Abstract
The prime objective of this study is to develop a water quality index (WQI) to identify the relationship between the drinking water quality and the prevalence of Chronic Kidney Disease of Uncertain Etiology (CKDu) in the Uva Province (UP). For this, all CKDu patients in the province were recorded. 251 groundwater samples were collected and analyzed for their major cations and anions. Following this procedure, the spatial distribution maps for CKDu patients, water quality parameters and WQI were generated. The results revealed that, 20.3% of groundwater samples are categorized under “excellent” in terms of the drinking water quality, 21.2% of the samples are categorized under “good”, 20.3% of the samples are categorized under “poor”, 9.9% of the samples are categorized under “very poor”, and 28.3% of the samples are categorized under “unsuitable” in terms of the WQI. According to the results, the most significant correlation was recorded between fluoride content in the samples and WQI (0.96). Statistical analysis showed that the WQI has a strong positive correlation (0.68) with the spatial distribution of CKDu patients in the UP inferring that groundwater quality has a significant effect on the prevalence of CKDu in the UP. Moreover, these maps can be effectively used by decision makers for groundwater quality management activities in the UP, Sri Lanka.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Groundwater is defined as the water that exists beneath the earth surface mostly in the aquifers (Chaurasia et al. 2018; Narsimha and Sudarshan 2017). It plays a vital role in water supply for various purposes viz. drinking, industrial and agricultural purposes (Adimalla et al. 2018). According to the previous studies, approximately 65% of groundwater in the world is utilized for drinking purposes whereas 20% and 15% of groundwater are being used for agricultural and industrial activities respectively (Adimalla and Venkatayogi 2017; Adimalla et al. 2019; Salehi et al. 2018; Subramani et al. 2005). The suitability of groundwater especially for drinking purposes depends mainly on its quality and therefore the latter has gained immense importance in the recent past (Alcamo 2019). In Sri Lanka, 80% of groundwater sources are utilized for domestic, commercial, and other industrial purposes due to the increasing the demand for groundwater daily (Panabokke and Perera 2005). Furthermore, as explained by Panabokke and Perera (2005), the majority of rural people in Sri Lanka heavily depend on dug and tube wells since the groundwater is the safest drinking water source that can be self-managed. Therefore, the quality of the groundwater deteriorates rapidly due to geological and anthropogenic activities (Udeshani et al. 2020). According to the finding of past researches, groundwater resources have been highly vulnerable within the country due to anthropogenic activities viz. agricultural activities, land-use practices, cultivation practices, and industrial activities as compared to natural causes (Gunatilake and Iwao 2009, 2010; Rubasinghe et al. 2015; Villholth and Rajasooriyar 2010). As explained by Aravinna et al. (2017), residues, pollutants, and contaminants of pesticides and other agrochemicals applied on agricultural lands reach groundwater by leaching, and move to offsite water bodies by direct runoff, soil erosion, and spray drift. Thus it has been revealed that contamination of this groundwater due to various causes may ultimately result in the availability of poor drinking water, reduction in the quantity of water sources, high cost for water purification, high cost for alternative water supplies and most importantly potential for human health problems (Chandrajith et al. 2020; Dissanayake and Chandrajith 2017; Rajasooriyar et al. 2013; Villholth and Rajasooriyar 2010). Moreover, groundwater pollution may also highly threatens economic development, and social prosperity (Milovanovic 2007; Shah 2007).
Chronic Kidney Disease of uncertain Etiology (CKDu) in Sri Lanka has received much attention over the last two decades and many scientists assumed and proposed that prolonged consumption of drinking water with high levels of contaminants like fluoride (Dissanayake 2005; Ileperuma et al. 2009), Cadmium (Bandara et al. 2008; Wanigasuriya et al. 2011), Arsenic (Jayasumana et al. 2013), hardness forming agents, high ionicity (Dharmawardana et al. 2015), and agrochemical residues like Glyphosate (Jayasumana et al. 2013) are the root causes for the progression of the CKDu. Furthermore, another contributory factor for the cause of CKDu explained by (Chandrajith et al. 2011a) is the Na/Ca ratio in drinking water with high levels of Fluoride. In Sri Lanka, North Central Province (NCP) is the most affected province with CKDu while Eastern and Central Provinces are the most prominent CKDu distributed provinces. As explained by Wanasinghe et al. (2018) the pattern of distribution of CKDu was spread outside the NCP again and it was diverted to the UP where 85% of the drinking water requirements of the rural communities are acquired from shallow and deep wells (Perera and Gonawala 2008). Therefore, regular monitoring and characterizing the groundwater quality in the UP is of utmost importance since they aid to examine its suitability for drinking and adopting appropriate measures for protection. Thus the UP was selected to investigate the root geochemical factor that contributes to the prevalence of CKDu.
Development of water quality indices is considered as the most effective tool of assessing water quality. In water quality indices, a number of water quality parameters viz. pH, major anion levels, major cation levels, and levels of trace elements are incorporated in a mathematical equation to rate the water quality defining its suitability for human consumption (Lkr et al. 2020; Logeshkumaran et al. 2015). Water quality indices condenses the bulk of various water quality parameters into a single value in a logical and simplified form (Sharma and Kansal 2011). The concept of WQI was first proposed by Horton (1965) and since then number of water quality indices have been suggested by experts which can identify the overall water quality status of a particular geographical location at a certain time easily, efficiently, and promptly. The water quality indices are easily interpretable and enable the comparison of the water quality status among different sites (Bora and Goswami 2017). In these water quality indices, the weights of each incorporated water quality parameters are derived based on the significance and impacts on such parameters on the overall water quality. Furthermore, WQI values can be classified into several categories as excellent water; Good water; Poor water; Very poor water; and Unsuitable water owing to the characteristics of particular WQI (Akther and Tharani 2017; Alobaidy et al. 2010; Udeshani et al. 2020). In a high CKDu endemic area like the UP of Sri Lanka, evaluating the availability of safe drinking water and sustainable management of water is one of the challenging areas towards development.
