Abstract
Over the past four decades, shrimp aquaculture has turned into a major industry providing jobs for millions of people worldwide especially in countries with large coastal boundaries. While the shrimp industry continues to expand, the sustainability of shrimp aquaculture has been threatened by the emergence of diseases. Diseases caused by single-stranded DNA containing viruses, such as infectious hypodermal and hematopoietic necrosis virus (IHHNV) and hepatopancreatic parvovirus (HPV), have caused immense losses in shrimp aquaculture since the early 1980s. In fact, the disease outbreak in the blue shrimp (Penaeus stylirostris) caused by IHHNV in early 1980s ultimately led to the captive breeding program in shrimp being shifted from P. stylirostris to the white shrimp (Penaeus vannamei), and today P. vannamei is the preferred cultured shrimp species globally. To date, four single-stranded DNA viruses are known to affect shrimp; these include IHHNV, HPV, spawner-isolated mortality virus (SMV) and lymphoidal parvo-like virus (LPV). Due to the economic losses caused by IHHNV and HPV, most studies have focused on these two viruses, and only IHHNV is included in the OIE list of Crustacean Diseases. Hence this review will focus on IHHNV and HPV. IHHNV and HPV virions are icosahedral in morphology measuring 20–22 nm in size and contain a single-stranded DNA (ssDNA) of 4–6 kb in size. Both IHHNV and HPV are classified into the sub-order Brevidensoviruses, family Densovirinae. The genome architecture of both viruses are quite similar as they contain two completely (as in IHHNV) or partially overlapping (as in HPV) non-structural and one structural gene. Histopathology and polymerase chain reaction (PCR)-based methods are available for both viruses. Currently, there is no anti-viral therapy for any viral diseases in shrimp. Therefore, biosecurity and the use of genetically resistant lines remains as the corner stone in the management of viral diseases. In recent years, gene silencing using the RNA interference (RNAi) approach has been reported for both IHHNV and HPV via injection. However, the delivery of RNAi molecules via oral route remains a challenge, and the utility of RNAi-based therapy has yet to be materialized in shrimp aquaculture.

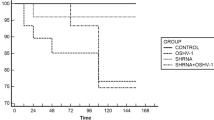
(Adapted from Anderson et al. [2])

(Taken from Anderson et al. [2])

(Taken from Anderson et al. [2])





Similar content being viewed by others
References
Alday-Sanz V. The shrimp book. 1st ed. Alday-Sanz V, editor. Nottingham: Nottingham University Press; 2010.
Anderson BJL, Valderrama D, Jory DE. Global shrimp production review and forecast: steady growth ahead [Internet]. Glob. Aquac. Alliance. 2018 [cited 2019 Feb 25]. pp. 5–10. https://www.aquaculturealliance.org/advocate/global-shrimp-production-review-and-forecast-steady-growth-ahead/.
Aranguren LF, Tang KFJ, Lightner DV. Quantification of the bacterial agent of necrotizing hepatopancreatitis (NHP-B) by real-time PCR and comparison of survival and NHP load of two shrimp populations. Aquaculture. 2010;307:187–92.
Attasart P, Kaewkhaw R, Chimwai C, Kongphom U, Panyim S. Clearance of Penaeus monodon densovirus in naturally pre-infected shrimp by combined ns1 and vp dsRNAs. Virus Res. 2011;159:79–82.
Attasart P, Namramoon O, Kongphom U, Chimwai C, Panyim S. Ingestion of bacteria expressing dsRNA triggers specific RNA silencing in shrimp. Virus Res. 2013;171:252–6.
Bell TA, Lightner DV. IHHN virus: infectivity and pathogenicity studies in Penaeus stylirostris and Penaeus vannamei. Aquaculture. 1984;38:185–94.
Bell TA, Lightner DV, Brock JA. A biopsy procedure for the nondestructive determination of infectious hypodermal and hematopoietic necrosis virus (IHHNV) infection in Penaeus vannamei. J Aquat Anim Health. 1990;2:151–3.
Bonami J-R, Trumper B, Mari J, Brehelin M, Lightner DV. Purification and characterization of the infectious hypodermal and haematopoietic necrosis virus of penaeid shrimps. J Gen Virol. 1990;71:2657–64.
Bonami JR, Mari J, Poulos BT, Lightner DV. Characterization of hepatopancreatic parvo-like virus, a second unusual parvovirus pathogenic for penaeid shrimps. J Gen Virol. 1995;76:813–7.
Browdy CL, Jory DE. The Rising Tide, Proceedings of the Special Session on Sustainable Shrimp Farming. World Aquac 2009. Lousiana, USA; 2009.
