Abstract
Assessing groundwater quality is critical to regional water resource conservation and human health safety, especially in areas with co-existence of toxic constituents fluoride (F−) and arsenic (As). In this study, fourteen groundwater samples were collected in Feng County, Northwestern Jiangsu Province to identify dominant contaminants and their spatial distribution and health risk. The composition and variation characteristics of major ions (K+, Na+, Ca2+, Mg2+, Cl−, SO42−, HCO3−, and NO3−) and trace elements (F−, As, and Mn) were analyzed. The hydrochemical results revealed that high F− groundwater was mainly distributed in the northern areas whereas As-riched groundwater was primarily distributed in southern areas. Notably, over 85.7% and 21.4% of the shallow groundwater samples exceeded the drinking water quality standard of 1.5 mg/L for F− and 10 µg/L for As, respectively. Based on the water quality index (WQI) appraisal result, 71.4% of the groundwater in the study area is classified as “poor”, and thus unsuitable for drinking directly. We assessed the human non-carcinogenic health risk of F− (HQFluoride) and As (HQArsenic) and the carcinogenic health risk of As (CRArsenic). The calculated hazard quotient (HQ) for F− indicated nearly all groundwater samples have an unacceptable risk (HQ > 1) for each age group. However, HQArsenic values revealed that 28.6%, 21.4%, 21.4%, and 21.4% of groundwater samples posed potential non-carcinogenic health risks for infants, children, females, and males, respectively. The calculated results of CRArsenic showed that 0%, 21.4%, 28.6%, and 28.6% of groundwater samples posed unacceptable health risks (CR > 1.0 × 10−4) to infants, children, females, and males, respectively. The groundwater irrigation suitability assessment results showed that 21.4% of samples were doubtful to unsuitable for irrigation, and 85.7% owed magnesium hazards. The findings of this study will assist policymakers in formulating proper remedial policies and mitigation strategies to ensure the safety of drinking and irrigation water.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide, over 2.5 billion people rely on groundwater as a reliable freshwater source for drinking (Liu et al. 2019a, b; Mahmud et al. 2020). Simultaneously, about 20% of agricultural irrigation and 15% of industrial water also depend on groundwater (Adimalla et al. 2018). In 2015, the United Nations made a universal call to ensure that all people have “clean drinking water and sanitation facilities” till 2030 in Sustainable Development Goal 6 (SDG-6) (Ruidas et al. 2024). However, an increasing number of countries and regions are experiencing serious water security problems due to natural environmental changes and intensive anthropogenic activities (industrial production, agricultural activities, wastewater discharge, etc.) (Xu et al. 2018; Liu et al. 2020b; Tanwer et al. 2023). In recent decades, human health risks triggered by groundwater contamination have received much attention in many countries, particularly in developing countries (Pal et al. 2022; Jaydhar et al. 2023; Ruidas et al. 2024). India and China are the worldwide hotspots in terms of fluoride (F−), nitrate (NO3−), and arsenic (As) contamination in groundwater aquifers (Liu et al. 2015a, b; Ruidas et al. 2022, 2023a; Jaydhar et al. 2023). According to Cao et al. (2023), 260 million people are reportedly exposed to endemic fluorosis in 25 countries worldwide, with China being one of the most vulnerable countries (> 60 million people). It is reported that the accumulation of F− in groundwater primarily resulted from the weathering of fluorine-hosting minerals like fluorite, topaz, and apatite (Bhattacharya et al. 2017; Enalou et al. 2018; Elumalai et al. 2019; Xu et al. 2023). Long-term consumption of high-fluoride groundwater (> 1.5 mg/L) may cause dental fluorosis, osteosclerosis, and mental retardation in children, despite fluoride’s role as an essential trace element (Jia et al. 2018; Hossain and Patra 2020; Jiang et al. 2022). Notably, the prolonged consumption of arsenic-containing groundwater (> 10 µg/L) can result in several health problems such as arsenic poisoning, cancer, and increased all-cause mortality (Pincetti-Zúniga et al. 2020; Podgorski and Berg 2022).
It is reported that natural processes or anthropogenic input of F− and As have a significant impact on human health and crop growth (Bhattacharya et al. 2017; Ruidas et al. 2023b, 2024). Irrigating crops and vegetables with F− and As contaminated groundwater is a key transfer route that F− and As entry into the human food chain via the water-soil-plant-food system (Huang et al. 2006; Bhattacharya et al. 2010). Therefore, the combined effect of F− and As in groundwater will enhance the probability of related diseases including chronic arsenic poisoning and endemic fluorosis (Bhattacharya et al. 2020; Ruidas et al. 2023a, 2024). A detailed scientific assessment of groundwater quality for various uses is imperative to guide water resource management. For example, Ruidas et al. (2024) and Pal et al. (2022) have been working on groundwater F− and As contamination along with associated health risks individually and combinedly in Bangladesh and India by utilizing various machine learning (ML), Random forest (RF), Multi-collinearity analysis, and GIS-based techniques.
Feng County, located in northwestern Jiangsu Province, is highly contaminated by F− since the Yellow River flood sediment contains a huge amount of F− to be released into the groundwater aquifer (Wang et al. 2023a; Chen et al. 2023). Until now, rural area still relies on groundwater for industrial, agricultural, and domestic use although 5% of the residents have suffered from skeletal fluorosis and 70% from dental fluorosis (Zhu 2007). Wang et al. (2023a) mainly focus on the groundwater cycle and hydrochemical evolution processes in Feng County using stable isotope and geochemical modeling. Chen et al. (2023) integrated self-organizing maps with the Monte Carlo method to evaluate groundwater fluoride exposure and related health risks in the Fengpei plain. Further, Zhou et al. (2023) reported that the mean F− concentration reached 2.81 mg/L in the shallow groundwater of Feng County. Previous studies mainly reported natural processes and anthropogenic factors affecting the hydrochemical characteristics of high-fluoride aquifers. However, little literature has been reported in this region regarding the co-existence of toxic constituents F− and As and their associated health risks. Additionally, groundwater suitability for drinking and irrigation purposes was also not studied.
Based on the above context, a systematic and comprehensive evaluation of groundwater quality in the study area is urgently needed to ensure the local water resources safety. The main objectives of this study are to (1) evaluate the groundwater quality and its spatial variation based on GIS; (2) assess the groundwater suitability for drinking and irrigation purposes; and (3) determine the potential human health risks of F− and As for different age groups. This study can provide an important foundation for formulating sustainable groundwater resource protection schemes in the study area and similar areas experiencing serious F− and As contamination.
Study area
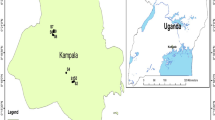
Feng County (116° 21′ 15″ –116° 52′ 03″ E, 34° 24′ 25″–34° 56′ 27″ N) is located in northwestern Jiangsu Province with an area about 1450 km2 (Fig. 1). This area is characterized by a semi-humid monsoon climate, with an average annual temperature of 15.3 °C, a maximum temperature of 38.3 °C, and a minimum temperature of − 9.9 °C. The average annual precipitation and evaporation from 1956 to 2020 were 733.82 mm and 1472.32 mm, respectively (Wang et al. 2023a). Evaporation is much greater than precipitation. The main surface water bodies in the study area include the Fuxin River and the Dasha River.