Thus the prime objective of this study is to develop a water quality index (WQI) to identify the relationship between the groundwater quality and the prevalence of CKDu in the UP, Sri Lanka. The results of the research findings on the groundwater quality status of the UP are presented based on spatial distribution maps of water quality parameters, WQI map, and statistical analysis of water quality results. Moreover, these maps aims to rapidly distinguish the location of most and least suitable water for drinking purposes in the UP and by mapping the index, the areas of high and low water quality can easily be distinguished by researchers as well as decision makers and/or the general public. In addition, these maps provide an important contribution for the understanding of relationships between the groundwater quality and the spatial distribution of the CKDu patients in UP, Sri Lanka.
Material and methods
Study area
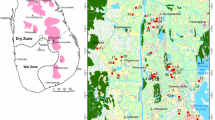
Sri Lanka, is a South Asian island country which lies at 7.8731° N and 80.7718° E. The country is surrounded by the Indian Ocean, southwest of the Bay of Bengal, and southeastern part of the Arabian Sea. UP is located in the south-east part of Sri Lanka and lies between longitude 80°40′0ʺE to 80°41′0ʺE and latitude 6°20′0ʺN to 7°40′0ʺN with an area of about 8500 Km2. The study area is mainly bordered by the Eastern Province, Southern Province, Sabaragamuwa Province and the Central province (UPC 2019). It comprises of 8,335 Km2 land and about 165 Km2 of inland water bodies. UP consists of two main administrative districts namely, Badulla and Moneragala. The total land area of the Badulla district is 2,861 Km2 and the total land area in Moneragala District is 5639 Km2.
Geology, soil and hydrology
Over 90% of Sri Lanka is made up of Precambrian high-grade metamorphic rocks in which primary porosity and permeability are negligible. The study area is also a part of the metamorphic terrain composed mainly of meta-sedimentary and meta-igneous rocks with few granitic intrusions. Migmatites, augen gneisses, granitic gneisses, biotite gneisses, and hornblende biotite gneisses are predominant in the region. Groundwater in this region is mostly from the weathered overburden and fractured zones (Fig. 1b).
The physical landscape of the Badulla district consists of mountain ranges, divided plateaus and narrow valleys. Geologically the Badulla district is located towards the East of the Central Highland complex and the prominent soil types include Red Earth and Brown Loams, Red-Yellow Padzolic and Reddish Brown soils (RDA 2017). The average annual rainfall in Badulla district varies from 900 mm to over 2500 mm. Rainfall is received in four seasons namely, first inter monsoon (March–April), Southwest Monsoon (July–September), second inter monsoon (October–November) and Northeast monsoon (December-January). Also, the average annual temperature of the district varies between 16 and 30 °C.
The Moneragala district is located in a transition zone within central highland to flat terrain. Mountainous terrain is marked in the western boundary of the region in which the elevation varies between 550 and 1500 m. Major soil types of the Moneragala district are Reddish Brown Earth and Red-Yellow Padzolic soils (RDA 2017). The annual precipitation of the district is about 1000 mm. Over 85% of rain in the district is received during the north-east monsoons while frequent drought conditions create an average evapotranspiration of 1200 mm per annum. The annual temperature of Moneragala district varies from 21.6 to 35 °C (RDA 2017). Since the climate is semi-arid to subtropical due to low rainfall and high evapotranspiration, water scarcity is a major problem in the district. The low rainfall and high evaporation rate drastically impacts on the groundwater storage and water quality, consequently the community health.
Ecological environment
The total extent of the land under protection in the UP exceeds 2000 Km2. The forest cover is mainly comprised of montane, sub-montane tropical, dry, evergreen, mixed forests. Also, important wildlife areas within the district include Gal Oya National Park, Yala National Park, Udawalawa National Park, and Maduru Oya National Park (Fig. 1a). The considerable land area of the province covers mainly forest areas (31.4%), scrublands (22.12%), home gardens (14.78%), rice (20.9%), and Chena cultivation for short-term crops (10.72%). In the Badulla district, the majority of the farmers are engaged in vegetable farming including potatoes, carrots, beans, leeks, cabbage, green chili, tomatoes, and beetroot. In the Moneragala district, paddy and field crops such as maize, cowpea, groundnut, and green grams are grown. Irrigation is largely practiced in the Moneragala district and it increases the sodicity of the soil.
Sampling procedure
The groundwater sampling program was designed following the stratified random sampling procedure. The total area of the entire UP was divided into 100 Km2 grids using the topographic maps prepared by the Survey Department of Sri Lanka. After completing the reconnaissance surveys, the sampling locations of dug wells and tube wells were randomly selected from each grid according to the availability. Then, groundwater samples were collected from each grid by applying American Public Health Association (APHA 2005) standard methods for sample collection and preservation. Groundwater samples were collected from both CKDu prevalent and non-prevalent areas covering the entire study area. The groundwater samples collected from CKDu non-prevalent areas were used as control samples of the study.
The sampling process has been carried out covering the whole of UP while the Geographical Positioning System (GPS) coordinates of the locations were recorded using a MAGELLAN™ GPS receiver. Groundwater samples were collected into properly labeled high-density polyethylene bottles which were acid-soaked overnight and then washed thoroughly with deionized water and oven-dried for 3 h at 50 °C. At the time of sampling, bottles were thoroughly rinsed 2–3 times with groundwater to be sampled. In the case of sampling from tube wells, samples were collected after pumping for 10 min to remove groundwater stored in the well. Two subsets of samples were collected for laboratory analysis in which one was filtered and acidified by adding several drops of Conc. Nitric acid (pH < 2) for cation analysis, while the un-acidified sample was used for anion analysis. The samples were placed in a cooled ice box during transportation and stored refrigerated until analysis (at 5 °C). The sampling points were plotted using GIS-ArcMap™ 10.4 mapping software to be used in data analysis (Fig. 2).
