PHA granules is also complex and puzzling. The surface of PHA is coated by different proteins, called phasins, which are involved in the PHA metabolism. Phasins regulate transcriptional response, depolymerization of the PHA, and regulation of the formation of granules (Hauf et al. 2015; Koch et al. 2020b). The open reading frame (ORF) ssl2501 encodes for phasin PhaP in Synechocystis sp. PCC 6803 (Hauf et al. 2015). PhaP modifies the size and number of granules in the cell by regulating the PHA synthetase activity (Hauf et al. 2015). Slr0060 is a PHA depolymerase, however, its function is unclear, as its deletion did not cause an increase in the amount or size of PHA granules (Koch et al. 2020b). Slr0058 expresses the phasin, PhaF, which may be involved in the initiation of PHA granule formation as PHA is not properly aggregated in its absence (Koch et al. 2020b).
The precise regulation of cyanobacteria PHA metabolism and its physiological functions are still puzzling, however, many research advances have been made in recent years, benefitting the further development of industrial cyanobacteria PHA production and the development of new modified strains. There is still work to be done to thoroughly understand the regulation of the PHA metabolism under different cultivation conditions.
3.2 Effect of the cultivation conditions on cyanobacterial PHAs production
Several strategies have been applied during the accumulation phase (Fig. 2) to enhance the PHA production in cyanobacteria, among them, nutrient deficiency and supplementation of organic carbon sources like glucose, acetate, fructose, or valerate are the most prevalent (Carpine et al. 2020). Recently, other factors, such as the content of inorganic carbon, salinity, or light intensity, have also been seen to have a role in accumulation. This section critically reviews the most recent findings on the effect of different factors on cyanobacteria PHA.
3.2.1 Effect of inorganic carbon
Inorganic carbon (CO2 or HCO3−) is essential for the generation of new organic molecules during photosynthesis (Markou et al. 2014). Nevertheless, only a few studies have evaluated the effect of varying amounts of inorganic carbon on PHA production, and the results are somehow contradictory (Eberly and Ely 2012; Kamravamanesh et al. 2017; Rueda et al. 2022b). Kamravamanesh et al., (2017) and Eberly and Ely, (2012), found that the increase in DIC concentration harmed PHA production (Table 3). On the contrary, Rueda et al. 2020, 2022b, reported a positive correlation between carbon and PHA production. The cause of the differing effects of DIC found in these studies remains unclear. One possible explanation is that adding high concentrations of CO2 can drastically lower the pH in the culture, which may lead to metabolic activity decrease or culture crash if pH is not well controlled. Another explanation is the use of various inorganic carbon sources (CO2 or NaHCO3). Nevertheless, CO2 exists as HCO3− at pH values between 6.5 and 9, which is the typical pH at which cyanobacteria are cultivated. Therefore, the only difference between the two DIC sources should be the increase in the ionic strength when NaHCO3 was added. The different capacities of the different strains to resist the osmotic shock may explain these contradictory results.
3.2.2 Effect of salinity
Several studies on heterotrophic bacteria indicate that the presence of PHAs in the cell enhances their survival under high osmotic pressure by improving the cell’s ability to maintain water content (Obruca et al. 2017).
Cyanobacteria also present several strategies for their protection against osmotic stress: (1) they produce compatible solutes (low molecular weight compounds such as sucrose, trehalose, glucosylglycerol, glucosylglycerate or glycine betaine, that increase osmolarity (Klähn and Hagemann 2011), (2) they activate or inactivate the ion transporters to regulate the water content inside the cell (Pade and Hagemann 2015), (3) they reorganize the thylakoid structure (Pandhal et al. 2008); (4) they increase the amount of nutrient binding proteins in the membrane (Pandhal et al. 2008); and (5) they modify the extracellular layers of the periplasmic space (Pandhal et al. 2008). By analogy to the observations made for heterotrophic bacteria, it can be hypothesized that PHAs accumulation also serves as a mechanism for cyanobacteria to adapt to osmotic stress. Meixner et al. (2022), observed that the optimal amount of salt to stimulate PHA production depends on the strain. For instance, Synechocystis IFA3 required 20 g NaCl L−1 to reach its maximum PHA content, a 3.2%dcw. Synechococcus sp., required an optimal NaCl content of 9 g L−1 (Rueda et al. 2022a). When 1 g L−1 of NaCl was added to Nostoc muscorum NCCU- 442, PHB content increased from 7.6%dcw to 8.15%dcw (Ansari and Fatma 2016). The addition of 50 g NaCl L−1 increased the PHB concentration from 5.9%dcw to 7.45%dcw in Spirulina subsalsa (Shrivastav et al. 2010). The halotolerant cyanobacterium Synechocystis cf. salina CCALA 192 required 40 g NaCl L−1 to reach a 6.9%dcw of PHA (Meixner et al. 2022). These results suggest that higher salinities are needed to boost the PHAs production in cultures adapted to salinity, indicating that this polymer may play a role in protecting the cell against osmotic stress. This agrees with the fact that Synechocystis sp., a moderately halotolerant cyanobacteria, requires a higher NaCl concentration to stimulate its PHAs production than Synechococcus sp. or N. muscorum NCCU- 442, non-halotolerant species, but less than the amount required by Spirulina subsalsa or Synechocystis cf. salina CCALA 192, both halotolerant strains.
However, the observed effect of salinity on cyanobacterial PHAs production is very limited (Table 3) (Shrivastav et al. 2010; Ansari and Fatma 2016; Rueda et al. 2022a, c). Although a salinity increase stimulates PHA production, it may also affect the properties of the polymer (Rueda et al. 2022c). Hence, the NaCl concentration should be wisely selected and controlled for each strain, since above certain values it harms polymer accumulation.
3.2.3 Effect of light
The presence of day and night cycles, instead of constant illumination, increased PHA production (Arias et al. 2018b; Koch et al. 2020a). The need for dark cycles seems to be related to the fact that the resulting low oxygen, or even anoxic conditions, boosts glycogen catabolism (glycolysis) to produce ATP. The reducing power needed in glycolysis can be regenerated by converting pyruvate into PHA. In this process, the carbon is conserved in the form of PHA, rather than being consumed by other fermentative processes (Koch et al. 2020a). A series of studies done with Nostoc muscorum and Synechocytis sp. CCALA192, indicate that a darkness period (3–5 days) at the end of the accumulation phase stimulates PHA production (Sharma and Mallick 2005; Sharma et al. 2007; Troschl et al. 2018). Another study observed a negative effect of a dark period for Synechocystis sp. and Synechococcus sp. cultures on PHAs accumulation (Rueda et al. 2022a). These differences may be explained by the presence of oxygen in the cultures of Synechocystis sp. and Synechococcus sp. due to the bubbling of compressed air (Rueda et al. 2022a). Dark cultivation must be performed under anoxic conditions since, otherwise, glycogen will be used during respiration, hindering its transformation into PHAs if oxygen is present.
Light intensity had differing effects on PHAs accumulation. Similar PHAs concentrations were obtained when light intensity was increased from 100 to 500 µmols m−2 s−1 in cyanobacteria cultivated under autotrophic conditions (Monshupanee and Incharoensakdi 2014; Rueda et al. 2022c). While, a higher PHA content was obtained by increasing light intensity in cultures grown mixotrophically (Faradinho et al. 2016; Gracioso et al. 2021). These differences may be caused by the fact that increased light intensity increases cyanobacteria growth rate and nutrient consumption, which will cause stronger nutrient starvation forcing the PHAs accumulation, rather than by the effect of light by itself. Further research is, therefore, necessary to clarify why light becomes beneficial under mixotrophic growth.
3.2.4 Effect of organic carbon sources
Organic carbon sources such as acetate, valerate, glucose, and fructose have been extensively used in many investigations to enhance PHAs production (Carpine et al. 2020) (Table 3). The supplementation of these compounds increases the pool of acetyl-CoA and the [acetyl CoA]/[CoA] ratio. PHA synthesis is subsequently activated to rebalance this ratio (Ren et al. 2009; Yashavanth et al. 2021). The positive effect of these organic carbon supplementations is extendible to most cyanobacteria studied (Miyake et al. 1997; Panda and Mallick 2007; Samantaray and Mallick 2014; Taepucharoen et al. 2017; Rueda et al. 2022a), however, the PHA yield increase depends on the cyanobacteria strain, culture conditions and probably other unknown factors. The amount of supplemented organic carbon plays an important role in PHA accumulation. In general, the higher the amount of organic carbon added, the higher the amount of PHA accumulated (Table 3).
Although the supplementation of organic carbon sources is a promising alternative to achieve high PHAs contents, their usage could increase production costs, which would hinder the competitivity of cyanobacteria PHAs production process compared to heterotrophic bacteria (Price et al. 2022). Moreover, the addition of organic carbon would increase the potential for bacterial growth when the process is scaled up to non-sterile outdoor conditions (Troschl et al. 2017). Therefore, the addition of organic carbon in cyanobacterial cultures should be minimized and very well controlled to the point necessary to achieve good productivity.