The study region belongs to the North China stratigraphic area geologically. The Quaternary strata are extensively developed and are approximately 270 m thick (Wang et al. 2023a). The thickness of the Quaternary Holocene strata is 11.0–15.0 m, mainly chalky sand and loamy soil. The thickness of Middle and Upper Pleistocene strata is about 130 m, and the thickness of Lower Pleistocene and Tertiary Pliocene strata is about 180 m. The bedrock is Tertiary, and the lithology is mainly red sandstone and conglomerate interbedded with colored shale, sandstone, and mudstone. There are three aquifers in the formation: the first aquifer is located above 50 m, with medium water-richness, mainly used for rural life and agricultural irrigation. The second aquifer (50–120 m) has low water-bearing and high mineralization. The third aquifer (100–200 m) has a strong water abundance and good water quality, with a single well with a production rate of 70 m3/h, which is mainly used as urban industrial and domestic water.
Based on the land use data of the raster image in 2018 (30m × 30m), the land use types in the study area mainly included agricultural, construction, and other land uses (Fig. 1). The agricultural land was the highest accounting for 77.4% of the total area, followed by construction and other lands use with the proportion of 18.2% and 4.4%, respectively. As reported by Liu et al. (2020a), land use patterns may influence the distribution and transport of pollutants and have a major impact on groundwater quality.
Material and methods
Sample collection and testing
Groundwater samples from 14 shallow wells were collected to investigate the concentrations of major ions and trace elements in November 2021 (Fig. 1). Most groundwater samples were taken from wells for domestic and irrigation supplies with groundwater levels ranging from 33 to 40 m. A calibrated portable multi-parameter digital meter (HQ30d, HACH) was used to measure the pH and electrical conductivity (EC) of groundwater samples during the field investigation. The parameter values were recorded when the data on the dashboard remained stable. After pumping for approximately 5 min, the bottles were rinsed 2–3 times with sample water before being placed in polyethylene sampling bottles. The samples were filtered using 0.45 μm microporous filters and stored at 4 °C before being analyzed. 50 ml groundwater samples were acidified to pH < 2 with ultrapure HNO3− to measure cations (K+, Na+, Ca2+, Mg2+). However, the other was kept without acidification for testing Cl−, SO42−, NO3−, F−, As, and Mn.
The major cations (K+, Na+, Ca2+, and Mg2+) were measured by using an inductively coupled plasma optical emission spectrometer (Perkin-Elmer Optima 5300DV, USA). whereas major anions (Cl−, SO42−, NO3−, and F−) were determined by ion chromatography (Thermo Fisher ICS600, USA). Alkalinity (HCO3−) was measured by using a titration method within 24 h of sampling. The As and Mn content of the groundwater samples was analyzed by inductively coupled plasma mass spectrometer ICP-MS (NexION 2000, Perkin Elmer, USA). The original dataset in this study is sourced from Wang et al. (2023a), except Mn and As.
To ensure the reliability of the results, the charge balance errors (CBE) of cation and anion were checked by Eq. (1). The acceptable ion balance errors in this study were within ± 10%.
where Nc and Nb represent the concentrations of anions and cations in meq/L, respectively.
Water quality index
The water quality index (WQI) approach assessed groundwater suitability for drinking purposes by integrating data sets (Verma et al. 2020; Gao et al. 2021; Tanwer et al. 2023). In this study, 14 key parameters (pH, EC, TDS, K+, Na+, Ca2+, Mg2+, Cl−, SO42−, HCO3−, NO3−, F−, As, and Mn) were incorporated into the WQI to assess the quality of groundwater. All these chemical parameters have a direct and indirect influence on groundwater quality.
Based on its relative importance in human health assessment, each physicochemical parameter was assigned a specific weighting (2 to 5) (Hossain and Patra 2020; Piyathilake et al. 2022). In this study, the weights of SO42−, NO3−, F−, As, and Mn were assigned 5 due to their importance in water quality assessment (Table 1). The WQI calculation consists of the following three steps:
where \({W}_{i}\) represents the relative weight of each water quality parameter; \({w}_{i}\) is the weight of each water quality parameter; \({C}_{i}\) is the concentration of each chemical parameter “\(i\)” in each sample (mg/L); \({S}_{i}\) is the World Health Organization standard (WHO 2011) for each chemical parameter; \({Q}_{i}\) is the quality rating scale of the chemical parameter “\(i\)”, and “\(n\)” is the total number of parameters.
According to the WQI value, groundwater quality can be classified into the following five categories: “Excellent” (WQI < 50); “Good” (50 ≤ WQI < 100); “Poor” (100 ≤ WQI < 200); “Very poor “(200 ≤ WQI < 300); “Unsuitable” (WQI ≥ 300).
Irrigation water quality evaluation
Excessive dissolved ions in irrigation water will have physical and chemical effects on plants and agricultural soil, thus reducing productivity. Therefore, it is critical to evaluate the suitability of groundwater for irrigation. In this study, irrigation assessment parameters such as sodium adsorption ratio (SAR), sodium percentage (%Na), residual sodium carbonate (RSC), permeability index (PI), and magnesium hazard (MH) were used to evaluate the suitability of irrigation water (Bian et al. 2018; Hou et al. 2023; Hasan et al. 2023; Taloor et al. 2024). These indexes were calculated by Eqs. (5)–(9), respectively, where all ion concentrations are expressed in meq/L:
Health risk assessment
Non-carcinogenic risk
Non-carcinogenic risk (NCR) was characterized by the hazard quotient (HQ) and HQ > 1 indicates the presence of non-carcinogenic risk (Nawale et al. 2021; Wang et al. 2023b). In this study, HQFluoride and HQArsenic represent the non-carcinogenic health risks from F− and As contamination, respectively. In general, ingestion and dermal are the primary pathways of human exposure to contaminants (Li et al. 2021b; Liu et al. 2022). Given the potential hazards from oral intake are greatly higher than those from dermal contact (Liu et al. 2022), only the oral ingestion exposure pathway is considered to assess the potential health risks in this study. Fluoride exposure in different age groups was calculated using Eq. (10) (Jaydhar et al. 2022). HQ can be calculated by dividing the estimated daily intake (CDI) by the safe dose (RfD), as expressed in Eq. (11).
where CDI indicates the daily intake dose from ingestion in drinking water (mg/kg/day); \({C}_{w}\) is the contaminant (F− or As) level in groundwater (mg/L); IR is the intake rate (L/day; 0.65 L/day for infants, 1.5 L/day for children, and 2.66 L/day for adults) (Xiao et al. 2022a; Pasupuleti et al. 2022); ED is the exposure duration (years; 0.5, 6, 30, and 30 years for infants, children, females, and males, respectively) (Xiao et al. 2022b; Zeng et al. 2023); EF is exposure frequency (days/year; EF = 365 days/year); BW indicates average body weight (kg; 6.94 kg, 25.9 kg, 52 kg and 73 kg for infants, children, female, and male, respectively) (Liu et al. 2022; Hu et al. 2022; Zeng et al. 2023); AT is the average exposure time (AT = 365 × ED); RfD denotes the reference dose (mg/kg/d), F− is 0.06 mg/kg/d (Zeng et al. 2023; Chen et al. 2023) and As is 0.0003 mg/kg/d (Zhang et al. 2019; Qaiser et al. 2023).