Analysis of physico-chemical parameters of groundwater
Onsite measurements of water quality parameters (Temperature, pH, Electrical Conductivity) of collected groundwater samples were recorded using a pre-calibrated Thermo Scientific Orion Star A325™ Multiparameter test kit. The reliability of pH and EC analysis was tested after every five samples using standard buffer solutions of pH 7 and 10, whereas that of standard EC solution EC = 1413 µS/cm. All other chemical parameters were determined using standard procedures described by APHA (2005). Total alkalinity, total hardness, and Chloride (Cl−) of the samples were analyzed using a Hach™ digital titrator within 24 h of sampling. To measure alkalinity, the sulfuric acid method was used whereas the EDTA method and Silver nitrate method was used, respectively, for the analysis of total hardness and chloride. Nitrate (NO3-N), Sulfate (SO42−), Phosphate (PO43−), and Fluoride (F−) contents were determined using a Hach™ DR 2700 spectrophotometer within 24 h of sampling. Major cations (Sodium-Na+, Potassium-K+, Calcium-Ca2+, and Magnesium-Mg2+) were measured using Atomic Absorption Spectrometer (AAS-Varian 240FS) at the Sabaragamuwa University of Sri Lanka, while trace metals (Aluminum-Al, Chromium-Cr, Manganese- Mn, Iron-Fe, Cobalt-Co, Nickel-Ni, Copper-Cu, Zinc-Zn, Arsenic-As, Cadmium-Cd, and Lead-Pb) were measured by Thermo ICapQ Inductively Coupled Plasma Mass Spectrometer (ICP-MS) at the University of Peradeniya, Sri Lanka. The detection limits for Na+, K+, Ca2+, and Mg2+ on the AAS-Varian 240FS are 0.05, 0.05, 0.10, and 0.10, ppm at 589, 769, 422, and 285 nm, respectively. Overall, measurement reproducibility and precision for each analysis was less than 2%. Furthermore, blank samples and standard solutions were used to check for possible errors during the analysis. Moreover, based on the method explained by Appelo and Postma (1996), the ion balance errors was calculated to verify the accuracy of the chemical analysis. The ion balance error yielded about ± 4% for all the ion concentrations. This means that the data quality is sufficient for drawing simple conclusions about water quality.
Preparation of spatial distribution maps
Spatial distribution maps of water quality parameters such as pH, EC, TDS, alkalinity, Total Hardness (TH), anions (NO3-N, SO42−, PO43−, Cl− and F−) major cations (Na+, K+, Ca2+ and Mg2+) were prepared using GIS techniques. In this, ArcMap™ 10.4 mapping software was used and the Inverse Distance Weighted (IDW) which is more accurate method with power 2 was used to generate spatial distribution maps of measured water quality parameters. Furthermore, Spatial distribution of CKDu patients map was generated based on the data collected from the main hospitals, regional hospitals, and previously published data of the UP, Sri Lanka.
Estimation of water quality index (WQI)
To get a comprehensive overall quality of the groundwater in the UP, the WQI method was used. As explained by Walsh and Wheeler (2012), the WQI method has been widely used as a prominent approach to convey the water quality status of an area of interest to the general public and policymakers. Furthermore, WQI can be effectively used in the determination of the suitability of drinking water for human consumption (Poonam et al. 2013; Ramakrishnaiah et al. 2009).
In this study, the weighted arithmetic WQI method was applied based on the recommended guidelines for drinking water quality by the SLS 614:2013 (Sri Lankan Standard) for drinking purposes. The weighted arithmetic WQI is calculated using Eq. (1) (Adimalla et al. 2018; Akther and Tharani 2017; Sadat-Noori et al. 2014; Udeshani et al. 2020)
where, wi is the unit weightage of ith water quality parameter and qi is the quality rating scale of the ith parameter. The WQI calculation procedure can be further divided into five steps as shown in Fig. 3.
In the first step, each of the twelve parameters (pH, NO3-N, SO42−, PO43−, Cl−, F−, HCO3−, Hardness, Na+, K+, Ca2+ and Mg2+) has been assigned a weight (wi) based on their relative effects on primary health over other parameters (Akther and Tharani 2017). The weightage for various parameters is assumed to be inversely proportional to the SLS 614:2013 maximum permissible limit for the corresponding parameters (Sadat-Noori et al. 2014). As explained by Harshan et al. (2016), the wi for each parameter is calculated using Eqs. (2) and Eq. (3).
where, wi is the weightage for the ith parameter, k is a constant proportionality and vi is the standard value of ith water quality parameter.
where, k is a constant proportionality and vi is the standard value of ith water quality parameter.
In the second step, the relative weight (Wi) of each parameter is computed using Eq. (4).
where, Wi is the relative weights of each water quality parameter, wi is the calculated weights of each water quality parameter and, n is the number of water quality parameters. The calculated weights (wi) and relative weights (Wi) values are presented in Table 1.
In the third step, a water quality rating scale (Qi) was calculated for each parameter using Eq. (5).
where, Qi is the quality rating scale, va is the actual value obtained from laboratory analysis of ith parameter, vi is the recommended standard value of ith water quality parameter, and, vs is the ideal value (pH = 7 and 0 for all other parameters).
In the fourth step, a sub-index SIi for each ith water quality parameter and WQI were calculated using Eq. (6) and Eq. (7) respectively.
Computed WQI values were classified into five categories as excellent water (WQI < 25); Good water (WQI < 25–50); Poor water (WQI < 50–75); Very poor water (WQI < 75–100); and water Unsuitable for drinking (WQI > 100) (Akther and Tharani 2017; Alobaidy et al. 2010; Udeshani et al. 2020).
Data analysis
Statistical correlation analyses has been carried out to interpret the relationship among various water quality parameters, the relationship between the water quality parameters and WQI and, the relationship between the WQI and the prevalence of CKDu in the UP. To identify the most significant water quality parameters that influence the WQI, the correlation matrix of 12 water quality variables and WQI was determined. Furthermore, the statistical correlation has been calculated for the spatial distribution of WQI and the spatial distribution of the CKDu patients in the UP based on the Pearson correlation coefficient using the Minitab 17 statistical package.