3.2.5 Effect of cultivation conditions on polymer properties
The quality and mechanical properties of the produced biopolymer are determinants for their final application. Polymer properties are affected by their monomer composition and chain length. Polyhydroxybutyrate (PHB) is the primary PHA polymer synthesized by cyanobacteria, but it is known to be highly brittle and crystalline. Adding other monomers different than the 3-hydroxybutyrate (3HB) to the chains can improve PHB properties. For instance, the introduction of 3-hydroxyvalerate (3HV) improves PHB crystallinity and thermal properties (Chan et al. 2019). On the other hand, the incorporation of 3-hydroxyvalerate resulted in a decrease in Young’s Modulus and tensile strength, indicative of a heightened elasticity in the material (Bhati and Mallick 2015). Elongation to break can also be increased by adding a higher proportion of co-monomers (such as 3-hydroxyhexanoate) (Asrar et al. 2002). Variations in biopolymer molecular weight can also significantly influence mechanical properties. PHAs exhibiting high molecular weights (600 kDa) can serve as thermoplastics, whereas those with low molecular weight (< 400 kDa) are characterized as brittle (Luzier 1992; COX 1994; Penloglou et al. 2012).
The type of cyanobacteria strains and culture conditions affect the polymer composition and properties (Miu et al. 2022). For instance, Rueda et al., (2022c), observed that for Synechocystis sp. cultivated under a salinity of 12 mS cm−1 (approximately 40 g salt L−1) and a salinity of 60 mS cm−1 (approximately 40 g salt L−1) the molecular weight of the produced polymer was reduced from around 100 to 38 kDa. The type of carbon source especially affects the type of co-polymer. For example, Nostoc muscorum produced the homopolymer PHB under photosynthetic conditions (CO2) and also under acetate supplementation, while the co-polymer poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (P(3HB-co-3HV) was produced when propionate was added (Mallick et al. 2007). On the other hand, poly(3-hydroxybutyrate-co-4-hydroxybutyrate) (P(3HB-co-4HB)) was obtained by supplementing Synechocystis sp. PCC 6803 cultures with γ-butyrolactone (Tanweer and Panda 2020). The amount of each co-polymer could be tailored by changing the concentration of each of the organic carbon sources. For instance, Tanweer and Panda, (2020), found that by increasing the concentration of γ-butyrolactone from 0.1 to 0.4%, the monomer 4HB increased from 16 to 43%mol. When both acetate and γ-butyrolactone were added at a concentration of 0.4% and 0.1% respectively, the content of 4HB decreased from 16 to 11%mol and the 3HB monomer content increased from 84 to 89%mol (Tanweer and Panda 2020). A strong relationship of the carbon source with polymer properties has also been observed for heterotrophic bacteria (Penloglou et al. 2012; McAdam et al. 2020). Penloglou et al. (2012), observed that by increasing the C/N and the C/P ratio the molecular weight of the synthetized polymer can be increased. Sanhueza et al.( 2020) reported that for P. xenovorans LB400, the use of mannitol, xylose or glucose as a carbon source changed the molecular weight of the polymer from, 2122 kDa, 1708 kDa and 1589 kDa respectively. Garcia-Gonzalez et al. (2015) tested the possibility of producing PHB autotrophically using Cupriavidus necator DSM 545 previously grown using different carbon sources. This study demonstrates that the polymers produced autotrophically have a lower molecular weight compared to polymers produced from organic substrates. They also reported that cells previously grown on glucose accumulated polymers with a higher molecular weight and melting temperature than the cells previously grown on glycerol (Garcia-Gonzalez et al. 2015).
Culture conditions seem to have a pivotal role in customizing bioplastic production to meet specific requirements for each of the desired applications. However, there is a notable absence of comprehensive information regarding the influence of cultivation conditions on the thermo-mechanical properties of PHA, especially for PHB produced for cyanobacteria, but also for heterotrophic bacteria (McAdam et al. 2020).
3.2.6 Optimal cultivation conditions
Figure 4 shows the relative effect of each of the reviewed parameters on PHAs accumulation. The addition of Na+ rather than the increase in carbon availability itself was primarily responsible for how NaHCO3 increased PHAs formation (Fig. 4). Optimal salinity is strain-dependent and strongly related to the capacity of each strain to survive osmotic stress (Rueda et al. 2022c; Meixner et al. 2022). A period without illumination enhanced PHAs production in some studies (Sharma and Mallick 2005; Sharma et al. 2007; Troschl et al. 2018). It is possible that in the dark and anoxic conditions are appropriate for PHA accumulation. The supplementation of organic carbon sources (citrate, acetate, fructose, glucose, valerate) stimulates PHA production in all cyanobacteria, however, the extent of this effect depends on the strain and the amount of organic carbon. Moreover, the modification of the cultivation conditions, especially the addition of different organic carbon sources at different concentrations can also be used to tailor the polymer properties.
Box-plot of the difference in the PHA content when modifying the studied parameters. The dotted line indicates the limit between positive and negative effects. Lower and upper box boundaries represent the 25th and 75th percentiles, respectively; the line inside the box represents the average; lower and upper error lines show the maximum and minimum of the studied data; and black circles represent the actual value of each study. The PHA difference was obtained by comparing the optimal results with the control condition obtained in each of the studies reviewed in Table 3 (Miyake et al. 1997; Sharma and Mallick 2005; Sharma et al. 2007; Eberly and Ely 2012; Samantaray and Mallick 2012, 2014; Monshupanee and Incharoensakdi 2014; Kamravamanesh et al. 2017; Gracioso et al. 2021; Rueda et al. 2022c, a, b)
Although general trends can be observed, there is high variability between studies using similar cultivation conditions. Indeed, using the same cultivation conditions and strain, Rueda et al., (2022b) obtained 14%dcw of PHA, while Rueda et al., (2022c) obtained only 6.4%dcw PHA. These differences may be related to uncontrolled variables, reactor design, previous cultivation modes, temperature, other microorganisms growing in the culture, nutrient consumption, etc. On the other hand, in some cases, the maximum PHA accumulated was maintained for some period (Miyake et al. 1997; Carpine et al. 2018; Kamravamanesh et al. 2018a; Rueda et al. 2020, 2022a), while in other studies, a PHAs production peak was obtained followed by a rapid decline (Samantaray and Mallick 2012; Monshupanee and Incharoensakdi 2014; Rueda et al. 2022b). The explanation for these mixed results is unclear, but it does not appear to be related to the amount of PHA accumulated or the carbon source used. A feasible hypothesis is that PHA is degraded and cells begin to lyse after a certain time under nutrient limitation. The extent to which this happens may depend on the age and the state of the culture. Future research should target the effects of varying parameters to create a standardized process that will allow better comparison between studies.
3.3 Use of wastewater to produce PHA with cyanobacteria
The use of wastewater has been reported for microalgae biomass production to be an excellent strategy to reduce production costs from 3.2 to 1.8 € kg−1 of biomass (Acién et al. 2012). In alignment with this trend, cyanobacteria can potentially grow and treat wastewater at a reduced cost with higher efficiency than traditional wastewater treatment processes (Oswald 1988; Selvaratnam et al. 2015; Li et al. 2019). Cyanobacteria are useful bioaccumulators for the bioremediation of emerging contaminants such as dyes, hydrocarbons, phenolic compounds, pesticides, and herbicides (Touliabah et al. 2022). However, using wastewater to grow cyanobacteria for PHA production presents several challenges. One of the most crucial is the need to keep cultures cyanobacteria-dominated during extensive, non-sterile cultivation.
There are several strategies/recommendations to maintain cyanobacteria as the dominant microorganism in cultures: (1) use of wastewaters with low organic carbon content to reduce the growth of heterotrophic bacteria (Arias et al. 2020); (2) keep a high N:P ratio (> 32:1 molar) and low phosphorus concentrations (Arias et al. 2017); (3) optimize the hydraulic (HRT) and solids retention times (SRT) to retain filamentous cyanobacteria and avoid the growth of unicellular microalgae and (4) as usually done with mixed heterotrophic bacterial cultures, a feast-famine strategy could be applied to specifically select those microorganisms that accumulate more PHAs (Fardinho et al. 2016; Arias et al. 2018a). This strategy consists of adding the substrate in an intermittent regime. During the feast phase, microorganisms accumulate PHA. Then, in the famine phase, only the microorganisms that can grow using stored PHAs will survive (Fradinho et al. 2016).
Although several studies have evaluated the potential of cyanobacteria to treat wastewater, only a few of them assess the use of nutrients from wastewater to grow and produce PHA. The results of these few studies are found in Table 4. Synechocystis sp. PCC 6803 ΔSphU, which is a mutant with phosphate transport regulation genes deleted, growing in filtered shrimp wastewater produced 32.5%dcw of PHA after 14 days of cultivation (Krasaesueb et al. 2019). Aulosira fertilissima cultivated outdoors for 27 days in aquaculture wastewater reached an 80%dcw PHA (Samantaray et al. 2011). Synechocystis salina accumulated 5.5%dcw of PHA in 36 days growing in sterilized digestate from the anaerobic digestion of thin stillage (Meixner et al. 2018). Nostoc muscorum obtained 65%dcw of PHA accumulated in 20 days (Bhati and Mallick 2016). Wastewater generated during PHA extraction can also be used for cyanobacteria cultivation. During PHAs extraction, many chemicals and non-polymeric biomass are generated. This waste can potentially be recirculated as a source of nutrients for new cultures (Da Silva et al. 2018). All the reviewed studies evidenced that PHA production using cyanobacteria cultivated in wastewater is a promising strategy to enhance the environmental benefits and reduce production costs. There are still numerous issues that need to be addressed further, particularly those related to the robustness and scaling up of the process (Arias et al. 2020). Additionally, the use of wastewater also presents sociological challenges, such as consumers’ acceptance of products produced from wastewater, the lack of a robust regulatory framework, and the interest of investors in these processes (Josa and Garfí 2023).