Carr WH, Sweeney JN, Nunan L, Lightner DV, Hirsch HH, Reddington JJ. The use of an infectious hypodermal and hematopoietic necrosis virus gene probe serodiagnostic field kit for the screening of candidate specific pathogen-free Penaeus vannamei broodstock. Aquaculture. 1996;147:1–8.
Catap ES, Lavilla-Pitogo CR, Maeno Y, Traviña RD. Occurrence, histopathology and experimental transmission of hepatopancreatic parvovirus infection in Penaeus monodon postlarvae. Dis Aquat Organ. 2003;57:11–7.
Chen B, Dong Z, Pang N, Nian Y, Yan D. A novel real-time PCR approach for detection of infectious hypodermal and haematopoietic necrosis virus (IHHNV) in the freshwater crayfish Procambarus clarkii. J Invertebr Pathol. 2018;157:100-1003.
Chong Y, Loh P. Hepatopancreas chlamydial and parvovirus infections of farmed marine prawns in Singapore. Singapore Vet J. 1984;51–6.
Cotmore SF, Agbandje-McKenna M, Chiorini JA, Mukha DV, Pintel DJ, Qiu J, et al. The family Parvoviridae. Arch Virol. 2014;159:1239–47.
Cowley JA, Rao M, Coman GJ, Cowley J. Real-time PCR tests to specifically detect infectious hypodermal and haemopoietic necrosis virus (IHHNV) lineages and an IHHNV endogenous viral element (EVE) integrated in the genome of black tiger shrimp (Penaeus monodon). 2018;129:145–58.
Dhar AK, Robles-Sikisaka R, Saksmerprome V, Lakshman DK. Biology, Genome Organization, and Evolution of Parvoviruses in Marine Shrimp. Adv Virus Res. 1st ed. Elsevier Inc.; 2014. p. 85–139.
Dhar AK, Roux MM, Klimpel KR. Detection and quantification of infectious hypodermal and hematopoietic necrosis virus and white spot virus in shrimp using real-time quantitative PCR and SYBR green chemistry. J Clin Microbiol. 2001;39:2835–45.
Dhar AK, Lakshman DK, Natarajan S, Allnutt FCT, van Beek NAM. Functional characterization of putative promoter elements from infectious hypodermal and hematopoietic necrosis virus (IHHNV) in shrimp and in insect and fish cell lines. Virus Res. 2007;127:1–8.
Dhar AK, Kaizer KN, Lakshman DK. Transcriptional analysis of Penaeus stylirostris densovirus genes. Virology. 2010;402:112–20.
Escobedo-Bonilla CM, Rangel JLI. Susceptibility to an inoculum of infectious hypodermal and haematopoietic necrosis virus (IHHNV) in three batches of whiteleg shrimp Litopenaeus vannamei (Boone, 1931). Zookeys. 2014;365:355–65.
Flegel TW. Special topic review: major viral diseases of the black tiger prawn (Penaeus monodon) in Thailand. World J Microbiol Biotechnol. 1997;13:433–42.
Flegel TW. Detection of major penaeid shrimp viruses in Asia, a historical perspective with emphasis on Thailand. Aquaculture. 2006;258:1–33.
Flegel TW. Hypothesis for heritable, anti-viral immunity in crustaceans and insects. Biol Direct. 2009;4:32.
Flegel TW, Nielsen L, Thamavit V, Kongtim S, Pasharawipas T. Presence of multiple viruses in non-diseased, cultivated shrimp at harvest. Aquaculture. 2004;240:55–68.
Ho T, Yasri P, Panyim S, Udomkit A. Double-stranded RNA confers both preventive and therapeutic effects against Penaeus stylirostris densovirus (PstDNV) in Litopenaeus vannamei. Virus Res. 2011;155:131–6.
Itsathitphaisarn O, Thitamadee S, Weerachatyanukul W, Sritunyalucksana K. Potential of RNAi applications to control viral diseases of farmed shrimp. J Invertebr Pathol. 2017;147:76–85.
Jang IK, Jeeva S, Seo HC, Lee Y-S, Choi T-J, Kang S-W. Complete nucleotide sequence analysis of a Korean strain of hepatopancreatic parvovirus (HPV) from Fenneropenaeus chinensis. Virus Genes. 2011;44:89–97.
Kalagayan H, Godin D, Kanna R, Hagino G, Sweeney J, Wyban J, et al. IHHN virus as an etiological factor in runt-deformity syndrome (RDS) of Juvenile Penaeus vannamei cultured in Hawaii. J World Aquac Soc. 1991;22:235–43.