Results and discussion
Spatial analysis of groundwater quality
The pH is one of the most important water quality parameters which determines the acidity or alkalinity of the groundwater. A pH 7 in the groundwater at 25 °C is considered neutral (Nelson 2002). Measuring the pH of the groundwater is essential when carrying out a water quality assessment because this parameter regulates the quantity and chemical structure of some organic and inorganic constituents that have been dissolved in the groundwater (Sadat-Noori et al. 2014). As recommended by the SLS 614:2013 water quality guidelines, the pH of the drinking water should range from 6.5 to 8.5. The pH of the groundwater in the UP varies between 5.04 and 8.87 whereas the mean pH of the UP was recorded as 7.04 with a standard deviation of 0.49 (Table 2). According to the results, 12% of the collected 230 samples were not in the permissible level (greater than 8.5) with slightly alkaline conditions. Spatial distributions of pH variation is shown in Fig. 4. The map reveals that except for very insignificant areas, most of the areas consist of the desirable limit of pH. As explained by Chaurasia et al. (2018), the electrical conductivity (EC) in the groundwater is a measurement of the dissolved constituents in an aqueous solution.. As shown in Table 2, the EC of the groundwater in the UP ranged between 3.4 and 5129.0 μS/cm which has the mean value of 724.2 ± 620.7 μS/cm. According to SLS 614:2013 specification, only a single groundwater sample location exceeds the allowable limit (3500 μS/cm) that indicates the suitability of the groundwater for drinking purposes. The spatial distribution map of the EC is shown in the Fig. 4.
The nitrate (NO3-N) concentration of the groundwater in the UP ranged from 0.12 to 11.51 mg/L with the mean value of 2.79 ± 1.56 mg/L (Table 2). According to the SLS 614:2013 drinking water quality guidelines, the maximum contaminated limit of the NO3-N in the groundwater should not exceed 10 mg/L as NO3−-N. The results of the NO3-N concentrations in all the sampling locations reveals that 2% of the samples are not contaminated (less than 1 ppm), 34% of samples are less contaminated (less than 3 ppm), 63% of the samples are contaminated (3–10 ppm) and 1% of the samples exceed the desirable levels of NO3-N in the drinking water. However, the majority of the people in the UP are farmers, and the major occupation of the UP is the cultivation of crops viz. rice, vegetables, tea, etc. (Piyathilake et al. 2020). Therefore, it can be hypothesized that there is a harmful effect of the application of nitrogenous fertilizers on the crop fields in terms of the NO3-N accumulation in the groundwater. The spatial distribution of the NO3-N concentration in the samples is shown in Fig. 4.
Phosphate (PO43−) is another significant water quality parameter that has a significant potential to effect the overall quality of groundwater. In this study, PO43− concentrations of all the samples ranged between 0.02 and 5.86 mg/L whereas the mean was recorded as 0.67 ± 0.86 mg/L. According to World Health Organization guidelines WHO (2004), PO43− concentration in all water samples exceeds the contaminated levels for an aquatic organism. According to the results, 77.4% of water samples were showed a PO43− concentration less than 1.00 ppm, and 14.6% of the sample were between 1.00 and 2.00 ppm with higher PO43− concentration. 8% of the samples were not in the permissible level (2 mg/L) with very high phosphate conditions. This may be due to the long-term application of chemical fertilizers on crop fields for many decades. Since the majority of phosphate levels in groundwater samples were higher than the recommended level, the contamination of groundwater by the application of phosphate fertilizer should be controlled. The spatial distribution of the phosphate in the UP is shown in Fig. 4.
Sulfate (SO42−) in groundwater is mostly available due to the dissolution of rocks which contain compounds viz. gypsum, iron sulfides, and other sulfur-containing materials (Chaurasia et al. 2018). As revealed by the results of this study, only 1% of the groundwater samples collected exceeded the permissible limit of SO42− (250 mg/L) for drinking water. The values of SO42− concentrations ranged from 0 to 540 mg/L with the mean SO42− concentration of 33 ± 52 mg/L. The spatial distribution of SO42− concentrations in the samples is shown in Fig. 4.
Fluoride (F−) is an essential element for human beings for development and growth (Chattopadhyay et al. 2011). Although it is beneficial at low recommended doses (0.5–1.0 ppm), the higher levels of F− may cause human kidney damage and accumulation of F− in hard tissues of the body and endemic skeletal/ teeth fluorosis may be caused (Chandrajith et al. 2011a). However, 40% of the groundwater samples out of analyzed 230 samples exceeds the permissible limit of 1 mg/L with the mean F− concentration of 0.97 ± 0.83 mg/L. According to Cooray (1994), Sri Lanka is dominated by Precambrian metamorphic rocks and can be divided into four main lithotechtonic units namely, the Highland Complex, the Vijayan Complex, the Kadugannawa complex and, the Wanni Complex. Among these, the Highland complex is the largest while it is comprised of mostly Garnet—sillimanite–graphite gneiss, charnockite, quartzites, marbles, and calc gneisses whereas the Vijayan and the Wanni complexes consist of mainly biotite—hornblende gneisses, scattered bands of metasediments, charnockitic gneisses, and granites. Furthermore, as mentioned by Chandrajith et al. (2012), most of these rocks consist of F-bearing minerals such as micas, hornblende, and apatite, and minerals such as fluorite, tourmaline, and topaz also contribute to the general geochemical cycle of fluorine in the physical environment. Thus, the mechanism of leaching of F− into groundwater is clearly explained by Chandrajith et al. (2012), and according to their explanation, intense weathering of rocks and minerals in the tropical climate tends to enhance the entry of F− into the aqueous phase and is therefore leached out from the F-bearing minerals. The spatial distribution map of the F− variability in water samples is shown in the Fig. 4.
Chloride (Cl−) is also considered as one of the most important water quality parameters since higher levels of Cl− may cause severe health effects to human beings (Pius et al. 2012). Furthermore, as in the case of SO42− the high Cl− levels of the groundwater may impart a change in the taste of the water (Sadat-Noori et al. 2014). However, only 3% of the groundwater samples exceeded the recommended higher permissible limit (250 mg/L) with the mean value of 60.21 ± 992.15 mg/L whereas the minimum and maximum values were 7.00 and 1100.00 respectively. The spatial distribution of the Cl− in the UP is shown in Fig. 4.