4 PHA recovery
PHA recovery consists of 4 main steps: (1) biomass harvesting, (2) pre-treatment, (3) PHA extraction, and (4) PHA purification (Fig. 5). PHA recovery from cyanobacteria is a relatively immature process, and only a few studies have evaluated its efficiency. Recovery is a crucial phase of the PHA production process because it affects product yield, quality, environmental impact, and process costs (Koller et al. 2016). In this section, the strategies used in each step of PHA recovery will be reviewed.
Conventional steps for PHA downstream and most common methods for each stage (adapted from (Pérez-Rivero et al. 2019))
The first step of the recovery process is harvesting the cyanobacteria biomass. This step is challenging as cyanobacteria cells are small and may have relatively low cell densities (Drosg et al. 2015). 20–30% of the costs associated with the production of microalgae and cyanobacteria are due to the harvesting of the cell (Gudin and Thepenier 1986). Microalgal and cyanobacteria species characteristics, such as cell size, affect the harvesting efficiency. Larger microalgae or cyanobacteria (such as Spirulina platensis) are usually easier to recover than smaller diameter species (such as Synechocystis and Synechococcus) (Drexler and Yeh 2014).
Conventional methods for harvesting microalgae or cyanobacteria are membrane filtration, centrifugation or sedimentation usually enhanced with coagulation and flocculation (Pérez-Rivero et al. 2019). At present, novel cost-effective microalgal/cyanobacterial biomass harvesting technologies are being investigated. For instance, a new cyanobacteria concentrator based on microfluidics was developed in which a diluted suspension is introduced into a microchannel and the inertial forces generated from the structure geometry move the cyanobacteria laterally to a known position from where the concentrated stream is recovered (Wang and Dandy 2017). Magnetic nanoparticles were also used to improve harvesting, reaching a harvesting efficiency > 93% (Wang et al. 2020). Another possible alternative to reduce harvesting costs is to grow cyanobacteria in biofilm reactors (Strieth et al. 2018), however, not all cyanobacteria can easily form biofilms.
After harvesting, biomass needs to be pre-treated to enhance accessibility to the polymer and increase polymer properties. Cell disruption is needed to completely dissolve PHA (Meixner et al. 2018). Different mechanical methods such as freeze-thawing, sonication, homogenization, bead milling etc. have been seen to effectively disrupt the cell with low operational cost. However, they present certain drawbacks associated with their practical application in large-scale product recovery. Bead milling and homogenization, for instance, have a high energy demand. Ultrasonication is easily scalable but may be not effective in disrupting some strains and cause heat that may damage other sub-products (Corrêa et al. 2020). In this context, the pulsed electric field technique (PEF) has gained recognition for extracting a wide range of metabolites, including lipids, proteins, and carotenoids, from cyanobacteria. This approach utilizes an electric field to enhance the irreversible pore formation and the subsequent release of compounds from the cell. PEF has emerged as a prominent method due to its cost-effectiveness, high efficiency, minimal time requirements, and environmentally friendly characteristics (Corrêa et al. 2020). Another interesting alternative method is the use of viruses that lyse cyanobacteria cells. This method significantly reduces the use of chemicals and energy consumption and they are not likely to cause health problems to humans or ecosystems. Moreover, viruses are easy to acquire and maintain, so they are cheaper than enzymes (Sun and Zhou 2019). The main drawback of this method is that it is a slow method; it requires several days, compared to ultrasonication which requires only some minutes.
Meixner et al., (2018) also observed that removal of carotenoids and chlorophyll was necessary before a complete PHA dissolution into chloroform. This pre-treatment step could be leveraged to recover other interesting bioproducts from cyanobacteria, such as pigments or exopolysaccharides (EPS), etc. After recovering all these co-products, PHAs could be purified from the remaining biomass (Strieth et al. 2018).
PHA can be extracted by solubilizing the polymer using organic solvents or by directly digesting the cellular components surrounding the polymer (Table 5). Solvent extraction is the most widely used method for PHA recovery, as it is simple, quick, and extracts polymers with a high purity and molecular weight (Pérez-Rivero et al. 2019; Carpine et al. 2020). The majority of the employed solvents are halogenated organic compounds, with chloroform being a particularly common choice. Halogenated solvents pose several challenges due to their high cost, adverse effects on aquatic environments, contribution to ozone layer depletion and they are potentially harmful to human health, as they are suspected carcinogens. Therefore, it is crucial to find safer alternatives (Pérez-Rivero et al. 2019). Methylene chloride or 1,2-dichloromethane were tested for PHA extraction from cyanobacteria and obtained similar yields to chloroform (7.11% and 6.98% of PHB extracted respectively, in front of 7.5% with chloroform) (Ansari and Fatma 2016). However, these are halogenated solvents that have the same aforementioned adverse effects. Alternative non-halogenated solvents for PHA extraction from cyanobacteria have never been evaluated. 1,3-dioxolane, which is a non-chlorinated green solvent, obtained recovery yields of 89.7% in front of 53.9% for chloroform and similar purity, 94% and 93.8% respectively for 1,3-dioxolane and chloroform when extracting PHA from Cupriavidus necator. Similar trends were obtained for PHA extracted from cyanobacteria using 1,3-dioxolane in a preliminary study (Bantysh 2021). Butyl acetate at 103 °C, for 30 min, was also found to be a good alternative to halogenated solvents in C. necator, leading to a recovery level of 96%, a purity of 99% and a higher molecular weight than chloroform. 1400 kDa using butyl acetate in front of 1000 kDa for chloroform (Aramvash et al. 2015). However, this solvent has not been yet tested to extract PHA from cyanobacteria.
Another strategy to recover PHA is to digest the cellular components surrounding the polymer. Kobayashi et al., (2015) evaluated ionic liquids as alternative solvents to dissolve cyanobacteria and recover PHA by filtration (Kobayashi et al. 2015). More than 98% of the PHA was retained after membrane filtration with the solvent 1-ethyl-3-methylimidazolium methylphosphonate. (Kobayashi et al. 2015). Other innovative biological methods have also been applied to recover PHAs. Murugan et al., (2016), demonstrated the feasibility of recovering PHA using mealworms, which eat and digest the non-PHA cell and excrete the PHA granules that can be cleaned using detergents. This process decreases the use of solvents and chemicals and reuses the non-PHA residual cellular materials to feed the worms, which can be an alternative source of proteins for animal feed. Using this methodology PHA was extracted from C.necator with a purity of 100% and a molecular weight of 240 kDa, equal to that of chloroform (Murugan et al. 2016). Genetically modified Bdellovibrio bacteriovorus Bd3709 was also used to recover PHA with an 80% efficiency from heterotrophic bacteria. These bacteria predate the gram-negative bacteria, releasing the PHA granules to the culture media (Martínez et al. 2016). Enzymes (i.e. proteases) are also promising to recover PHA, as they are very specific and use mild conditions, which give rise to high reaction rates with little product damage (Kapritchkoff et al. 2006). For instance, using 2.0% of bromelain per mass of biomass, 88% of PHA is recovered with a purity of 66% from Ralstonia eutropha DSM545 (Kapritchkoff et al. 2006).
Although these PHA extraction strategies seem promising to reduce PHA extraction costs, they have been tested only on heterotrophic bacteria. There are very few studies applied to cyanobacteria and most of them do not evaluate polymer properties after extraction. Compared to heterotrophic bacteria, cyanobacteria have a thicker and more complex cell wall, composed of peptidoglycan and cellulose, which is difficult to dissolve (Mehta et al. 2015). Therefore, methods used on heterotrophic bacteria may not be as efficient in cyanobacteria. It is, therefore, necessary to direct future research efforts in the evaluation of the new green purification protocols with cyanobacteria biomass. Moreover, the effect of the PHA extraction method applied, on the polymer properties and quality should be evaluated. Additionally, independently of the extraction method, PHA content in the cell has also a strong effect on the purity and extraction efficiency of the purification process. Cells that have a lower PHA content leave more cellular debris after extraction, thus, more energy and digesting agents are needed to break the cells, which leads to lower efficiency of the process and lower purity (Mohammadi et al. 2012). Therefore, to reach high purities and recovery yields, it is essential to still work on improving the PHA content in the cell.
5 Economic and environmental process sustainability
Cyanobacterial PHA offers a promising pathway towards the production of sustainable bioplastic, as they utilize inorganic carbon sources for growth (CO2 and HCO3−) and they do not require intensive aeration for oxygenation. However, there are still significant technical barriers to be solved before production can be economically and environmentally viable (Price et al. 2022). In comparison with heterotrophic microorganisms, cyanobacteria growth rate is very slow, lower cell concentrations can be achieved in the photobioreactors due to light limitation (Acién Fernández et al. 1999; Bähr et al. 2016) and PHA accumulated in the cell is in general lower than in heterotrophic microorganisms. These technical disadvantages can hinder the economic viability of the process and reduce its sustainability.