Carcinogenic risk
Arsenic (As) is a typical carcinogen in groundwater that affects the public health of inhabitants through oral exposure pathway (Rapant and Krčmová 2007; Bhattacharya et al. 2020). Chronic exposure to As-rich groundwater can lead to carcinogenic risks (CR), such as skin cancer and kidney cancer (Zhu et al. 2023). Therefore, As was selected to assess the carcinogenic risk of groundwater in the study. The carcinogenic risk value of As was calculated using the following formula:
where SF indicates the slope factor for carcinogenic contaminants (mg/kg/day)−1 (Zhu et al. 2023). The \(SF\) value for As was set at 1.5 (mg/kg/day)−1 in this study (Li et al. 2021a; Rashid et al. 2023). For carcinogenic effects, AT = 70 × 365 (25550 days) because 70 years represent the adverse human health effects of As on a person’s lifetime (Zhang et al. 2019; Yuan et al. 2023; Rashid et al. 2023). The carcinogenic risk is an acceptable level if CR values of As via ingestion pathway between 1 × 10−6 and 1 × 10−4 (USEPA 2004); while an unacceptable range when the CR values are larger than 1 × 10−4 (Kumar et al. 2017; Rahman et al. 2022; Goswami et al. 2022).
Geostatistical analysis
The Geographic Information System (GIS) techniques have been widely adopted to identify the spatiotemporal variation of groundwater quality (Ruidas et al. 2021, 2023a; Jaydhar et al. 2022; Biswas et al. 2023). In this study, the spatial distribution map of hydrochemical constituents and water quality index was generated by the inverse distance weighted (IDW) interpolation method in ArcGIS 10.2 software (Rahman et al. 2022). The IDW method estimates the values of an attribute at unsampled points using the distance-weighted average of all data of the neighboring sampled sites (Atikul Islam et al. 2017; Biswas et al. 2023).
Results and discussion
Groundwater quality assessment
Hydrochemical characteristics and spatial distribution
The summary statistics of the physical and chemical parameters in the groundwater samples are shown in Table 1. The pH varied from 7.3 to 7.8 with an average value of 7.5, indicating groundwater was slightly alkaline. As specified by WHO (2011), the pH of all samples is below the limit (6.5–8.5). Total dissolved solids (TDS) varied from 391.7 to 2132.3 mg/L, with a mean value of 1041.2 mg/L. Approximately 42.9% of the water samples exceeded the permissible limit of 1000 mg/L. As shown in Fig. 2a, the TDS exceedance points were mainly distributed in the northern of the study area, which is an important industrial zone, implying that the discharge of industrial pollutants will inevitably lead to groundwater contamination.
The trace elements can be effective in determining the potential contamination status of groundwater. It is noteworthy that F− concentrations in groundwater in the study area ranged from 1.3 to 5.5 mg/L, with a mean value of 3.4 mg/L. Notably, more than 85.7% of the groundwater samples had fluoride concentrations higher than the WHO guideline-recommended standard of 1.5 mg/L. As shown in Fig. 2b, the F− concentration of the shallow groundwater rose from south to north in the study area. According to previous studies, high levels of F− concentration in groundwater are primarily due to fluoride-rich minerals existing in groundwater aquifers, including clay particles, muscovite, biotite, and fluorite (Wang et al. 2023a; Chen et al. 2023). Arsenic concentrations in groundwater ranged from 0.1 to 72.4 µg/L, with a mean value of 8.6 µg/L (Table 1). The geological origins of high-As groundwater are mainly associated with the sedimentary environment of strata, such as the reductive dissolution of Fe (hydr)oxides and the oxidation of sulfide minerals containing As (Zhang et al. 2019; Goswami et al. 2022). Nearly 21.4% of groundwater samples showed arsenic concentrations above the allowable limit of 10 µg/L set by the WHO (2011). Figure 2c shows that the As contamination in groundwater was mainly concentrated in the southeastern part of the study area, such as Liangzhai and Dashahe. Particularly, the As concentration in Huashan town reached 72.4 µg/L, indicating severe As contamination. Notably, co-occurrence of high F− and As in 21.4% of groundwater samples increased the health risk relative to one toxicant, which was also observed in areas of Dharmanagar region (Bhattacharya et al. 2020) and Huaihe River Basin (Xu et al. 2023). The concentration of Mn in the current study varied between 0.3 and 1536.4 µg/L with a mean value of 250.1 µg/L, and 42.9% of water samples were above WHO guideline for drinking water (100 µg/L) (Fig. 2d). It was notable that the high Mn concentrations (> 100 μg/L) of groundwater were mainly found in the south of the study area (Fig. 2d).
In the present study, K+ and Na+ concentrations in groundwater ranged from 2.8 to 32.4 mg/L (mean 16.9 mg/L) and 51.1 to 547.8 mg/L (mean 263.6 mg/L), respectively, indicating that Na+ concentrations in groundwater were significantly higher than K+ concentrations. More than 78.6% of sampling sites exceeded the WHO-recommended K+ permissible limit (12 mg/L), whereas 57.1% of samples had Na+ exceeding the threshold value of 200 mg/L (WHO 2011). As shown in Fig. 3a and b, the areas with excessive K+ and Na+ contents are mainly distributed in the southeast and northwest agricultural areas. According to Wang et al. (2023a) and Chen et al. (2023), the dissolved Na+ in groundwater may not only be related to the widespread distribution of feldspar and halite in the aquifer but also be probably attributed to cation exchange processes between groundwater and soil. It is noted that the widespread use of potassium fertilizers in agriculture can result in elevated K+ levels in groundwater, which can harm the nervous and digestive systems of humans through oral intake (Sharma et al. 2017). On the other hand, Ca2+ and Mg2+ concentrations ranged from 29.8 to 95.5 mg/L and 24.7 to 103.8 mg/L with mean levels of 56.9 and 59.2 mg/L, respectively. It can be seen that Ca2+ and Mg2+ levels are all below the acceptable limits of 200 mg/L and 150 mg/L (WHO 2011), respectively (Fig. 3). Related studies have shown that Ca2+ and Mg2+ in groundwater are mainly derived from the dissolution of carbonate and gypsum by weathering (Liu et al. 2020a; Guo et al. 2023).