In water quality analysis, HCO3− is given a minimum weight since it plays an insignificant role in water pollution (Ketata et al. 2012). As explained by Chaurasia et al. (2018), HCO3− may enter groundwater due to the action of carbon dioxide in the water on carbonated rocks viz. dolomite and limestone. However, 57% of the collected groundwater samples, the HCO3− level exceeded the permissible limit (200 mg/L) with the range of 12.0 and 820.0 mg/L. The mean value of the HCO3− concentration of the UP was recorded as 262.6 ± 166.5 mg/L. The spatial distribution of the HCO3− ions of the samples is shown in the Fig. 4.
The Total Hardness (TH) of the groundwater is also considered an important factor that determines the groundwater quality for drinking purposes. As explained by Ravikumar et al. (2011), Ca2+, Mg2+, SO42−, Cl−, CO32− and HCO3− of the water may cause water hardness. The hardness values ranged between 4.0 to 1464.0 mg/L and the water with hardness above 250 mg/L is considered as the highest permissible limit according to the SLS 614:2013. However, in this study, the mean hardness of the study was recorded as 234.4 ± 169.7 mg/L in which 41% of the water samples have exceeded the recommended limits. The spatial distribution of groundwater hardness is shown in Fig. 5. As revealed by Ramesh and Elango (2006), the regular consumption of water with a hardness above 300 mg/L may lead to human heart diseases and kidney diseases.
The major cation concentrations of the collected groundwater samples are mentioned in Table 2 and the spatial distribution of each major cation is shown in Fig. 5.
The major cation trend in the groundwater of the UP is Na+ > Ca2+ > Mg2+ > K+. Sodium is the dominant cation and the levels of sodium ranged between 0.17 to 608.74 mg/L with a mean value of 66.26 ± 84.65 mg/L where the maximum permissible limit of the sodium is 200 mg/L. In this study, 6% of the groundwater samples were recorded as exceeding the limits. Calcium is the second dominant cation in the UP which shows a mean value of 56.01 ± 36.65 mg/L with a range of 14.18 to 206.23 mg/L. The maximum permissible limit for calcium is 100 mg/L and it was calculated that 12% of groundwater samples exceeded this limit. The mean value of magnesium is recorded as 23.05 ± 29.60 mg/L whereas the values are ranged between 0.26 to 262.68 mg/L. In this study, 24% of the groundwater samples exceeded the maximum permissible limit of magnesium (30 mg/L) in the drinking water. The least dominant major cation of the UP is potassium and the mean value of the potassium was recorded as 2.11 ± 1.82 mg/L that the value ranged between 0.21 to 11.56 mg/L. However, all the groundwater samples were within the desirable limits recommended by the SLS 614:2013 water quality guidelines. Furthermore, the trace element concentrations of the groundwater in the UP were analyzed and all the statistical data are presented in Table 2. According to the results, none of the trace elements in all the groundwater samples had exceeded their highest permissible limits recommended by the SLS 614:2013. Due to this insignificant effect of the trace elements in the overall quality of the groundwater in the UP, trace elements are not considered when developing the WQI for the UP, Sri Lanka.
Water quality index (WQI)
Water quality index for the groundwater in the UP is calculated and mapped to determine the suitability of groundwater for drinking purposes. The calculated WQI values for each of the 251 groundwater sampling locations are shown in Table 3. According to the sampling procedure, there are 164 dug wells, 87 tube wells which were sampled and analyzed to generate the WQI. Furthermore, as shown in the Fig. 6, groundwater samples can be comprehensively categorized into five water quality levels (excellent, good, poor, very poor and unsuitable) by considering the type of groundwater sources (dug wells and tube wells). According to the results, 20.3% of groundwater samples are categorized under excellent in terms of the drinking water quality, 21.2% of the samples are categorized under good, 20.3% of the samples are categorized under poor, 9.9% of the samples are categorized under very poor, and 28.3% of the samples are categorized under unsuitable category in terms of the WQI.
Figure 7 illustrates the spatial distribution of the WQI map. The WQI map revealed that the central high elevated areas of the UP are the safest zone in terms of groundwater quality for drinking purposes. In those high elevated areas, nearly all the WQI values of the groundwater samples are in excellent and good categories where groundwater can be highly recommended for drinking purposes. In general, the groundwater quality decreases from high elevated areas to flat terrain mostly in the Moneragala district and upper part of the Badulla district.
Relationship between WQI and water quality parameters
A correlation matrix of twelve water quality parameters, namely, pH, NO3-N, SO42−, PO43−, Cl−, F−, HCO3−, hardness, Na+, K+, Ca2+ and Mg2+ among themselves and WQI was generated and it is shown in Table 4.
According to the results, TH exhibits significantly strong positive correlation with HCO3− (r = 0.78), SO42− (r = 0.70), Mg2+ (r = 0.73), and Ca2+ (r = 0.74). Also, significantly a strong positive correlation was observed between HCO3− and F− (r = 0.70) whereas HCO3− is moderately correlated with Na+ (r = 0.63), Mg2+ (r = 0.62), Ca2+ (r = 0.54), SO42− (r = 0.59), and Cl− (r = 0.51). Moreover, the correlation analysis exhibits significantly strong positive correlation between SO42− and Cl− (r = 0.79), Na+ and Cl− (r = 0.71), Mg2+ and Cl− (r = 0.77), and Mg2+ and Na+ (r = 0.80). Furthermore, the correlation of all the water quality parameters with the WQI was evaluated to identify the significant impact of each water quality parameter on the overall quality of the groundwater in the UP. According to the results, the most significant correlation was recorded between F− and WQI (0.96). This is a strong positive correlation and it can be recognized that F− has the most significant impact on the overall quality of the groundwater in the UP since F− has been identified as and water quality parameter which has severe human health impacts. Furthermore, WQI has significant positive correlations with HCO3− (0.71), and Mg2+ (0.62). But these are not parameters that severely impact human health. Moreover, SO42−, Cl−, and TH show a moderate positive correlation with the WQI whereas pH, NO3-N, PO43−, K+, and Ca2+ show a week positive correlation with the WQI.