Recently, some authors have evaluated the economic viability of a hypothetical industrial plant for this process. Panuschka et al. (2019), determined that under optimal cultivation conditions (60%dcw of PHA, cell cultivated in a thin layer photobioreactor in south Europe) the minimum selling price for the produced PHB would be 24 € · kg−1 PHB. Similarly, Price et al. (2022), evaluated different plausible scenarios to reduce PHA production costs, such as the use of solar energy to reduce electricity costs, the use of wastewater as a cultivation media, deep ‘PHB ripening ponds’ to reduce capital and operating costs, biogas production from the remaining biomass and the pigment production using a biorefinery approach and carbon credits for the use of CO2. The combination of all these improvements reduced the PHA production costs up to 7 €· kg−1 PHB, which is still approximately twice the current market price of PHA. Rueda et al. (2023), determined that to obtain competitive costs, PHA productivity should be increased up to 810 mg · L−1 · d−1, while the actual maximum PHA productivity achieved is 59 mg L−1 d−1 (Kamravamanesh et al. 2017). The three studies agree that the highest costs were related to the major equipment costs.
Regarding process sustainability, (Rueda et al. 2023), observed that increasing PHB productivity drastically reduce all environmental impacts of the process. These impacts were mainly caused by construction materials and the use of chemicals, especially the use of chloroform for PHB purification. Compared with other PHA production processes and fossil-based polymers, the production of PHB with cyanobacteria has similar environmental impacts (Rueda et al. 2023). Energy Consumption is slightly lower than that of other PHA production processes and fossil-based plastics. Impacts on Fine Particulate Matter and Freshwater Eutrophication are similar to other PHA production processes. Indeed, the impact on these categories would be lower than the one of heterotrophic bacteria if the technology is further developed to become cost-competitive. Impacts in Stratospheric Ozone Depletion were higher for cyanobacteria. This is due to the chloroform extraction methodology used. Nevertheless, comparability between PHA production processes is limited. Differences in the technology maturity should also be considered. Those processes with a high maturity (i.e. fossil-based plastics production) are more likely to be highly optimized and to have improved efficiency concerning the immature processes (i.e. PHA production from cyanobacteria) (Walker and Rothman 2020).
Both environmental and economic evaluations of the process, underscore the necessity of increasing both the content of PHA and biomass productivity. This can be achieved by using genetically modified strains able to attain high PHA content, such as the strain recently developed by Koch et al. (2020b), which achieved an 81%dcw of PHA under photoheterotrophic growth conditions. Additionally, using reactors with a lower volume-to-surface ratio is also necessary to achieve these higher productivities. Alternative extraction methods, not using chloroform, as the ones described in the previous section, are also essential to reduce the environmental impact of the process.
6 Conclusions and future perspectives
The productive sectors of the 21st Century rely heavily on plastics; however, conventional petroleum-based plastics pose a growing environmental challenge due to their limited biodegradability and escalating pollution issues. PHA is a promising substitute for these plastics since they can be readily degraded in a variety of environments, such as marine waters, freshwaters, landfills, soils, or home composting devices. Moreover, PHA has similar properties to conventional polymers and can be processed with the same techniques.
PHA can be produced from cyanobacteria cultures from CO2 and sunlight with the potential to reduce production costs and environmental footprint in comparison to heterotrophic bacteria because (1) utilize inorganic carbon sources for growth (CO2 and HCO3−), and (2) they do not require intensive aeration for oxygenation. Achieving an industrial-scale production process for PHA with cyanobacteria necessitates a comprehensive optimization of the entire workflow, encompassing cyanobacteria cultivation, PHA production stimulation, as well as the extraction and purification of PHA. Concerns arise from the fact that much of the existing literature predominantly addresses isolated aspects of the process, leaving uncertainties about the practical feasibility and applicability of the overall concept of the process. Also, the fundamental knowledge of the function and regulation of metabolisms is still incomplete and limits the development of industrial PHA production.
In the next paragraphs, we list and describe the crucial challenges that have to be overcome:
-
Increased biomass productivity: cyanobacteria biomass productivity must be increased to make PHA from cyanobacteria commercially viable (Rueda et al. 2023). New photobioreactor designs should be, therefore, developed to obtain cultures with an increased cell density. This is especially challenging when using photosynthetic microorganisms, because of light limitation due to self-shading (Acién Fernández et al. 1999; Bähr et al. 2016). Therefore, new designs should consider an efficient light transmission meanwhile, they enable a good CO2 supply and O2 exchange (Hoschek et al. 2019). New promising reactor configurations reaching high cell densities have been recently developed. For instance, capillary microreactors made of borosilicate (2 mm inner diameter) (David et al. 2015) were able to reach up to 48 g · L−1 when cultivating Synechocystis sp. PCC 6803 together with P. taiwanensis (Hoschek et al. 2019). Thin layer cascade reactors reached a cell density of 17 g L−1 and biomass productivities of 4 g L−1 d−1 with Nannochloropsis salina (Apel et al. 2017). Two-tier reactors consisting of two chambers divided by a membrane attained a cell density of 30 g L−1 with Synechococcus sp. PCC 7002 (Bähr et al. 2016). However, none of them have been yet applied at an industrial scale, most of them had a volume < 1 L. Moreover, an economic analysis of the new alternative photobioreactors has not yet been done. It is essential to evaluate if the higher biomass production economically and environmentally compensates for the use of materials and energy consumption.
-
Improvement of PHA accumulation: increasing PHA yield is one of the main challenges of cyanobacteria cultures for minimizing production costs and making the process economically feasible. Although literature describes general trends on the factors that affect PHA production, there is high variability in results between studies using similar cultivation conditions. Future research should target the effects of varying parameters to create a standardized process that will allow better comparison between studies.
PHA production has been seen to be stimulated by an increase in salinity, but the optimal salinity depends on the capability of each strain to resist osmotic stress. A period of darkness under anoxic conditions may also stimulate accumulation. Light intensity seems to enhance PHA production, especially under mixotrophic conditions. Organic carbon supplementations also stimulated PHB and other co-polymers synthesis. Culture conditions seem to have a pivotal role in customizing bioplastic production to meet specific requirements for each of the desired applications. However, there is a notable absence of comprehensive information regarding the influence of cyanobacteria cultivation conditions on quality and mechanical properties.
We envisage two different approaches to further increase the PHA content in cyanobacterial cells: (1) screening of new cyanobacteria strains and (2) genetically modifying the cells. There may be unexplored strains exhibiting higher productivity. Genetic modification, either by random mutagenesis or by target mutation, is also a good strategy to increase the PHA content of cyanobacteria (Kamravamanesh et al. 2018a; Koch et al. 2020b). As discussed in Sect. 3.1, PirC is a central carbon flow regulator that controls the carbon flux towards PHA production. When concentrations of 2-OG are high the PII-PirC complex, encountered under normal growth conditions, is broken and PirC inhibits PGAM and slows down the flow of carbon toward PHA formation. Therefore, the disruption of this gene increases the flux of newly fixed carbon towards the PHA building blocks. Indeed, Koch et al. (2020b) obtained a 63 %dcw PHA content in Synechocysts sp. PCC 6803 by applying this modification. On the other hand, SphU regulon is activated in response to phosphate-limiting conditions leading to the upregulation of genes involved in the phosphate transportation and to the accumulation of polyphosphate and acetyl phosphate in the cell (Summers et al. 1999). Silencing this gene may allow PHA accumulation even when P is abundant, which can increase productivity (Krasaesueb et al. 2019). Genes from other highly productive microorganisms, such as Cupriavidus necator, can also be introduced to increase the capacity of the modified strain to accumulate PHAs (Koch et al. 2020b). These genetic modifications will lead to strains with a high capability to produce PHA under laboratory conditions. Nonetheless, it is essential to test the stability of these new strains during long-term cultivation under real-scale photobioreactors, which are complex to control and the sterility is difficult to maintain (Troschl et al. 2018; Khan et al. 2019).
-
Wastewater as culture media: Growth in wastewater to produce PHA has been a promising strategy to reduce production costs and environmental impact. The major challenge of using wastewater as a culture media is maintaining cyanobacteria as the dominant species during long cultivation periods. Most studies used lab-scale batch cultivation, where cultures were maintained for less than 30 days, so the stability of the culture in wastewater during long cultivation times needs further evaluation. Lastly, the social acceptance of the products coming from wastewater should be further improved by introducing more robust regulations.
-
Improvement of biomass and bioproducts recovery: PHA extraction is one of the most important challenges to make cyanobacteria PHA production economically viable. The extraction of PHA from cyanobacteria has hardly been evaluated. Methods for heterotrophic bacteria cannot be directly transferred to cyanobacteria since they present a thicker and more complex cell wall. Therefore, in future research, the most promising and sustainable methods developed for heterotrophic bacteria, such as new green solvents, mealworms or enzymatic processes, should be further tested on cyanobacteria. In addition, the recuperation of other by-products (e.g. pigments) together with PHA should be addressed to increase process profitability.