Kaufmann B, Bowman VD, Li Y, Szelei J, Waddell PJ, Tijssen P, et al. Structure of Penaeus stylirostris densovirus, a shrimp pathogen. J Virol. 2010;84:11289–96.
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, et al. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–9.
Kelly DC, Elliott RM. DNA contained by two densonucleosis viruses. JVirol. 1977;21:408–10.
Khawsak P, Deesukon W, Chaivisuthangkura P, Sukhumsirichart W. Multiplex RT-PCR assay for simultaneous detection of six viruses of penaeid shrimp. Mol Cell Probes. 2008;22:177–83.
Kiatmetha P, Santimanawong W, Chotwiwatthanakun C, Jariyapong P, Ounjai P, Weerachatyanukul W. Nanocontainer designed from an infectious hypodermal and hematopoietic necrosis virus (IHHNV) has excellent physical stability and ability to deliver shrimp tissues. PeerJ. 2018;6:e6079.
Kim JH, Choresca CH Jr, Shin SP, Han JE, Jun JW, Han SY, et al. Detection of infectious hypodermal and hematopoietic necrosis virus (IHHNV) in Litopenaeus vannamei shrimp cultured in South Korea. Aquaculture. 2011;313:161–4.
Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis Version 7. 0 for Bigger Datasets. Mol B. 2018;33:1870–4.
La Fauce KA, Owens L. The use of insects as a bioassay for Penaeus merguiensis densovirus (PmergDNV). J Invertebr Pathol. 2008;98:1–6.
La Fauce KA, Elliman J, Owens L. Molecular characterisation of hepatopancreatic parvovirus (PmergDNV) from Australian Penaeus merguiensis. Virology. 2007;362:397–403.
Lightner DV. Epizootiology, distribution and the impact on international trade of two penaeid shrimp viruses in the Americas. Rev Sci Tech l’OIE. 1996;15:579–601.
Lightner DV. Biosecurity in shrimp farming : pathogen exclusion through use of SPF stock and routine surveillance. J World Aquac Soc. 2005;36:230–48.
Lightner DV, Redman RM. A parvo-like virus disease of penaeid shrimp. J Invertebr Pathol. 1985;45:47–53.
Lightner DV, Redman RM. Penaeid virus diseases of the shrimp culture industry of the Americas. In: Fast A, Lester L, editors. Mar shrimp cult princ pract. Amsterdam: Elsevier; 1992. p. 569–88.
Lightner DV, Redman RM. Shrimp diseases and current diagnostic methods. Aquaculture. 1998;164:201–20.
Lightner DV, Redman RM, Bell T, Brock J. Detection of IHHN virus in Penaeys stylirostris and P. vannamei imported into Hawaii. J World Maric Soc. 1983;225:212–25.
Mari J, Bonami JR, Lightner DV. Partial cloning of the genome of infectious hypodermal and haematopoietic necrosis virus, an unusual parvovirus pathogenic for penaeid shrimps; diagnosis of the disease using a specific probe. J Gen Virol. 1993;74:2637–43.
Motte E, Yugcha E, Luzardo J, Castro F, Leclercq G, RodrÍguez J, et al. Prevention of IHHNV vertical transmission in the white shrimp Litopenaeus vannamei. Aquaculture. 2003;219:57–70.
Ongvarrasopone C, Saejia P, Chanasakulniyom M, Panyim S. Inhibition of Taura syndrome virus replication in Litopenaeus vannamei through silencing the LvRab7 gene using double-stranded RNA. Arch Virol. 2011;156:1117–23.
Owens L, De Beer S, Smith J. Lymphoidal parvovirus-like particles in Australian penaeid prawns. Dis Aquat Organ. 1991;11:129–34.
Owens L, Haqshenas G, McElnea C, Coelen R. Putative spawner-isolated mortality virus associated with mid-crop mortality syndrome in farmedPenaeus monodon from northern Australia. Dis Aquat Organ. 1998;34:177–85.
Pantoja CR, Lightner DV. A non-destructive method based on the polymerase chain reaction for detection of hepatopancreatic parvovirus (HPV) of penaeid shrimp. 2000;39:177–82.
Phromjai J, Boonsaeng V, Withyachumnarnkul B, Flegel TW. Detection of hepatopancreatic parvovirus in Thai shrimp Penaeus monodon by in situ hybridization, dot blot hybridization and PCR amplification. Dis Aquat Organ. 2002;51:227–32.