Furthermore, the WHO maximum permissible limits of water quality parameters were compared with WQI to comply with water quality data for drinking purposes (Table 5). In excellent WQI category, 100% of the samples were ranged within the WHO safe limits in terms of all the assessed parameters. In good WQI category, pH, NO3—N, PO43−, SO42−, F−, Cl−, Na+, K+, Ca2+ and Mg2+ of all the samples ranged within the WHO safe limits whereas 33%, 20% and 11% of samples exceeded the WHO safe limits of HCO3− (200 mg/L), Ca2+ (100 mg/L), and Mg2+ (30 mg/L) respectively. In poor WQI category, pH, NO3—N, SO42−, Cl−, Na+, and K+ of all the samples ranged within the WHO safe limits whereas 12%, 26%, 65%, 13%, and 20% of samples exceeded the WHO safe limits of PO43− (2 mg/L), F− (1 mg/L), HCO3− (200 mg/L), Ca2+ (100 mg/L), and Mg2+ (30 mg/L) respectively. In very poor WQI category, pH, NO3—N, SO42−, Cl−, Na+, and K+ of all the samples ranged within the WHO safe limits whereas 11%, 85%, 81%, 12%, and 38% of samples exceeded the WHO safe limits of PO43− (2 mg/L), F− (1 mg/L), HCO3− (200 mg/L), Ca2+ (100 mg/L), and Mg2+ (30 mg/L) respectively. In unsuitable WQI category, pH, NO3—N, SO42−, Cl−, Mg2+ and K+ of all the samples ranged within the WHO safe limits whereas 8%, 97%, 93%, 51%, and 13% of samples exceeded the WHO safe limits of PO43− (2 mg/L), F− (1 mg/L), HCO3− (200 mg/L), Na+ (200 mg/L), and Ca2+ (100 mg/L) respectively.
As explained by Young et al. (2011), high F- levels in the dry zone areas of Sri Lanka is recorded due to the effects of underlying rocks. The UP is mainly composed of meta-sedimentary and meta-igneous rocks with few granitic intrusions with dominant hornblende and biotite bearing migmatites and gneisses (Malaviarachchi et al. 2021). These hornblende and biotite rocks has been identified as F− bearing minerals (Wilson et al. 2013). Thus, basement rocks in the area seem highly favorable subsurface sources supplying F- to the groundwater. Though it is not known with certainty that the host rock where rock–water interaction taking place is same as the bedrock exposed where the wells are located, thick rock profiles of hornblende and biotite bearing rocks with low dip angles are very likely to host for the wells. Also, as explained by Karunaratne and Pathmarajah (2002), the underlying basement rock in the study area consist of shallow regolith aquifers (2–10 m) and deep fracture zone aquifers (> 30–40 m) with fractures allowing a long residence time for groundwater. Therefore, these bedrocks are capable of activating the processes of dissolution of F- in the groundwater and increase the F− concentrations of the groundwater (Rao et al. 1993). Saxena and Ahmed (2003) explained that the alkaline nature of the groundwater could increase the F- levels as the alkaline water can mobilize F− from minerals. Moreover, Gibbs plots were employed to understand the relationships between functional sources of dissolved chemicals due to influences of controlling processes such as water–rock interaction, evaporation, and precipitation in water (Gibbs 1970). In this study, the majority of the groundwater samples were distributed in the rock weathering zone indicating the groundwater is primarily controlled by the chemical weathering of rocks (Srinivasamoorthy et al. 2012) (Fig. 8).
Relationship between WQI and the spatial distribution of chronic kidney disease of uncertain etiology
The prevalence of the CKDu in the UP, Sri Lanka has received much attention recently and many scientists assumed and proposed that persistent consumption of poor quality drinking water might be the root causes for the progression of the disease (Bandara et al. 2008; Chandrajith et al. 2011b; Dissanayake 2005; Gunatilake et al. 2014; Ileperuma et al. 2009; Jayasumana et al. 2013; Wanigasuriya et al. 2011). As explained by Perera and Gonawala (2008), approximately 85% of rural people in the UP obtain drinking water from dug wells and tube wells. Therefore this study aims at investigating the relationship between WQI and the prevalence of CKDu to check whether water quality directly impacts on CKDu in the UP. The UP of Sri Lanka consists of twenty-six Divisional Secretariat Divisions (DSDs) as shown in Fig. 9a. The spatial distribution of CKDu patients of the UP was developed based on the results of the survey that has been conducted by de Alwis and Panawala (2019). According to de Alwis and Panawala (2019), each DSD of the UP have been categorized into five categories as shown in Fig. 9b. According to the developed map, Mahiyanganaya is the highest vulnerable DS division for CKDu while Madulla, Medagama, and Katharagama are the lowest vulnerable DS divisions for CKDu in the UP. Katharagama may be recorded as the lowest vulnerable due to the majority of the Katharagama area is covered by the Yala national park. Also, using the ArcGIS 10.4 mapping software the statistical data of WQI of each DS division were extracted as shown in Table 6.
Ultimately the correlation coefficient between WQI and the CKDu distribution was evaluated. According to the results, WQI has a strong positive correlation (0.68) with the spatial distribution of CKDu patients in the UP. That means, it can be inferred that groundwater quality has a significant effect on CKDu distribution in the UP. Since F− has the most significant correlation (0.96) with WQI, ultimately it can be hypothesized that in the UP CKDu directly has a relationship with F− content in groundwater. Moreover, Chandrajith et al. (2011b) explained that kidney tabular damages are possible due to the formation of CaF2 which is insoluble in water. Further, he observed that a low Na/Ca ratio in groundwater is favorable to form CaF2 complex, which enhances the toxicity of F- ions in the human body and the incidence of CKDu in endemic CKDu regions. The spatial distribution map of the F− in UP shows that the areas where high F− varied in the Moneragala district overlapped with high CKDu prevalent areas indicating to some extent, that the F− content of drinking water might contribute to the CKDu (Dissanayake and Chandrajith 2017).