Abbreviations
- 2-OG:
-
2-Oxoglutarate
- 2-PGA:
-
2-Phosphoglycerate
- c-di-AMP:
-
Second messenger nucleotide
- CBC:
-
Calvin–Benson cycle
- DIC:
-
Dissolved inorganic carbon
- ED:
-
Entner–Doudoroff
- EMP:
-
Emden–Meyerhof–Parnas
- GlgA:
-
Glycogen synthase
- glob:
-
Glycogen branching enzyme
- GlgC:
-
Glucose-1-phosphate adenylyltransferase
- GlgP:
-
Glycogen phosphorylase
- GlgX:
-
Glycogen debranching enzyme
- GltA:
-
Citrate synthase
- HDPE:
-
High-density polyethylene
- LDPE:
-
Low-density polyethylene
- mcl-PHA:
-
Medium-chain length polyhydroxyalkanoates
- OPP:
-
Oxidative pentose phosphate
- P(3HB-co-3HV):
-
Co-polymer of polyhydroxybutyrate and polyhydroxyvalerate
- P(3HB):
-
Poly(3-hydroxybutyrate)
- P(3HD):
-
Poly(3-hydroxydecanoate)
- P(3HHx):
-
Poly(3-hydroxyhexanoate)
- P(3HO-co-3HD):
-
Co-polymer of poly(3-hydroxyoctanoate) and poly(3-hydroxydecanoate)
- P(3HO):
-
Poly(3-hydroxyoctanoate)
- P(3HV):
-
Poly(3-hydroxyvalerate)
- PBAT:
-
Polybutylene adipate terephthalate
- PBR:
-
Photobioreactor
- PBS:
-
Polybutylene succinate
- PCL:
-
Polycarbonate
- PE:
-
Polyethylene
- PEP:
-
Phenolpyruvate
- PET:
-
Polyethylene terephthalate
- PGAM:
-
2,3-Phosphoglycerate-independent phosphoglycerate mutase
- pgm:
-
Phosphoglucomutase
- PHA:
-
Polyhydroxyalkanoates
- PhaB:
-
Acetyl-CoA reductase
- PhaC/PhaE:
-
Poly(3-hydroxyalkanoate) synthase
- phaF:
-
Phasin
- PhaP:
-
Poly(3-hydroxyalkanoate) inclusion protein
- phoR:
-
Phosphate transport
- PHV:
-
Polyhydroxyvalerate
- PirC:
-
PII interacting regulator of carbon storage
- PLA:
-
Polylactic acid
- PP:
-
Polypropylene
- ppx:
-
Polyphosphate phosphatase
- pstA:
-
Phosphate transport
- pstC:
-
Phosphate transport
- SBR:
-
Sequencing batch reactor
- sbtA:
-
HCO3− transporter
- scl_PHA:
-
Short-chain length polyhydroxyalkanoates
- Slr0058:
-
Phasin family protein
- sphU:
-
Phosphate regulator
- TCA:
-
Tricarboxylic acid cycle
- Tg:
-
Glass transition temperature
- Thl/PhaA:
-
Acetyl-CoA acetyltransferase
- TIN:
-
Total inorganic nitrogen
- TIP:
-
Total inorganic phosphate
- Tm:
-
Melting temperature
- Trk AG/H:
-
K+ transporter
References
Abe MM, Martins JR, Sanvezzo PB et al (2021) Advantages and disadvantages of bioplastics production from starch and lignocellulosic components. Polymers. https://doi.org/10.3390/polym13152484
Achilias DS, Roupakias C, Megalokonomos P et al (2007) Chemical recycling of plastic wastes made from polyethylene (LDPE and HDPE) and polypropylene (PP). J Hazard Mater 149:536–542. https://doi.org/10.1016/j.jhazmat.2007.06.076
Acién Fernández FG, García Camacho F, Chisti Y (1999) Photobioreactors: light regime, mass transfer, and scaleup. pp 231–247
Acién FG, Fernández JM, Magán JJ, Molina E (2012) Production cost of a real microalgae production plant and strategies to reduce it. Biotechnol Adv 30:1344–1353
Afreen R, Tyagi S, Singh G, Singh M (2021) Challenges and perspectives of polyhydroxyalkanoate production from microalgae/cyanobacteria and bacteria as microbial factories: an assessment of hybrid biological system. Front Bioeng Biotechnol 9:624885. https://doi.org/10.3389/fbioe.2021.624885
Agawin NSR, Rabouille S, Veldhuis MJW et al (2007) Competition and facilitation between unicellular nitrogen-fixing cyanobacteria and non-nitrogen-fixing phytoplankton species. Limnol Oceanogr 52:2233–2248. https://doi.org/10.4319/lo.2007.52.5.2233
Ansari S, Fatma T (2016) Cyanobacterial polyhydroxybutyrate (PHB): screening, optimization and characterization. PLoS ONE 11:1–20
Apel AC, Pfaffinger CE, Basedahl N et al (2017) Open thin-layer cascade reactors for saline microalgae production evaluated in a physically simulated Mediterranean summer climate. Algal Res 25:381–390. https://doi.org/10.1016/j.algal.2017.06.004
Aramvash A, Gholami-Banadkuki N, Moazzeni-Zavareh F, Hajizadeh-Turchi S (2015) An environmentally friendly and efficient method for extraction of PHB biopolymer with non-halogenated solvents. J Microbiol Biotechnol 25:1936–1943
Arias DM, Fradinho JC, Uggetti E et al (2018a) Polymer accumulation in mixed cyanobacterial cultures selected under the feast and famine strategy. Algal Res 33:99–108
Arias DM, García J, Uggetti E (2020) Production of polymers by cyanobacteria grown in wastewater: current status, challenges and future perspectives. New Biotechnol 55:46–57
Arias DM, Uggetti E, García-Galán MJ, García J (2018b) Production of polyhydroxybutyrates and carbohydrates in a mixed cyanobacterial culture: effect of nutrients limitation and photoperiods. New Biotechnol 42:1–11. https://doi.org/10.1016/j.nbt.2018.01.001
Arias DM, Uggetti E, García-Galán MJ, García J (2017) Cultivation and selection of cyanobacteria in a closed photobioreactor used for secondary effluent and digestate treatment. Sci Total Environ 587–588:157–167
Asrar J, Valentin HE, Berger PA et al (2002) Biosynthesis and Properties of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) Polymers. Biomacromol 3:1006–1012. https://doi.org/10.1021/bm025543a
Bähr L, Wüstenberg A, Ehwald R (2016) Two-tier vessel for photoautotrophic high-density cultures. J Appl Phycol 28:783–793. https://doi.org/10.1007/s10811-015-0614-5
Bantysh O (2021) Sustainable extraction of PHB (polyhydroxybutyrate ) from Synechocystis sp. Master thesis Oceonografy and Marine management, University of Bercelona Facultat
Bhati R, Mallick N (2015) Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer production by the diazotrophic cyanobacterium Nostoc muscorum Agardh: process optimization and polymer characterization. Algal Res 7:78–85. https://doi.org/10.1016/j.algal.2014.12.003
Bhati R, Mallick N (2016) Carbon dioxide and poultry waste utilization for production of polyhydroxyalkanoate biopolymers by Nostoc muscorum Agardh: a sustainable approach. J Appl Phycol 28:161–168. https://doi.org/10.1007/s10811-015-0573-x
Byrom D (1993) The synthesis and biodegradation of polyhydroxyalkanoates from bacteria. Int Biodeterior Biodegrad 31:199–208
Carpine R, Olivieri G, Hellingwerf KJ et al (2020) Industrial production of poly-β-hydroxybutyrate from CO2: Can cyanobacteria meet this challenge? Processes 8:1–23
Carpine R, Raganati F, Olivieri G et al (2018) Poly-β-hydroxybutyrate (PHB) production by Synechocystis PCC6803 from Co2. Model development (2018).pdf. Algal Res 29:46–60
Castilho LR, Mitchell DA, Freire DMG (2009) Production of polyhydroxyalkanoates (PHAs) from waste materials and by-products by submerged and solid-state fermentation. Bioresour Technol 100:5996–6009. https://doi.org/10.1016/j.biortech.2009.03.088
Chan CM, Vandi LJ, Pratt S et al (2019) Understanding the effect of copolymer content on the processability and mechanical properties of polyhydroxyalkanoate (PHA)/wood composites. Compos Part A Appl Sci Manuf 124:105437. https://doi.org/10.1016/J.COMPOSITESA.2019.05.005
Chen G-Q (2009) A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem Soc Rev 38:2434. https://doi.org/10.1039/b812677c
Choi T-R, Park Y-L, Song H-S, et al (2021) Fructose-based production of short-chain-length and medium-chain-length polyhydroxyalkanoate copolymer by arctic Pseudomonas sp. B14–6. 13. https://doi.org/10.3390/polym13091398
Cook CR, Halden RU (2020) Ecological and health issues of plastic waste. In: Plastic waste and recycling. Elsevier, pp 513–527
Corrêa PS, Morais Júnior WG, Martins AA et al (2020) Microalgae biomolecules: extraction. Sep Purif Methods Process 9:10. https://doi.org/10.3390/pr9010010
COX MK (1994) Properties and applications of polyhydroxyalkanoates, pp 120–135
Damrow R, Maldener I, Zilliges Y (2016) The multiple functions of common microbial carbon polymers, glycogen and PHB, during stress responses in the non-diazotrophic cyanobacterium Synechocystis sp. PCC 6803. Front Microbiol 7:1–10
Da Silva CK, De Almeida ACA, Costa JAV, De Morais MG (2018) Cyanobacterial biomass by reuse of wastewater-containing hypochlorite. Ind Biotechnol 14:265–269. https://doi.org/10.1089/ind.2018.0014
David C, Bühler K, Schmid A (2015) Stabilization of single species Synechocystis biofilms by cultivation under segmented flow. J Ind Microbiol Biotechnol 42:1083–1089. https://doi.org/10.1007/s10295-015-1626-5