The statistical values and spatial variation of major anions (Cl−, SO42−, HCO3−, and NO3−) are depicted in Table 1 and Fig. 4, respectively. According to WHO guidelines, the maximum permissible limit for Cl− is 250 mg/L (Liu et al. 2022). In this study, Cl− concentration in the shallow groundwater ranged from 54.5 to 506.4 mg/L, with about 21.4% of the samples exceeding the recommended permissible limit. Groundwater Cl− levels were high in Zhaozhuang, Wanggou, and Huankou, although mean values did not exceed the limit (Fig. 4a). High Cl− concentration in groundwater can induce hypertension, osteoporosis, and asthma in humans. Besides halite dissolution, industrial wastewater, compost, and waste leachate may also contribute to high Cl− concentrations in groundwater (Chen et al. 2023). The concentrations of SO42− in groundwater in the study area ranged from 8.1 to 945.7 mg /L (mean 262.8 mg/L). About 42.9% of sampled groundwaters exceeded the safety limit of 250 mg/L (WHO 2011). Groundwater samples with high SO42− content mainly occurred in Shouxian, Changdian, Huankou, Shizhai, Shunhe, and Fanlou (Fig. 4b). Although SO42− has no toxicity it does change the level of methemoglobin and sulphaemoglobin in the human body when consumed in large amounts through drinking water (WHO 2011; Sharma and Kumar 2020). Gypsum dissolution and other anthropogenic sources (e.g., mining, fertilizer, etc.) may contribute significantly to high SO42− concentrations in groundwater (Kumar et al. 2017; Goswami et al. 2022). The concentration of HCO3− in groundwater ranged from 195.2 to 585.6 mg/L with a mean value of 398.9 mg/L. About 7.1% of the samples exceeded the limit of 500 mg/L allowed by WHO (2011). As reported by Wang et al. (2023a), silicate and carbonate weathering in the study area may have been an important factor in increasing HCO3− concentration of groundwater. Furthermore, the alkaline environment promotes the enrichment of F− content (Chen et al. 2023). According to the spatial distribution map of HCO3− in the study area (Fig. 4c), all samples were within the permissible range, except for the Fanlou. Shallow groundwater NO3− concentrations in the study area ranged from 0.9 to 88.5 mg/L, with a mean of 24.9 mg/L. Naturally, precipitation or soil organic nitrogen are the main sources of groundwater NO3−, which is present in the environment at low amounts (below 10 mg/L) (Mao et al. 2023). However, more than 28.6% of samples exceeded the natural environment concentrations and the contaminated areas were mainly located in the southeast (Fig. 4d). Therefore, NO3− contamination in groundwater in the study area cannot be ignored. Chronic ingestion of NO3−-contaminated groundwater can cause methemoglobinemia in humans (Wang et al. 2023b). According to the field investigation, farmers in the study area use various nitrogenous fertilizers to increase crop yields, which inevitably elevated NO3− levels in groundwater (Wang et al. 2023a).
In summary, the dominant orders of major cations and anions concentration in the shallow groundwater of the study area were Na+ > Ca2+ > Mg2+ > K+ and HCO3− > SO42− > Cl− > NO3− > F−. The comparative analysis revealed that the groundwater suffered serious contamination from TDS, K+, Na+, Cl−, SO42−, NO3−, F−, Mn, and As, with exceedance rates of 42.9%, 78.6%, 57.1%, 21.4%, 42.9%, 28.6%, 85.7%, 42.9%, and 21.4%, respectively.
Drinking water suitability
WQI is a worthwhile approach for assessing groundwater quality and its suitability for drinking purposes (Hossain and Patra 2020; Liu et al. 2021). Several researchers (such as Nizam et al. 2022 and Taloor et al. 2024) have employed this method to assess groundwater quality in their studies. The WQI values of the groundwater samples ranged from 61.6 to 206.7, with a mean value of 125.7. According to the WQI classification, 28.6% of the groundwater samples were “good” quality, 64.3% were “poor” quality, and 7.1% were “very poor” quality. As shown in Fig. 5, the areas with “good” water quality were mainly concentrated in Sunlou and Fengcheng in the central part of the study area and Liangzhai in the southeast. However, a majority of the shallow groundwater samples were not suitable for drinking due to WQI values between 100 and 200. Human health is expected to be jeopardized if contaminated groundwater is consumed for an extended period. Consequently, seeking alternative clean water sources for drinking is urgent for this area.
Irrigation water quality evaluation
Shallow groundwater is one of the main water sources for agricultural irrigation in the study area. Therefore, groundwater irrigation suitability has a direct impact on the sustainable development of local agriculture. High Na+ concentration in irrigation water reduces the permeability of the soil, resulting in alkaline soils unsuitable for cultivation (Li et al. 2018). High salinity inhibits water from reaching the branches and leaves of plants, which is detrimental to crop growth (Sellamuthu et al. 2022). To check the suitability of the groundwater for irrigation uses, a set of determinants for irrigation water suitability was studied and outlined in Table 3 (Atikul Islam et al. 2017; Mohamed et al. 2022). Typically, SAR and %Na are used to evaluate alkali damage of irrigation water (Wang et al. 2022; Mukherjee et al. 2022). SAR were classified as excellent (< 10), good (10–18), doubtful (18–26), and unsuitable (> 26) (Table 3) (Liu et al. 2022). Results showed that SAR values ranged from 1.21 to 12.82, with a mean of 5.61, indicating that all groundwater was suitable for irrigation (Table 2). Furthermore, the %Na in irrigation water should not exceed 60%, and when it does, groundwater becomes unsuitable for agricultural irrigation (Zhou et al. 2021). In Table 2, the variation of %Na ranged from 25.73 to 80.77%, with a mean value of 55.51%. In terms of %Na, about 7.1% of the water samples in the study area were unsuitable for irrigation (Table 3). EC can be used to assess the groundwater salinity suitability for irrigation purposes. Based on the EC values, salinity hazards can be classified as excellent (EC < 250 µS/cm), good (250 < EC < 750 µS/cm), doubtful (750 < EC < 2250 µS/cm), and unsuitable (EC > 2250 µS/cm) (Sharma et al. 2017). The EC of groundwater in this study area ranged from 729 to 3030 µS/cm with a mean value of 1667.4 µS/cm and 21.4% of the groundwater was unsuitable for irrigation regarding salinity hazards (Table 3).
According to the RSC classification (Table 3), groundwater with an RSC value < 1.25 is considered suitable for irrigation purposes. In contrast, if the RSC value varies between 1.25 and 2.5, the water quality is doubtful; water with an SAR value above 2.5, signifies that the water quality is unsuitable for irrigation (Panneerselvam et al. 2021). The results show that the RSC ranged between − 5.48 to 3.88 with a mean value of − 1.24 (Table 2), and 92.9% of the samples were suitable for irrigation (Table 3). Only one sample (G12) had a SAR value of 3.88, indicating a higher risk of sodium toxicity and a negative impact on crop growth. The permeability index (PI) is an index to assess the permeability of the soil. Long-term groundwater irrigation with rich minerals such as Na+, Ca2+, Mg2+, and HCO3− will lower the soil permeability and reduce the crop yield. According to the PI classification (Table 3), PI > 75% was considered suitable for irrigation; 25% < PI < 75% was permissible for irrigation; and when PI < 25%, it was not suitable for irrigation (Liu et al. 2022). Table 2 shows that the PI values in the study area ranged from 48.81 to 90.94 with a mean of 69.87, indicating that groundwater is suitable for irrigation. Overall, 28.6% of the samples were “good”, and the remaining 71.4% of samples were in the “permissible” category (Table 3). Therefore, the groundwater samples show no evidence of a soil infiltration problem. The MH in groundwater samples ranged from 31.11 to 76.12 (average 62.31) (Table 2), with 85.7% of the water samples exceeding the permissible limit (> 50%) (Table 3). This meant that the majority of the groundwater in the study area posed a significant magnesium hazard.