Conclusions
In this study, WQI method was applied to investigate the water quality status of the UP of Sri Lanka. Out of the analyzed samples 21.5%, 21.2%, 20.0%, 10.0%, and 27.3% are categorized under excellent, good, poor, very poor, and unsuitable category respectively in terms of the WQI. Moreover, based on the results it can be determined that the groundwater quality has a significant effect on the CKDu distribution in the UP. Since F− has the most significant correlation with WQI, ultimately it can be inferred that, in the UP, CKDu directly has a relationship with F− in groundwater. These various water quality indices can be effectively used as a valuable tool for policymakers to be able to recognize the status of the water quality in a specific area of interest and to have the capability to make suitable decisions regarding the management of natural groundwater sources. This study has shown the valuable combination of GIS and WQI to monitor and assess groundwater quality in any area of the world.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Adimalla N, Venkatayogi S (2017) Mechanism of fluoride enrichment in groundwater of hard rock aquifers in Medak, Telangana State, South India. Environ Earth Sci 76:45. https://doi.org/10.1007/s12665-016-6362-2
Adimalla N, Li P, Venkatayogi S (2018) Hydrogeochemical evaluation of groundwater quality for drinking and irrigation purposes and integrated interpretation with water quality index studies. Environ Process 5:363–383. https://doi.org/10.1007/s40710-018-0297-4
Adimalla N, Venkatayogi S, Das S (2019) Assessment of fluoride contamination and distribution: a case study from a rural part of Andhra Pradesh, India. Appl Water Sci 9:1–15. https://doi.org/10.1007/s13201-019-0968-y
Akther M, Tharani G (2017) Assessment of water quality parameters and determination of water quality index of tube well water in Vengalacheddikulam DS division, Vavuniya District, Sri Lanka. Int J Sci: Basic Appl 32(3):317–328
Alcamo J (2019) Water quality and its interlinkages with the sustainable development goals. Curr Opin Environ Sustain 36:126–140. https://doi.org/10.1016/j.cosust.2018.11.005
Alobaidy AHMJ, Maulood BK, Kadhem AJ (2010) Evaluating raw and treated water quality of Tigris River within Baghdad by index analysis. J Water Resour Prot 2:629. https://doi.org/10.4236/jwarp.2010.27072
APHA (2005) Standard methods of water and wastewater, 21st edn. APHA, Washington
Appelo CAJ, Postma D (1996) Geochemistry, groundwater and pollution. A.A. Balkema, Rotterdam
Aravinna P, Priyantha N, Pitawala A, Yatigammana SK (2017) Use pattern of pesticides and their predicted mobility into shallow groundwater and surface water bodies of paddy lands in Mahaweli river basin in Sri Lanka. J Environ Sci Health B 52:37–47. https://doi.org/10.1080/03601234.2016.1229445
Bandara J, Senevirathna D, Dasanayake D, Herath V, Bandara J, Abeysekara T, Rajapaksha K (2008) Chronic renal failure among farm families in cascade irrigation systems in Sri Lanka associated with elevated dietary cadmium levels in rice and freshwater fish (Tilapia). Environ Geochem Health 30:465–478. https://doi.org/10.1007/s10653-007-9129-6
Bora M, Goswami DC (2017) Water quality assessment in terms of water quality index (WQI): case study of the Kolong River, Assam, India. Appl Water Sci 7:3125–3135. https://doi.org/10.1007/s13201-016-0451-y
Chandrajith R et al (2011a) Chronic kidney diseases of uncertain etiology (CKDue) in Sri Lanka: geographic distribution and environmental implications. Environ Geochem Health 33:267–278
Chandrajith R, Dissanayake C, Ariyarathna T, Herath H, Padmasiri J (2011b) Dose-dependent Na and Ca in fluoride-rich drinking water—another major cause of chronic renal failure in tropical arid regions. Sci Total Environ 409:671–675. https://doi.org/10.1016/j.scitotenv.2010.10.046
Chandrajith R, Padmasiri J, Dissanayake C, Prematilaka K (2012) Spatial distribution of fluoride in groundwater of Sri Lanka. J Natl Sci Found. https://doi.org/10.4038/jnsfsr.v40i4.5044
Chandrajith R, Diyabalanage S, Dissanayake C (2020) Geogenic fluoride and arsenic in groundwater of Sri Lanka and its implications to community health. Groundw Sustain Dev 10:100359
Chattopadhyay A, Podder S, Agarwal S, Bhattacharya S (2011) Fluoride-induced histopathology and synthesis of stress protein in liver and kidney of mice. Arch Toxicol 85:327–335. https://doi.org/10.1007/s00204-010-0588-7
Chaurasia AK, Pandey H, Tiwari S, Prakash R, Pandey P, Ram A (2018) Groundwater Quality assessment using Water Quality Index (WQI) in parts of Varanasi District, Uttar Pradesh, India. J Geol Soc India 92:76–82. https://doi.org/10.1007/s12594-018-0955-1
Cooray P (1994) The Precambrian of Sri Lanka: a historical review. Precambrian Res 66:3–18
de Alwis A, Panawala P (2019) A review of the national response to CKDu in Sri Lanka Sri Lanka. J Soc Sci 42:83–100. https://doi.org/10.4038/sljss.v42i2.7966
Dharmawardana M, Amarasiri SL, Dharmawardene N, Panabokke C (2015) Chronic kidney disease of unknown aetiology and ground-water ionicity: study based on Sri Lanka. Environ Geochem Health 37:221–231. https://doi.org/10.1007/s10653-014-9641-4
Dissanayake CB (2005) Water quality in the dry zone of Sri Lanka—some interesting health aspect. J Natl Sci Found 33(3):161–168
Dissanayake C, Chandrajith R (2017) Groundwater fluoride as a geochemical marker in the etiology of chronic kidney disease of unknown origin in Sri Lanka. Ceylon J Sci. https://doi.org/10.4038/cjs.v46i2.7425
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170:1088–1090. https://doi.org/10.1126/science.170.3962.1088
Gunatilake S, Iwao Y (2009) Geoenvironmental assessment for nitrate pollution of surface and groundwater by fertilization (a study in Shiroishi Plain, Japan). Int J Civil Environ Eng 9:12–19