Drexler ILC, Yeh DH (2014) Membrane applications for microalgae cultivation and harvesting: a review. Rev Environ Sci Biotechnol 13:487–504. https://doi.org/10.1007/s11157-014-9350-6
Drosg B, Fritz I, Gattermayr F, Silvestrini L (2015) Photo-autotrophic production of poly(hydroxyalkanoates) in cyanobacteria. Chem Biochem Eng Q 29:145–156. https://doi.org/10.15255/CABEQ.2014.2254
Dutt V, Srivastava S (2018) Novel quantitative insights into carbon sources for synthesis of poly hydroxybutyrate in Synechocystis PCC 6803. Photosynth Res 136:303–314
Eberly JO, Ely RL (2012) Photosynthetic accumulation of carbon storage compounds under CO2 enrichment by the thermophilic cyanobacterium Thermosynechococcus elongatus. J Ind Microbiol Biotechnol 39:843–850
Forchhammer K, Selim KA, Huergo LF (2022) New views on PII signaling: from nitrogen sensing to global metabolic control. Trends Microbiol 30:722–735. https://doi.org/10.1016/j.tim.2021.12.014
Fradinho JC, Reis MAM, Oehmen A (2016) Beyond feast and famine: selecting a PHA accumulating photosynthetic mixed culture in a permanent feast regime. Water Res 105:421–428
Garcia-Gonzalez L, Mozumder MdSI, Dubreuil M et al (2015) Sustainable autotrophic production of polyhydroxybutyrate (PHB) from CO2 using a two-stage cultivation system. Catal Today 257:237–245. https://doi.org/10.1016/j.cattod.2014.05.025
Ghosh S, Chakraborty S (2020) Production of polyhydroxyalkanoates (PHA) from aerobic granules of refinery sludge and Micrococcus aloeverae strain SG002 cultivated in oily wastewater. Int Biodeterior Biodegrad 155:105091. https://doi.org/10.1016/J.IBIOD.2020.105091
Gracioso LH, Bellan A, Karolski B et al (2021) Light excess stimulates Poly-beta-hydroxybutyrate yield in a mangrove-isolated strain of Synechocystis sp. Bioresour Technol 320:124379
Gudin C, Thepenier C (1986) Bioconversion of solar energy into organic chemicals by microalgae. Adv Biotechnol Process
Halami PM (2008) Production of polyhydroxyalkanoate from starch by the native isolate Bacillus cereus CFR06. World J Microbiol Biotechnol 24:805–812. https://doi.org/10.1007/s11274-007-9543-z
Hauf W, Schlebusch M, Hüge J et al (2013) Metabolic changes in Synechocystis PCC6803 upon nitrogen-starvation: excess NADPH sustains polyhydroxybutyrate accumulation. Metabolites 3:101–118. https://doi.org/10.3390/metabo3010101
Hauf W, Watzer B, Roos N et al (2015) Photoautotrophic polyhydroxybutyrate granule formation is regulated by cyanobacterial phasin PhaP in Synechocystis sp. strain PCC 6803. Appl Environ Microbiol 81:4411–4422. https://doi.org/10.1128/AEM.00604-15
Hoschek A, Heuschkel I, Schmid A et al (2019) Mixed-species biofilms for high-cell-density application of Synechocystis sp. PCC 6803 in capillary reactors for continuous cyclohexane oxidation to cyclohexanol. Bioresour Technol 282:171–178. https://doi.org/10.1016/j.biortech.2019.02.093
Jiang G, Hill DJ, Kowalczuk M et al (2016) Carbon sources for polyhydroxyalkanoates and an integrated biorefinery. Int J Mol Sci. https://doi.org/10.3390/ijms17071157
Josa I, Garfí M (2023) Social life cycle assessment of microalgae-based systems for wastewater treatment and resource recovery. J Clean Prod 407:137121. https://doi.org/10.1016/j.jclepro.2023.137121
Kaewbaingam A, Incharoensakdi A, Monshupanee T (2016) Increased accumulation of polyhydroxybutyrate in divergent cyanobacteria under nutrient-deprived photoautotrophy: an efficient conversion of solar energy and carbon dioxide to polyhydroxybutyrate by Calothrix scytonemicola TISTR 8095. Bioresour Technol 212:342–347. https://doi.org/10.1016/j.biortech.2016.04.035
Kamravamanesh D, Kovacs T, Pflügl S et al (2018a) Increased poly-Β-hydroxybutyrate production from carbon dioxide in randomly mutated cells of cyanobacterial strain Synechocystis sp. PCC 6714: mutant generation and characterization. Bioresour Technol 266:34–44
Kamravamanesh D, Lackner M, Herwig C (2018b) Bioprocess engineering aspects of sustainable polyhydroxyalkanoate production in cyanobacteria. Bioengineering 5:111
Kamravamanesh D, Pflügl S, Nischkauer W et al (2017) Photosynthetic poly-β-hydroxybutyrate accumulation in unicellular cyanobacterium Synechocystis sp. PCC 6714. AMB Express 7
Kamravamanesh D, Slouka C, Limbeck A et al (2019) Increased carbohydrate production from carbon dioxide in randomly mutated cells of cyanobacterial strain Synechocystis sp. PCC 6714: bioprocess understanding and evaluation of productivities. Bioresour Technol 273:277–287
Kapritchkoff FM, Viotti AP, Alli RCP et al (2006) Enzymatic recovery and purification of polyhydroxybutyrate produced by Ralstonia eutropha. J Biotechnol 122:453–462. https://doi.org/10.1016/j.jbiotec.2005.09.009
Khan A, Bilal M, Mehmood S et al (2019) State-of-the-art genetic modalities to engineer cyanobacteria for sustainable biosynthesis of biofuel and fine-chemicals to meet bio-economy challenges. Life 9:54. https://doi.org/10.3390/life9030054
Klähn S, Hagemann M (2011) Compatible solute biosynthesis in cyanobacteria. Environ Microbiol 13:551–562. https://doi.org/10.1111/j.1462-2920.2010.02366.x
Kobayashi D, Fujita K, Nakamura N, Ohno H (2015) A simple recovery process for biodegradable plastics accumulated in cyanobacteria treated with ionic liquids. Appl Microbiol Biotechnol 99:1647–1653. https://doi.org/10.1007/s00253-014-6234-1
Koch M, Berendzen KW, Forchhammer K (2020a) On the role and production of polyhydroxybutyrate (Phb) in the cyanobacterium Synechocystis sp. pcc 6803. Life 10
Koch M, Bruckmoser J, Scholl J et al (2020b) Maximizing PHB content in Synechocystis sp. PCC 6803: a new metabolic engineering strategy based on the regulator PirC. Microb Cell Fact 19:1–12
Koch M, Doello S, Gutekunst K, Forchhammer K (2019) PHB is produced from Glycogen turn-over during nitrogen starvation in Synechocystis sp. PCC 6803. Int J Mol Sci 20
Koch M, Forchhammer K (2021) Polyhydroxybutyrate: a useful product of chlorotic cyanobacteria. Microb Physiol
Koch M, Orthwein T, Alford JT, Forchhammer K (2020c) The Slr0058 protein from Synechocystis sp. PCC 6803 is a novel regulatory protein involved in PHB granule formation. Front Microbiol 11:1–13
Koller M, Maršálek L, Miranda De Sousa Dias M, Braunegg G (2016) Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner Sustainable production of microbial polyhydroxyalkanoate. https://doi.org/10.1016/j.nbt.2016.05.001
Krasaesueb N, Incharoensakdi A, Khetkorn W (2019) Utilization of shrimp wastewater for poly-β-hydroxybutyrate production by Synechocystis sp. PCC 6803 strain ΔSphU cultivated in photobioreactor. Biotechnol Rep 23:e00345
Krasaesueb N, Promariya A, Raksajit W, Khetkorn W (2021) Inactivation of phosphate regulator (SphU) in cyanobacterium Synechocystis sp. 6803 directly induced acetyl phosphate pathway leading to enhanced PHB level under nitrogen-sufficient condition. J Appl Phycol 33:2135–2144
Lee SY, Choi J (1998) Effect of fermentation performance on the economics of poly(3-hydroxybutyrate) production by Alcaligenes latus. Polym Degrad Stab 387–393
Li K, Liu Q, Fang F et al (2019) Microalgae-based wastewater treatment for nutrients recovery: a review. Bioresour Technol 291:121934. https://doi.org/10.1016/j.biortech.2019.121934
López JC, Arnáiz E, Merchán L, et al (2018) Biogas-based polyhydroxyalkanoates production by Methylocystis hirsuta: a step further in anaerobic digestion biorefineries. 333:529–536
Luzier WD (1992) Materials derived from biomass/biodegradable materials. Proc Natl Acad Sci 89:839–842. https://doi.org/10.1073/pnas.89.3.839
Lynd LR, Wyman CE, Gerngross TU (1999) Biocommodity engineering. Biotechnol Prog 15:777–793. https://doi.org/10.1021/bp990109e
Mallick N, Gupta S, Panda B, Sen R (2007) Process optimization for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) co-polymer production by Nostoc muscorum. Biochem Eng J 37:125–130. https://doi.org/10.1016/j.bej.2007.04.002
Manikandan NA, Pakshirajan K, Pugazhenthi G (2021) Techno-economic assessment of a sustainable and cost-effective bioprocess for large scale production of polyhydroxybutyrate. Chemosphere 284:131371. https://doi.org/10.1016/j.chemosphere.2021.131371