Besides the above parameters, USLL and Wilcox plots correlating alkalinity and salinity have been widely used to evaluate groundwater suitability for irrigation (Mukherjee et al. 2022; Sellamuthu et al. 2022). The United States Salinity Laboratory plot (Fig. 6a) showed that 71.4% of the water samples are plotted in zone C3S1, indicating high salinity and low sodium hazards. One sample (7.1%) is scattered in zone C3S2, illustrating high salinity and medium sodium hazards. However, 21.4% of the samples are plotted in zone C4S2, showing very high salinity and medium sodium hazards. Wilcox plots (Fig. 6b) showed that 7.1%, 14.3%, and 42.9% of the water samples were plotted in the categories of “unsuitable”, “doubtful to unsuitable”, and “permissible to doubtful”, respectively. Therefore, alternate irrigation with fresh water is necessary to prevent the accumulation of sodium in the soil (Li et al. 2016).
To sum up, the most important factors affecting the irrigation water quality are the high salinity and the magnesium hazard. High Mg2+ in agricultural water will reduce soil fertility and thus affect crop yields (Shaikh et al. 2020). As a result, groundwater that has not been treated for salinity reduction and Mg2+ control is not suitable for irrigation directly. Furthermore, sodium hazards in irrigation water should not be ignored.
Health risk assessment for F− and As in terms of drinking water
Non-carcinogenic risk of F− and As
Given the study area is facing severe groundwater F− and As contamination, an assessment of the human health risks is needed. According to Table 4, HQFluoride ranged from 2.03 to 8.62 (average 5.35), 1.25 to 5.33 (average 3.31), 1.08 to 4.60 (average 2.85), and 0.77 to 3.28 (average 2.03) for infants, children, females, and males, respectively. Therefore, it can be presumed that all age groups have an alarming non-carcinogenic if prolonged ingestion of high F− groundwater in the study area.
Also, the values of HQArsenic were 0.03 to 22.61 (average 2.69) for infants, 0.02 to 13.98 (average 1.66) for children, 0.02 to 12.07 (average 1.43) for females, and 0.01 to 8.60 (average 1.02) for males. Notably, 28.6%, 21.4%, 21.4%, and 21.4% of the groundwater samples have high arsenic non-carcinogenic risks for infants, children, females, and males, respectively.
In summary, it can be seen from average that the order of non-carcinogenic health risks caused by F− and As is infants > children > females > males, which is consistent with the findings of Jiang et al. (2022) and Chen et al. (2023).
Carcinogenic risk of As
The results of the carcinogenic risk assessment showed that the potential CRArsenic values for infants, children, females, and males ranged from 1.0E−07 to 7.3E−05 (mean 8.6E−06), 7.4E−07 to 5.4E−04 (mean 6.4E−05), 3.2E−06 to 2.3E−03 (mean 2.8E−04), and 2.3E-06 to 1.7E-03 (mean 2.0E-04), respectively. Accordingly, 0%, 21.4%, 28.6%, and 28.6% of groundwater samples surpassed the carcinogenic tolerable risk values of 10−4 recommended by USEPA (2004). This indicated that As might pose a potential carcinogenic risk to the residents who used groundwater for their daily purposes (Rahman et al. 2022). Therefore, continuous monitoring of groundwater arsenic levels is recommended to identify spatiotemporal variations of potential As risks, particularly in the southeast of the study area where samples (G01, G10, G11, and G14) exceeded the limit values.
Groundwater sustainable management strategies
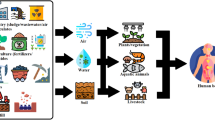
Based on the above analysis, an integrated conceptual model of groundwater quality and associated health risk assessment was developed (Fig. 7). As stated in Sect. ”Health risk assessment for F− and As in terms of drinking water”, groundwater F− and As has a significant impact on groundwater quality and associated health hazards in Feng County, particularly in the infants group. Hence, constant monitoring of groundwater F− and As levels is needed to gain better insight into the contamination dynamics and variation of health hazards (Goswami et al. 2022). Additionally, the Water Affairs Bureau should establish special funding for machine learning projects to predict groundwater quality and enhance sustainable groundwater management (Haggerty et al. 2023).
Monitoring and evaluating environmental pollutants are merely the first steps in improving groundwater quality. Promoting low-cost technologies to remove F− and As could be a viable alternative for this region. Particularly, rural households should be provided with low-cost water purifiers, such as deionization and de-fluoridation filters. Water administration authorities should prioritize initiating water quality improvement projects to reduce the human health risk of F− and As contamination in groundwater. More importantly, this region urgently needs to use the unpolluted deep groundwater and surface water resources for drinking and irrigation purposes. Irrigation problems caused by high salinity and magnesium content in groundwater can be treated by diluting with different freshwater (Mukherjee et al. 2022).
Conclusions
In the present study, shallow groundwater quality and its suitability for drinking and irrigation, as well as F− and As health risks were evaluated in Feng County. Results indicated that about 85.7% of shallow groundwater samples exceeded the WHO drinking criterion for F−, followed by K+ (78.6%), Na+ (57.1%), TDS (42.9%), SO42− (42.9%), Mn (42.9%), Cl− (21.4%), and As (21.4%). Based on WQI assessment results, 28.6% of the groundwater was good quality, while 71.4% was unsafe for drinking. According to non-carcinogenic risk appraisal results, almost all age groups hold HQFluoride values > 1 and followed the order of infants > children > females > males. Nevertheless, only 28.6%, 21.4%, 21.4%, and 21.4% of samples had HQArsenic values > 1 for infants, children, females, and males, respectively. For carcinogenic risk, 0%, 21.4%, 28.6%, and 28.6% of groundwater samples have unacceptable CRArsenic values (> 1×10−4) for infants, children, females, and males, respectively. According to the salinity and alkalinity indicators (SAR, RSC, %Na, PI, MH, and SH) and USSL and Wilcox plot, about 21.4% and 85.7% of groundwater samples were unsuitable for irrigation due to they contained high salinity and magnesium hazards, respectively. The findings of this study could help government agencies or decision-makers adopt appropriate remedial measures to ensure residents’ health and help in achieving SDG-6 i.e., supplying clean and hygienic drinking water. Future in-depth studies could integrate aquifer geology and machine learning for groundwater quality forecasting and safeguard public and ecological health.
Data and materials availability
The data supporting this study’s findings are available from the corresponding author upon reasonable request.