Rao M, Cowley JA, Murphy BS, Stratford CN, Sellars MJ. Double-stranded RNA injected into female black tiger shrimp (Penaeus monodon) prior to spawning does not transfer to progeny. Aquaculture. 2019;500:126–34.
Robles-Sikisaka R, Bohonak AJ, McClenaghan LR, Dhar AK. Genetic signature of rapid IHHNV (infectious hypodermal and hematopoietic necrosis virus) expansion in wild penaeus shrimp populations. PLoS One. 2010;5:e11799.
Safeena MP, Rai P. Molecular biology and epidemiology of hepatopancreatic parvovirus of penaeid shrimp. Indian J Virol. 2012;23:191–202.
Saksmerprome V, Charoonnart P, Gangnonngiw W, Withyachumnarnkul B. A novel and inexpensive application of RNAi technology to protect shrimp from viral disease. J Virol Methods. 2009;162:213–7.
Sellars MJ, Cowley JA, Musson D, Rao M, Menzies ML, Coman GJ, et al. Reduced growth performance of black tiger shrimp (Penaeus monodon) infected with infectious hypodermal and hematopoietic necrosis virus. Aquaculture. 2019;499:160–6.
Shike H, Dhar AK, Burns JC, Shimizu C, Jousset FX, Klimpel KR, et al. Infectious hypodermal and hematopoietic necrosis virus of shrimp is related to mosquito brevidensoviruses. Virology. 2000;277:167–77.
Spann K, Adlard R, Hudson D, Pyecroft S, Jones T, Voigt M. Hepatopancreatic parvo-like virus (HPV) of Penaeus japonicus cultured in Australia. Dis Aquat Organ. 1997;31:239–41.
Sukhumsirichart W, Wongteerasupaya C, Boonsaeng V, Panyim S, Sriurairatana S, Withyachumnarnkul B, et al. Characterization and PCR detection of hepatopancreatic parvovirus (HPV) from Penaeus monodon in Thailand. Dis Aquat Organ. 1999;38:1–10.
Sukhumsirichart W, Attasart P, Boonsaeng V, Panyim S. Complete nucleotide sequence and genomic organization of hepatopancreatic parvovirus (HPV) of Penaeus monodon. Virology. 2006;346:266–77.
Tang KFJ, Lightner DV. Detection and quantification of infectious hypodermal and hematopoietic necrosis virus in penaeid shrimp by real-time PCR. Dis Aquat Organ. 2001;44:79–85.
Tang KFJ, Lightner DV. Low sequence variation among isolates of infectious hypodermal and hematopoietic necrosis virus (IHHNV) originating from Hawaii and the Americas. Dis Aquat Organ. 2002;49:93–7.
Tang KFJ, Lightner DV. Infectious hypodermal and hematopoietic necrosis virus (IHHNV) -related sequences in the genome of the black tiger prawn Penaeus monodon from Africa and Australia. Virus Res. 2006;118:185–91.
Tang KFJ, Durand SV, White BL, Redman RM, Pantoja CR, Lightner DV. Postlarvae and juveniles of a selected line of Penaeus stylirostris are resistant to infectious hypodermal and hematopoietic necrosis virus infection. Aquaculture. 2000;1:203–10.
Tang KFJ, Poulos B, Wang J, Redman R, Shih H, Lightner DV. Geographic variations among infectious hypodermal and hematopoietic necrosis virus (IHHNV) isolates and characteristics of their infection. Dis Aquat Organ. 2003;53:91–9.
Tang KFJ, Navarro SA, Lightner DV. PCR assay for discriminating between infectious hypodermal and hematopoietic necrosis virus (IHHNV) and virus-related sequences in the genome of Penaeus monodon. Dis Aquat Organ. 2007;74:165–70.
Tang KFJ, Pantoja C, Lightner DV. Nucleotide sequence of a Madagascar hepatopancreatic parvovirus (HPV) and comparison of genetic variation among geographic isolates. Dis Aquat Organ. 2008;80:105–12.
Tirasophon W, Yodmuang S, Chinnirunvong W, Plongthongkum N, Panyim S. Therapeutic inhibition of yellow head virus multiplication in infected shrimps by YHV-protease dsRNA. Antiviral Res. 2007;74:150–5.
Acknowledgements
Partial funding for this work was provided by College of Agricultural and Life Sciences to Arun K Dhar.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dhar, A.K., Cruz-Flores, R., Caro, L.F.A. et al. Diversity of single-stranded DNA containing viruses in shrimp. VirusDis. 30, 43–57 (2019). https://doi.org/10.1007/s13337-019-00528-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13337-019-00528-3