Markou G, Vandamme D, Muylaert K (2014) Microalgal and cyanobacterial cultivation: the supply of nutrients. Water Res 65:186–202
Martínez V, Herencias C, Jurkevitch E, Prieto MA (2016) Engineering a predatory bacterium as a proficient killer agent for intracellular bio-products recovery: the case of the polyhydroxyalkanoates. Sci Rep 6:24381. https://doi.org/10.1038/srep24381
McAdam B, Brennan Fournet M, McDonald P, Mojicevic M (2020) Production of polyhydroxybutyrate (PHB) and factors impacting its chemical and mechanical characteristics. Polymers (basel) 12:2908. https://doi.org/10.3390/polym12122908
Mehta KK, Evitt NH, Swartz JR (2015) Chemical lysis of cyanobacteria. J Biol Eng 9:10
Meixner K, Daffert C, Dalnodar D et al (2022) Glycogen, poly(3-hydroxybutyrate) and pigment accumulation in three Synechocystis strains when exposed to a stepwise increasing salt stress. J Appl Phycol 34:1227–1241. https://doi.org/10.1007/s10811-022-02693-3
Meixner K, Kovalcik A, Sykacek E et al (2018) Cyanobacteria biorefinery—production of poly(3-hydroxybutyrate) with Synechocystis salina and utilisation of residual biomass. J Biotechnol 265:46–53. https://doi.org/10.1016/j.jbiotec.2017.10.020
Miu DM, Eremia MC, Moscovici M (2022) Polyhydroxyalkanoates (PHAs) as biomaterials in tissue engineering: production, isolation, characterization. Materials (basel). https://doi.org/10.3390/MA15041410
Miyake M, Kataoka K, Shirai M, Asada Y (1997) Control of poly-beta-hydroxybutyrate synthase mediated by acetyl phosphate in cyanobacteria. Bacteriology 179:5009–5013
Mohammadi M, Hassan MA, Phang L-Y et al (2012) Intracellular polyhydroxyalkanoates recovery by cleaner halogen-free methods towards zero emission in the palm oil mill. J Clean Prod 37:353–360. https://doi.org/10.1016/j.jclepro.2012.07.038
Monshupanee T, Incharoensakdi A (2014) Enhanced accumulation of glycogen, lipids and polyhydroxybutyrate under optimal nutrients and light intensities in the cyanobacterium Synechocystis sp. PCC 6803. J Appl Microbiol 116:830–838
Mudliar SN, Vaidya AN, Suresh Kumar M et al (2008) Techno-economic evaluation of PHB production from activated sludge. Clean Technol Environ Policy 10:255–262. https://doi.org/10.1007/s10098-007-0100-0
Muhr A, Rechberger EM, Salerno A et al (2013) Biodegradable latexes from animal-derived waste: biosynthesis and characterization of mcl-PHA accumulated by Ps. citronellolis. React Funct Polym 73:1391–1398. https://doi.org/10.1016/j.reactfunctpolym.2012.12.009
Mukhopadhyay M, Patra A, Paul AK (2005) Production of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxy-valerate) by Rhodopseudomonas palustris SP5212. World J Microbiol Biotechnol 21:765–769. https://doi.org/10.1007/s11274-004-5565-y
Murugan P, Han L, Gan C-Y et al (2016) A new biological recovery approach for PHA using mealworm, Tenebrio molitor. J Biotechnol 239:98–105. https://doi.org/10.1016/j.jbiotec.2016.10.012
Muthuraj R, Valerio O, Mekonnen TH (2021) Recent developments in short- and medium-chain- length Polyhydroxyalkanoates: production, properties, and applications. Int J Biol Macromol 187:422–440. https://doi.org/10.1016/j.ijbiomac.2021.07.143
Naser AZ, Deiab I, Darras BM (2021) Poly(lactic acid) (PLA) and polyhydroxyalkanoates (PHAs), green alternatives to petroleum-based plastics: a review. RSC Adv 11:17151–17196. https://doi.org/10.1039/d1ra02390j
Obruca S, Sedlacek P, Mravec F et al (2017) The presence of PHB granules in cytoplasm protects non-halophilic bacterial cells against the harmful impact of hypertonic environments. New Biotechnol 39:68–80
Oliveira GHD, Zaiat M, José et al (2020) Towards the production of mcl-PHA with enriched dominant monomer content: process development for the sugarcane biorefinery context. J Polym Environ 28:844–853. https://doi.org/10.1007/s10924-019-01637-2
Orthwein T, Scholl J, Spät P et al (2021) The novel PII-interactor PirC identifies phosphoglycerate mutase as key control point of carbon storage metabolism in cyanobacteria. Proc Natl Acad Sci U S A 118:1–9. https://doi.org/10.1073/pnas.2019988118
Oswald WJ (1988) Micro-algal and waste-water treatment. Micro-algal biotechnology 305–328
Pade N, Hagemann M (2015) Salt acclimation of cyanobacteria and their application in biotechnology. Life 5:25–49
Panda B, Mallick N (2007) Enhanced poly-ß-hydroxybutyrate accumulation in a unicellular cyanobacterium, Synechocystis sp. PCC 6803. Lett Appl Microbiol 44:194–198. https://doi.org/10.1111/j.1472-765X.2006.02048.x
Pandhal J, Wright PC, Biggs CA (2008) Proteomics with a pinch of salt: a cyanobacterial perspective. Saline Syst 4:1–18
Panuschka S, Drosg B, Ellersdorfer M et al (2019) Photoautotrophic production of poly-hydroxybutyrate—first detailed cost estimations. Algal Res 41:101558. https://doi.org/10.1016/j.algal.2019.101558
Penloglou G, Kretza E, Chatzidoukas C et al (2012) On the control of molecular weight distribution of polyhydroxybutyrate in Azohydromonas lata cultures. Biochem Eng J 62:39–47. https://doi.org/10.1016/j.bej.2011.12.013
Pérez-Rivero C, López-Gómez JP, Roy I (2019) A sustainable approach for the downstream processing of bacterial polyhydroxyalkanoates: state-of-the-art and latest developments. Biochem Eng J 150:107283. https://doi.org/10.1016/j.bej.2019.107283
Poltronieri P, Kumar P, Poltronieri P, Kumar P (2017) Polyhydroxyalcanoates (PHAs) in industrial applications. Handbook of ecomaterials, pp 1–30. https://doi.org/10.1007/978-3-319-48281-1_70-1
Price S, Kuzhiumparambil U, Pernice M, Ralph P (2022) Techno-economic analysis of cyanobacterial PHB bioplastic production. J Environ Chem Eng 10:107502
Price S, Kuzhiumparambil U, Pernice M, Ralph PJ (2020) Cyanobacterial polyhydroxybutyrate for sustainable bioplastic production: critical review and perspectives. J Environ Chem Eng 8
Rajendran N, Han J (2022) Techno-economic analysis of food waste valorization for integrated production of polyhydroxyalkanoates and biofuels. Bioresour Technol 348:126796. https://doi.org/10.1016/j.biortech.2022.126796
Ren Q, De Roo G, Ruth K et al (2009) Simultaneous accumulation and degradation of polyhydroxyalkanoates: futile cycle or clever regulation? Biomacromol 10:916–922. https://doi.org/10.1021/bm801431c
Ritchie H, Roser M (2019) Plastic pollution. In: OurWorldInData.org
Rodríguez-Contreras A, Koller M, Miranda-de Sousa Dias M et al (2015) Influence of glycerol on poly(3-hydroxybutyrate) production by Cupriavidus necator and Burkholderia sacchari. Biochem Eng J 94:50–57. https://doi.org/10.1016/j.bej.2014.11.007
Rueda E, Altamira-Algarra B, García Joan (2022a) Process optimization of the polyhydroxybutyrate and glycogen production in the cyanobacteria Synechocystis sp. and Synechococcus sp. Bioresour Technol 356
Rueda E, Álvarez-González A, Vila J, et al (2022b) Inorganic carbon stimulates the metabolic routes related to the Polyhdroxybutyrate (PHB) production in Cyanobacteria Synechocystis isolated from wastewater. Sci Total Environ 829
Rueda E, García-Galán MJ, Díez-Montero R et al (2020) Polyhydroxybutyrate and glycogen production in photobioreactors inoculated with wastewater borne cyanobacteria monocultures. Bioresour Technol 295:122233
Rueda E, González-Flo E, Roca L et al (2022c) Accumulation of polyhydroxybutyrate in Synechocystis sp. isolated from wastewaters: effect of salinity, light, and P content in the biomass. J Environ Chem Eng 10:107952
Rueda E, Senatore V, Zarra T et al (2023) Life cycle assessment and economic analysis of bioplastics production from cyanobacteria. Sustain Mater Technol. https://doi.org/10.1016/j.susmat.2023.e00579
Ruiz C, Kenny ST, Narancic T et al (2019) Conversion of waste cooking oil into medium chain polyhydroxyalkanoates in a high cell density fermentation. J Biotechnol 306:9–15. https://doi.org/10.1016/j.jbiotec.2019.08.020
Samantaray S, Mallick N (2014) Production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) co-polymer by the diazotrophic cyanobacterium Aulosira fertilissima CCC 444. J Appl Phycol 26:237–245. https://doi.org/10.1007/s10811-013-0073-9