References
Adimalla N, Li P, Venkatayogi S (2018) Hydrogeochemical evaluation of groundwater quality for drinking and irrigation purposes and integrated interpretation with water quality index studies. Environ Process 5:363–383. https://doi.org/10.1007/s40710-018-0297-4
Atikul Islam M, Zahid A, Rahman MdM et al (2017) Investigation of groundwater quality and its suitability for drinking and agricultural use in the south central part of the coastal region in Bangladesh. Expo Health 9:27–41. https://doi.org/10.1007/s12403-016-0220-z
Bhattacharya P, Samal AC, Majumdar J, Santra SC (2010) Arsenic contamination in rice, wheat, pulses, and vegetables: a study in an arsenic affected area of West Bengal, India. Water Air Soil Pollut 213:3–13. https://doi.org/10.1007/s11270-010-0361-9
Bhattacharya P, Samal AC, Banerjee S et al (2017) Assessment of potential health risk of fluoride consumption through rice, pulses, and vegetables in addition to consumption of fluoride-contaminated drinking water of West Bengal, India. Environ Sci Pollut Res 24:20300–20314. https://doi.org/10.1007/s11356-017-9649-2
Bhattacharya P, Adhikari S, Samal AC et al (2020) Health risk assessment of co-occurrence of toxic fluoride and arsenic in groundwater of Dharmanagar region, North Tripura (India). Groundw Sustain Dev 11:100430. https://doi.org/10.1016/j.gsd.2020.100430
Bian J, Nie S, Wang R et al (2018) Hydrochemical characteristics and quality assessment of groundwater for irrigation use in central and eastern Songnen plain, Northeast China. Environ Monit Assess 190:382. https://doi.org/10.1007/s10661-018-6774-4
Biswas T, Pal SC, Chowdhuri I et al (2023) Effects of elevated arsenic and nitrate concentrations on groundwater resources in deltaic region of Sundarban Ramsar site, Indo-Bangladesh region. Mar Pollut Bull 188:114618. https://doi.org/10.1016/j.marpolbul.2023.114618
Cao W, Zhang Z, Guo H et al (2023) Spatial distribution and controlling mechanisms of high fluoride groundwater in the coastal plain of Bohai Rim, North China. J Hydrol 617:128952. https://doi.org/10.1016/j.jhydrol.2022.128952
Chen J, Wang S, Zhang S et al (2023) Identifying the hydrochemical features, driving factors, and associated human health risks of high-fluoride groundwater in a typical Yellow River floodplain, North China. Environ Geochem Health 45:8709–8733. https://doi.org/10.1007/s10653-023-01748-9
Elumalai V, Nwabisa DP, Rajmohan N (2019) Evaluation of high fluoride contaminated fractured rock aquifer in South Africa – Geochemical and chemometric approaches. Chemosphere 235:1–11. https://doi.org/10.1016/j.chemosphere.2019.06.065
Enalou HB, Moore F, Keshavarzi B, Zarei M (2018) Source apportionment and health risk assessment of fluoride in water resources, south of Fars province, Iran: stable isotopes (δ18O and δD) and geochemical modeling approaches. Appl Geochem 98:197–205. https://doi.org/10.1016/j.apgeochem.2018.09.019
Gao Z, Han C, Xu Y et al (2021) Assessment of the water quality of groundwater in Bohai Rim and the controlling factors—a case study of northern Shandong Peninsula, north China. Environ Pollut 285:117482. https://doi.org/10.1016/j.envpol.2021.117482
Goswami R, Neog N, Bhagat C et al (2022) Arsenic in the groundwater of the Upper Brahmaputra floodplain: variability, health risks and potential impacts. Chemosphere 306:135621. https://doi.org/10.1016/j.chemosphere.2022.135621
Guo Y, Zhang S, Wang S et al (2023) Using stable isotopes (δ2H and δ18O) and hydrochemistry to understand the genesis and hydrochemical processes of groundwater in Chongming Island, Yangtze Estuary. Environ Sci Pollut Res 30:84300–84313. https://doi.org/10.1007/s11356-023-28401-3
Haggerty R, Sun J, Yu H, Li Y (2023) Application of machine learning in groundwater quality modeling - a comprehensive review. Water Res. https://doi.org/10.1016/j.watres.2023.119745
Hasan I, Reza S, Siddique AB et al (2023) Assessment of groundwater vulnerability for seawater intrusion using DRASTIC model in coastal area at Patuakhali District, Bangladesh. Environ Sci Pollut Res 30:109021–109040. https://doi.org/10.1007/s11356-023-29988-3
Hossain M, Patra PK (2020) Hydrogeochemical characterisation and health hazards of fluoride enriched groundwater in diverse aquifer types. Environ Pollut 258:113646. https://doi.org/10.1016/j.envpol.2019.113646
Hou L, Liu Z, Zhai B et al (2023) Contrasting water quality in response to long-term nitrogen fertilization in rainfed and irrigated apple-producing regions on China’s Loess Plateau. Agr Ecosyst Environ 354:108561. https://doi.org/10.1016/j.agee.2023.108561
Hu B, Song X, Lu Y et al (2022) Fluoride enrichment mechanisms and related health risks of groundwater in the transition zone of geomorphic units, northern China. Environ Res 212:113588. https://doi.org/10.1016/j.envres.2022.113588
Huang R-Q, Gao S-F, Wang W-L et al (2006) Soil arsenic availability and the transfer of soil arsenic to crops in suburban areas in Fujian Province, southeast China. Sci Total Environ 368:531–541. https://doi.org/10.1016/j.scitotenv.2006.03.013
Jaydhar AK, Chandra Pal S, Saha A et al (2022) Hydrogeochemical evaluation and corresponding health risk from elevated arsenic and fluoride contamination in recurrent coastal multi-aquifers of eastern India. J Clean Prod 369:133150. https://doi.org/10.1016/j.jclepro.2022.133150
Jaydhar AK, Pal SC, Saha A et al (2023) Hydrogeochemical properties of groundwater and associated human health hazards in coastal multiaquifers of India. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-023-27765-w
Jia Y, Xi B, Jiang Y et al (2018) Distribution, formation and human-induced evolution of geogenic contaminated groundwater in China: a review. Sci Total Environ 643:967–993. https://doi.org/10.1016/j.scitotenv.2018.06.201
Jiang W, Liu H, Sheng Y et al (2022) Distribution, source apportionment, and health risk assessment of heavy metals in groundwater in a multi-mineral resource area, North China. Expo Health 14:807–827. https://doi.org/10.1007/s12403-021-00455-z
Kumar M, Patel AK, Das A et al (2017) Hydrogeochemical controls on mobilization of arsenic and associated health risk in Nagaon district of the central Brahmaputra Plain, India. Environ Geochem Health 39:161–178. https://doi.org/10.1007/s10653-016-9816-2
Li J, Yang Y, Huan H et al (2016) Method for screening prevention and control measures and technologies based on groundwater pollution intensity assessment. Sci Total Environ 551–552:143–154. https://doi.org/10.1016/j.scitotenv.2015.12.152
Li P, He S, Yang N, Xiang G (2018) Groundwater quality assessment for domestic and agricultural purposes in Yan’an City, northwest China: implications to sustainable groundwater quality management on the Loess Plateau. Environ Earth Sci 77:775. https://doi.org/10.1007/s12665-018-7968-3
Li Y, Bi Y, Mi W et al (2021a) Land-use change caused by anthropogenic activities increase fluoride and arsenic pollution in groundwater and human health risk. J Hazard Mater 406:124337. https://doi.org/10.1016/j.jhazmat.2020.124337
Li Y, Ji L, Mi W et al (2021b) Health risks from groundwater arsenic on residents in northern China coal-rich region. Sci Total Environ 773:145003. https://doi.org/10.1016/j.scitotenv.2021.145003
Liu F, Song X, Yang L et al (2015a) Identifying the origin and geochemical evolution of groundwater using hydrochemistry and stable isotopes in the Subei Lake basin, Ordos energy base, Northwestern China. Hydrol Earth Syst Sci 19:551–565. https://doi.org/10.5194/hess-19-551-2015
Liu F, Song X, Yang L et al (2015b) The role of anthropogenic and natural factors in shaping the geochemical evolution of groundwater in the Subei Lake basin, Ordos energy base, Northwestern China. Sci Total Environ 538:327–340. https://doi.org/10.1016/j.scitotenv.2015.08.057
Liu F, Song X, Zhen P et al (2019a) Insights from stable isotopes of water and hydrochemistry to the evolutionary processes of groundwater in the Subei lake basin, Ordos energy base, Northwestern China. Isot Environ Health Stud 55:438–458. https://doi.org/10.1080/10256016.2019.1654472
Liu F, Wang S, Wang L et al (2019b) Coupling hydrochemistry and stable isotopes to identify the major factors affecting groundwater geochemical evolution in the Heilongdong Spring Basin, North China. J Geochem Explor 205:106352. https://doi.org/10.1016/j.gexplo.2019.106352
Liu F, Wang S, Yeh T-CJ et al (2020a) Using multivariate statistical techniques and geochemical modelling to identify factors controlling the evolution of groundwater chemistry in a typical transitional area between Taihang Mountains and North China Plain. Hydrol Process 34:1888–1905. https://doi.org/10.1002/hyp.13701
Liu J, Gao Z, Zhang Y et al (2021) Hydrochemical evaluation of groundwater quality and human health risk assessment of nitrate in the largest peninsula of China based on high-density sampling: A case study of Weifang. J Clean Prod 322:129164. https://doi.org/10.1016/j.jclepro.2021.129164
Liu F, Zhen P, Wang S (2022) Groundwater quality assessment and health risks from nitrate contamination in the Heilongdong Spring Basin, a typical headwater basin of the North China Plain. Environ Sci Pollut Res 29:17655–17670. https://doi.org/10.1007/s11356-021-17065-6
Mahmud A, Sikder S, Joardar JC (2020) Assessment of groundwater quality in Khulna city of Bangladesh in terms of water quality index for drinking purpose. Appl Water Sci 10:226. https://doi.org/10.1007/s13201-020-01314-z
Mao H, Wang G, Liao F et al (2023) Spatial variability of source contributions to nitrate in regional groundwater based on the positive matrix factorization and Bayesian model. J Hazard Mater 445:130569. https://doi.org/10.1016/j.jhazmat.2022.130569
Mohamed NA, Wachemo AC, Karuppannan S, Duraisamy K (2022) Spatio-temporal variation of groundwater hydrochemistry and suitability for drinking and irrigation in Arba Minch Town, Ethiopia: an integrated approach using water quality index, multivariate statistics, and GIS. Urban Clim 46:101338. https://doi.org/10.1016/j.uclim.2022.101338
Mukherjee I, Singh UK, Chakma S (2022) Evaluation of groundwater quality for irrigation water supply using multi-criteria decision-making techniques and GIS in an agroeconomic tract of Lower Ganga basin, India. J Environ Manag 309:114691. https://doi.org/10.1016/j.jenvman.2022.114691
Nawale VP, Malpe DB, Marghade D, Yenkie R (2021) Non-carcinogenic health risk assessment with source identification of nitrate and fluoride polluted groundwater of Wardha sub-basin, central India. Ecotoxicol Environ Saf 208:111548. https://doi.org/10.1016/j.ecoenv.2020.111548
Nizam S, Acharya T, Dutta S, Sen IS (2022) Occurrence, sources, and spatial distribution of fluoride in the Ganga alluvial aquifer, India. Environ Geochem Health 45(5):1975–1989. https://doi.org/10.1007/s10653-022-01319-4
Pal SC, Ruidas D, Saha A et al (2022) Application of novel data-mining technique based nitrate concentration susceptibility prediction approach for coastal aquifers in India. J Clean Prod 346:131205. https://doi.org/10.1016/j.jclepro.2022.131205
Panneerselvam B, Muniraj K, Thomas M et al (2021) Identifying influencing groundwater parameter on human health associate with irrigation indices using the Automatic Linear Model (ALM) in a semi-arid region in India. Environ Res. https://doi.org/10.1016/j.envres.2021.111778
Pasupuleti S, Singha SS, Singha S et al (2022) Groundwater characterization and non-carcinogenic and carcinogenic health risk assessment of nitrate exposure in the Mahanadi River Basin of India. J Environ Manag 319:115746. https://doi.org/10.1016/j.jenvman.2022.115746
Pincetti-Zúniga GP, Richards LA, Tun YM et al (2020) Major and trace (including arsenic) groundwater chemistry in central and southern Myanmar. Appl Geochem 115:104535. https://doi.org/10.1016/j.apgeochem.2020.104535
Piyathilake IDUH, Ranaweera LV, Udayakumara EPN et al (2022) Assessing groundwater quality using the water quality index (WQI) and GIS in the Uva province, Sri Lanka. Appl Water Sci 12:72. https://doi.org/10.1007/s13201-022-01600-y
Podgorski J, Berg M (2022) Global analysis and prediction of fluoride in groundwater. Nat Commun 13:4232. https://doi.org/10.1038/s41467-022-31940-x
Qaiser FUR, Zhang F, Pant RR et al (2023) Characterization and health risk assessment of arsenic in natural waters of the Indus River Basin, Pakistan. Sci Total Environ 857:159408. https://doi.org/10.1016/j.scitotenv.2022.159408
Rahman MdA, Siddique MdAB, Khan R et al (2022) Mechanism of arsenic enrichment and mobilization in groundwater from southeastern Bangladesh: water quality and preliminary health risks assessment. Chemosphere 294:133556. https://doi.org/10.1016/j.chemosphere.2022.133556
Rapant S, Krčmová K (2007) Health risk assessment maps for arsenic groundwater content: application of national geochemical databases. Environ Geochem Health 29:131–141. https://doi.org/10.1007/s10653-006-9072-y
Rashid A, Ayub M, Bundschuh J et al (2023) Geochemical control, water quality indexing, source distribution, and potential health risk of fluoride and arsenic in groundwater: Occurrence, sources apportionment, and positive matrix factorization model. J Hazard Mater 460:132443. https://doi.org/10.1016/j.jhazmat.2023.132443
Ruidas D, Pal SC, Islam ARMdT, Saha A (2021) Characterization of groundwater potential zones in water-scarce hardrock regions using data driven model. Environ Earth Sci 80:809. https://doi.org/10.1007/s12665-021-10116-8
Ruidas D, Pal SC, Saha A et al (2022) Hydrogeochemical characterization based water resources vulnerability assessment in India’s first Ramsar site of Chilka lake. Mar Pollut Bull 184:114107. https://doi.org/10.1016/j.marpolbul.2022.114107
Ruidas D, Pal SC, Chowdhuri I et al (2023a) Hydrogeochemical evaluation for human health risk assessment from contamination of coastal groundwater aquifers of Indo-Bangladesh Ramsar site. J Clean Prod 399:136647. https://doi.org/10.1016/j.jclepro.2023.136647
Ruidas D, Pal SC, Towfiqul Islam ARMd, Saha A (2023b) Hydrogeochemical evaluation of groundwater aquifers and associated health hazard risk mapping using ensemble data driven model in a water scares plateau region of eastern India. Expo Health 15:113–131. https://doi.org/10.1007/s12403-022-00480-6
Ruidas D, Pal SC, Biswas T et al (2024) Extreme exposure of fluoride and arsenic contamination in shallow coastal aquifers of the Ganges delta, transboundary of the Indo-Bangladesh region. Geosci Front 15:101725. https://doi.org/10.1016/j.gsf.2023.101725
Sellamuthu S, Joseph S, Gopalakrishnan S et al (2022) Appraisal of groundwater quality for drinking and irrigation suitability using multivariate statistical approach in a rapidly developing urban area, Tirunelveli, India. Environ Sci. https://doi.org/10.1007/s11356-022-23533-4
Shaikh H, Gaikwad H, Kadam A, Umrikar B (2020) Hydrogeochemical characterization of groundwater from semiarid region of western India for drinking and agricultural purposes with special reference to water quality index and potential health risks assessment. Appl Water Sci 10:204. https://doi.org/10.1007/s13201-020-01287-z
Sharma MK, Kumar M (2020) Sulphate contamination in groundwater and its remediation: an overview. Environ Monit Assess 192:74. https://doi.org/10.1007/s10661-019-8051-6
Sharma D, Rishi M, Keesari T (2017) Evaluation of groundwater quality and suitability for irrigation and drinking purposes in southwest Punjab, India using hydrochemical approach. Appl Water Sci. https://doi.org/10.1007/s13201-016-0456-6
Taloor AK, Sharma S, Pir RA, Kumar K (2024) Appraisal of health risks associated with exposure of fluoride and nitrate contaminated springs in the Doda Kishtwar Ramban (DKR) region of Jammu and Kashmir, India. J Geochem Explor 257:107380. https://doi.org/10.1016/j.gexplo.2023.107380
Tanwer N, Deswal M, Khyalia P et al (2023) Assessment of groundwater potability and health risk due to fluoride and nitrate in groundwater of Churu district of Rajasthan, India. Environ Geochem Health 45:4219–4241. https://doi.org/10.1007/s10653-023-01485-z
USEPA (2004) Risk assessment guidance for superfund volume I: human health evaluation manual (Part E). Environmental Protection Agency, Washington, DC, U.S.