Samantaray S, Mallick N (2012) Production and characterization of poly-β-hydroxybutyrate (PHB) polymer from Aulosira fertilissima. J Appl Phycol 24:803–814. https://doi.org/10.1007/s10811-011-9699-7
Samantaray S, Nayak JK, Mallick N (2011) Wastewater utilization for poly-β-hydroxybutyrate production by the cyanobacterium Aulosira fertilissima in a recirculatory aquaculture system. Appl Environ Microbiol 77:8735–8743. https://doi.org/10.1128/AEM.05275-11
Sangkharak K, Paichid N, Yunu T, Prasertsan P (2020) Enhancing the degradation of mixed polycyclic aromatic hydrocarbon and medium-chain-length polyhydroxyalkanoate production by mixed bacterial cultures using modified repeated batch fermentation. J Appl Microbiol 129:554–564. https://doi.org/10.1111/JAM.14638
Sanhueza C, Diaz-Rodriguez P, Villegas P et al (2020) Influence of the carbon source on the properties of poly-(3)-hydroxybutyrate produced by Paraburkholderia xenovorans LB400 and its electrospun fibers. Int J Biol Macromol 152:11–20. https://doi.org/10.1016/j.ijbiomac.2020.02.080
Santos AJ, Dalla Valentina LVO, Schulz A, Tomaz Duarte MA (2017) From obtaining to degradation of PHB: material properties. Part I. Ing Cienc 13:269–298. https://doi.org/10.17230/INGCIENCIA.13.26.10
Selim KA, Haffner M, Burkhardt M et al (2021) Diurnal metabolic control in cyanobacteria requires perception of second messenger signaling molecule c-di-AMP by the carbon control protein SbtB. Sci Adv 7:1–14. https://doi.org/10.1126/sciadv.abk0568
Selvaratnam T, Pegallapati A, Montelya F et al (2015) Feasibility of algal systems for sustainable wastewater treatment. Renew Energy 82:71–76. https://doi.org/10.1016/j.renene.2014.07.061
Sen KY, Hussin MH, Baidurah S (2019) Biosynthesis of poly(3-hydroxybutyrate) (PHB) by Cupriavidus necator from various pretreated molasses as carbon source. Biocatal Agric Biotechnol 17:51–59. https://doi.org/10.1016/j.bcab.2018.11.006
Shaikh S, Yaqoob M, Aggarwal P (2021) An overview of biodegradable packaging in food industry. Curr Res Food Sci 4:503–520. https://doi.org/10.1016/j.crfs.2021.07.005
Sharma L, Kumar Singh A, Panda B, Mallick N (2007) Process optimization for poly-ß-hydroxybutyrate production in a nitrogen fixing cyanobacterium, Nostoc muscorum using response surface methodology. Bioresour Technol 98:987–993. https://doi.org/10.1016/j.biortech.2006.04.016
Sharma L, Mallick N (2005) Accumulation of poly-β-hydroxybutyrate in Nostoc muscorum: regulation by pH, light-dark cycles, N and P status and carbon sources. Bioresour Technol 96:1304–1310
Shrivastav A, Mishra SK, Mishra S (2010) Polyhydroxyalkanoate (PHA) synthesis by Spirulina subsalsa from Gujarat coast of India. Int J Biol Macromol 46:255–260
Singh AK, Mallick N (2017) Advances in cyanobacterial polyhydroxyalkanoates production. FEMS Microbiol Lett 364:1–13
Singh AK, Sharma L, Mallick N, Mala J (2017) Progress and challenges in producing polyhydroxyalkanoate biopolymers from cyanobacteria. J Appl Phycol 29:1213–1232
Strieth D, Ulber R, Muffler K (2018) Application of phototrophic biofilms: from fundamentals to processes. Bioprocess Biosyst Eng 41:295–312. https://doi.org/10.1007/s00449-017-1870-3
Summers ML, Denton MC, McDermott TR (1999) Genes coding for phosphotransacetylase and acetate kinase in Sinorhizobium meliloti are in an operon that is inducible by phosphate stress and controlled by PhoB. J Bacteriol 181:2217–2224. https://doi.org/10.1128/JB.181.7.2217-2224.1999
Sun Z, Zhou Z (2019) Nature-inspired virus-assisted algal cell disruption for cost-effective biofuel production. Appl Energy 251:113330. https://doi.org/10.1016/j.apenergy.2019.113330
Taepucharoen K, Tarawat S, Puangcharoen M et al (2017) Production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) under photoautotrophy and heterotrophy by non-heterocystous N2-fixing cyanobacterium. Bioresour Technol 239:523–527
Tanweer S, Panda B (2020) Prospect of Synechocystis sp. PCC 6803 for synthesis of poly(3-hydroxybutyrate-co-4-hydroxybutyrate). Algal Res 50:101994. https://doi.org/10.1016/j.algal.2020.101994
Tharanathan RN (2003) Biodegradable films and composite coatings: past, present and future. Trends Food Sci Technol 14:71–78. https://doi.org/10.1016/S0924-2244(02)00280-7
Touliabah HES, El-Sheekh MM, Ismail MM, El-Kassas H (2022) A review of microalgae-and cyanobacteria-based biodegradation of organic pollutants. Molecules. https://doi.org/10.3390/molecules27031141
Troschl C, Meixner K, Drosg B (2017) Cyanobacterial PHA production—review of recent advances and a summary of three years’ working experience running a pilot plant. Bioengineering 4:26. https://doi.org/10.3390/bioengineering4020026
Troschl C, Meixner K, Fritz I et al (2018) Pilot-scale production of poly-β-hydroxybutyrate with the cyanobacterium Synechocytis sp. CCALA192 in a non-sterile tubular photobioreactor. Algal Res 34:116–125. https://doi.org/10.1016/j.algal.2018.07.011
Walker S, Rothman R (2020) Life cycle assessment of bio-based and fossil-based plastic: a review. J Clean Prod 261:121158
Wang C, Yang Y, Hou J et al (2020) Optimization of cyanobacterial harvesting and extracellular organic matter removal utilizing magnetic nanoparticles and response surface methodology: a comparative study. Algal Res 45:101756. https://doi.org/10.1016/j.algal.2019.101756
Wang L, Dandy DS (2017) A microfluidic concentrator for cyanobacteria harvesting. Algal Res 26:481–489. https://doi.org/10.1016/j.algal.2017.03.018
Wang Y, Chen G-Q (2017) Polyhydroxyalkanoates: sustainability, production, and industrialization. Sustain Polym Biomass. https://doi.org/10.1002/9783527340200.CH2
Yabueng N, Napathorn SC (2018) Toward non-toxic and simple recovery process of poly(3-hydroxybutyrate) using the green solvent 1,3-dioxolane. Process Biochem 69:197–207
Yashavanth PR, Das M, Maiti SK (2021) Recent progress and challenges in cyanobacterial autotrophic production of polyhydroxybutyrate (PHB), a bioplastic. J Environ Chem Eng 9:105379
Yuan Q, Sparling R, Oleszkiewicz J (2015) Polyhydroxybutyrate production from municipal wastewater activated sludge with different carbon sources. Air Soil Water Res 8:ASWR.S27218. https://doi.org/10.4137/ASWR.S27218
Zhang S, Bryant DA (2011) The tricarboxylic acid cycle in cyanobacteria. Science 334:1551–1553. https://doi.org/10.1126/science.1210858
Zhang S, Wang W, Zhang K et al (2018) Phosphorus release from cyanobacterial blooms during their decline period in eutrophic Dianchi Lake, China. Environ Sci Pollut Res 25:13579–13588
Zhao X, Cornish K, Vodovotz Y (2020) Narrowing the gap for bioplastic use in food packaging: an update. Environ Sci Technol 54:4712–4732. https://doi.org/10.1021/acs.est.9b03755
Acknowledgements
The authors would like to acknowledge PROMICON (funded by the European Union’s Horizon 2020 research and innovation programme under the grant agreement No 101000733), AL4BIO (RTI2018-099495-B-C21) and Cyan2Bio (PID2021-126564OB-C32), the last two funded by the Spanish "Ministerio de Ciencia e Innovación", the Spanish "Agencia Estatal de Investigación", and “ERDF A way of making Europe”. The knowledge derived from these projects has been instrumental in enriching the depth of this article. Estel Rueda wants to thank the Spanish Ministry of Education, Culture, and Sport [FPU18/04941]. Eva Gonzalez-Flo would like to thank the European Union-Next Generation EU, Ministry of Universities and Recovery, Transformation and Resilience Plan for her research grant [2021UPF-MS12]. Karl Forchhammer wants to acknowledge the Deutsche Forschungsgemeinschaft grant [DFG Fo195/23-1] and Korea Evaluation Institute of Industrial Technology (KEIT) grant funded by the Korean government (MOTIE) [No. RS-2022-00155902]. J. García acknowledges the support provided by the ICREA Academia program.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rueda, E., Gonzalez-Flo, E., Mondal, S. et al. Challenges, progress, and future perspectives for cyanobacterial polyhydroxyalkanoate production. Rev Environ Sci Biotechnol 23, 321–350 (2024). https://doi.org/10.1007/s11157-024-09689-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11157-024-09689-0