Verma P, Singh PK, Sinha RR, Tiwari AK (2020) Assessment of groundwater quality status by using water quality index (WQI) and geographic information system (GIS) approaches: a case study of the Bokaro district, India. Appl Water Sci 10:27. https://doi.org/10.1007/s13201-019-1088-4
Wang X, Zheng W, Tian W et al (2022) Groundwater hydrogeochemical characterization and quality assessment based on integrated weight matter-element extension analysis in Ningxia, upper Yellow River, northwest China. Ecol Ind 135:108525. https://doi.org/10.1016/j.ecolind.2021.108525
Wang S, Chen J, Jiang W et al (2023a) Identifying the geochemical evolution and controlling factors of the shallow groundwater in a high fluoride area, Feng County, China. Environ Sci Pollut Res 30:20277–20296. https://doi.org/10.1007/s11356-022-23516-5
Wang S, Chen J, Zhang S et al (2023b) Hydrochemical evolution characteristics, controlling factors, and high nitrate hazards of shallow groundwater in a typical agricultural area of Nansi Lake Basin, North China. Environ Res 223:115430. https://doi.org/10.1016/j.envres.2023.115430
WHO (2011) Guidelines for Drinking-water Quality, Fourth Edition. World Health Organization. https://scholar.google.com/scholar_lookup?&title=Guidelines%20for%20drinking-water%20quality&journal=WHO%20Chron.&volume=38&pages=104-108&publication_year=2011
Xiao Y, Liu K, Hao Q et al (2022a) Hydrogeochemical insights into the signatures, genesis and sustainable perspective of nitrate enriched groundwater in the piedmont of Hutuo watershed. China CATENA 212:106020. https://doi.org/10.1016/j.catena.2022.106020
Xiao Y, Liu K, Hao Q et al (2022b) Occurrence, controlling factors and health hazards of fluoride-enriched groundwater in the lower flood plain of yellow river, northern China. Expo Health 14:345–358. https://doi.org/10.1007/s12403-021-00452-2
Xu N, Gong J, Yang G (2018) Using environmental isotopes along with major hydro-geochemical compositions to assess deep groundwater formation and evolution in eastern coastal China. J Contam Hydrol 208:1–9. https://doi.org/10.1016/j.jconhyd.2017.11.003
Xu N, Liu L, Mengjiao T et al (2023) Co-occurrence, sources and co-enrichment mechanism of arsenic, fluoride in groundwater from Huaihe River Basin, China. Emerg Contam. https://doi.org/10.1016/j.emcon.2023.100276
Yuan R, Li Z, Guo S (2023) Health risks of shallow groundwater in the five basins of Shanxi, China: geographical, geological and human activity roles. Environ Pollut 316:120524. https://doi.org/10.1016/j.envpol.2022.120524
Zeng Y, Lu H, Zhou J et al (2023) Enrichment mechanism and health risk assessment of fluoride in groundwater in the Oasis zone of the Tarim basin in Xinjiang, China. Expo Health. https://doi.org/10.1007/s12403-023-00553-0
Zhang Y, Xu B, Guo Z et al (2019) Human health risk assessment of groundwater arsenic contamination in Jinghui irrigation district, China. J Environ Manag 237:163–169. https://doi.org/10.1016/j.jenvman.2019.02.067
Zhou Y, Li P, Chen M et al (2021) Groundwater quality for potable and irrigation uses and associated health risk in southern part of Gu’an County, North China Plain. Environ Geochem Health 43:813–835. https://doi.org/10.1007/s10653-020-00553-y
Zhou J, Chen J, Wang S et al (2024) Hydrochemical characteristics and enrichment mechanism of shallow high fluorine groundwater in Feng county. Earth Environ 52:122–132. https://doi.org/10.14050/j.cnki.1672-9250.2023.051.072
Zhu Y, Yang Q, Wang H et al (2023) A hydrochemical and isotopic approach for source identification and health risk assessment of groundwater arsenic pollution in the central Yinchuan basin. Environ Res 231:116153. https://doi.org/10.1016/j.envres.2023.116153
Zhu K (2007) Practice of rural drinking water safety project construction in Feng County. Jiangsu Water Resources 43–45 (in Chinese)
Acknowledgements
The authors thank our colleagues and graduate students for their help in data collection and fieldwork. Special thanks to the editor and anonymous reviewers for their critical comments and valuable suggestions.
Funding
This study was supported by the National Key Research and Development Program of China (2020YFD0900703); the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX23_0737); and the Science and Technology Project on Water Conservancy in Jiangsu Province (2021056).
Author information
Authors and Affiliations
Contributions
SW: Investigation, Methodology, Data analysis, Interpretation of results, Writing – original draft. JC: Funding acquisition, Project management, Conceptualization, Validation. SZ: Investigation, Sample analysis, Interpretation of results, Writing – original draft. YB: Methodology, Software. XZ: Investigation, Sample analysis. WJ: Investigation. SY: Formal analysis. All authors read and approved the final draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, S., Chen, J., Zhang, S. et al. Shallow groundwater quality and health risk assessment of fluoride and arsenic in Northwestern Jiangsu Province, China. Appl Water Sci 14, 119 (2024). https://doi.org/10.1007/s13201-024-02174-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-024-02174-7