Gunatilake S, Iwao Y (2010) A comparison of nitrate distribution in shallow groundwater of two agricultural areas in Sri Lanka and in Japan. Sabaragamuwa Univ J 9:81–95. https://doi.org/10.4038/suslj.v9i1.3736
Gunatilake SK, Samaratunga SS, Rubasinghe RT (2014) Chronic kidney disease (CKD) in Sri Lanka-current research evidence justification: a review. Sabaragamuwa Univ J 13:31–58
Harshan S, Thushyanthy M, Gunatilake J, Srivaratharasan T, Gunaalan K (2016) Assessment of water quality index of groundwater quality in Chunnakam and Jaffna Town, Sri Lanka. Vingnanam J Sci 13:84. https://doi.org/10.4038/vingnanam.v13i1-2.4126
Horton RK (1965) An index number system for rating water quality. J Water Pollut Control Fed 37:300–306
Ileperuma O, Dharmagunawardhane H, Herath K (2009) Dissolution of aluminium from sub-standard utensils under high fluoride stress: a possible risk factor for chronic renal failure in the North-Central Province. J Natl Sci Found. https://doi.org/10.4038/jnsfsr.v37i3.1217
Jayasumana MACS, Paranagama PA, Amarasinghe MD, Wijewardane KMRC, Dahanayake KS, Fonseka SI, Rajakaruna KDLMP, Mahamithawa AMP, Samarasinghe UD, Senanayake VK (2013) Possible link of chronic arsenic toxicity with chronic kidney disease of unknown etiology in Sri Lanka. J Natl Sci Res 3(1):64–73
Karunaratne A, Pathmarajah S (2002) Groundwater development through introduction of agrowells and micro-irrigation in Sri Lanka Use of Groundwater for Agriculture in Sri Lanka. pp 29–41
Ketata M, Gueddari M, Bouhlila R (2012) Use of geographical information system and water quality index to assess groundwater quality in El Khairat deep aquifer (Enfidha, Central East Tunisia). Arab J Geosci 5:1379–1390. https://doi.org/10.1007/s12517-011-0292-9
Lkr A, Singh M, Puro N (2020) Assessment of water quality status of Doyang river, Nagaland, India, using water quality index. Appl Water Sci 10:1–13. https://doi.org/10.1007/s13201-019-1133-3
Logeshkumaran A, Magesh N, Godson PS, Chandrasekar N (2015) Hydro-geochemistry and application of water quality index (WQI) for groundwater quality assessment, Anna Nagar, part of Chennai City, Tamil Nadu, India. Appl Water Sci 5:335–343. https://doi.org/10.1007/s13201-014-0196-4
Malaviarachchi SP, Satish-Kumar M, Takahashi T (2021) New Sr-Nd isotope data record juvenile and ancient crust-mantle melt interactions in the Vijayan complex, Sri Lanka. J Geol 129:000–000. https://doi.org/10.1086/714172
Milovanovic M (2007) Water quality assessment and determination of pollution sources along the Axios/Vardar River, Southeastern Europe. Desalination 213:159–173. https://doi.org/10.1016/j.desal.2006.06.022
Narsimha A, Sudarshan V (2017) Contamination of fluoride in groundwater and its effect on human health: a case study in hard rock aquifers of Siddipet, Telangana State, India. Appl Water Sci 7:2501–2512. https://doi.org/10.1007/s13201-016-0441-0
Nelson D (2002) Natural variations in the composition of groundwater. Drinking Water Program, In Groundwater Foundation Annual Meeting, Oregon Department of Human Services, Springfield, Oregon
Panabokke C, Perera A (2005) Groundwater resources of Sri Lanka. Water Resources Board, Colombo, Sri Lanka, pp 1–28
Perera A, Gonawala J, Wijekoon D (2008) Groundwater quality in Anuradhapura Districy with special reference to fluoride Groundwater in Sri Lanka. National Academy of Science, Washington, pp 48–64
Pius A, Jerome C, Sharma N (2012) Evaluation of groundwater quality in and around Peenya industrial area of Bangalore, South India using GIS techniques. Environ Monit Assess 184:4067–4077. https://doi.org/10.1007/s10661-011-2244-y
Piyathilake IDUH, Sumudumali RGI, Udayakumara EPN, Ranaweera LV, Jayawardana JMCK, Gunatilake SK (2020) Modeling predictive assessment of soil erosion related hazards at the Uva Province in Sri Lanka. Model Earth Syst Environ. https://doi.org/10.1007/s40808-020-00944-1
Poonam T, Tanushree B, Sukalyan C (2013) Water quality indices—important tools for water quality assessment: a review. Int J Adv Chem 1:15–28
Rajasooriyar LD, Boelee E, Prado MC, Hiscock KM (2013) Mapping the potential human health implications of groundwater pollution in southern Sri Lanka. Water Resour Rural Dev 1:27–42. https://doi.org/10.1016/j.wrr.2013.10.002
Ramakrishnaiah C, Sadashivaiah C, Ranganna G (2009) Assessment of water quality index for the groundwater in Tumkur Taluk, Karnataka State, India. J Chem 6:523–530. https://doi.org/10.1155/2009/757424
Ramesh K, Elango L (2006) Groundwater quality assessment in Tondiar basin. Indian J Environ Prot 26:497
Rao NR, Rao N, Rao KSP, Schuiling R (1993) Fluorine distribution in waters of Nalgonda district, Andhra Pradesh, India. Environ Geol 21:84–89. https://doi.org/10.1007/s002540050170
Ravikumar P, Somashekar R, Angami M (2011) Hydrochemistry and evaluation of groundwater suitability for irrigation and drinking purposes in the Markandeya River basin, Belgaum District, Karnataka State, India. Environ Monit Assess 173:459–487. https://doi.org/10.1007/s10661-010-1399-2
RDA (2017) Initial environmental examination of second integrated road investment program. Road Development Authority, Ministry of Higher Education and Highways for the Government of Sri Lanka and the Asian Development Bank, Sri Lanka
Rubasinghe R, Gunatilake S, Chandrajith R (2015) Geochemical characteristics of groundwater in different climatic zones of Sri Lanka. Environ Earth Sci 74:3067–3076. https://doi.org/10.1007/s12665-015-4339-1
Sadat-Noori S, Ebrahimi K, Liaghat A (2014) Groundwater quality assessment using the Water Quality Index and GIS in Saveh-Nobaran aquifer. Iran Environ Earth Sci 71:3827–3843. https://doi.org/10.1007/s12665-013-2770-8
Salehi S, Chizari M, Sadighi H, Bijani M (2018) Assessment of agricultural groundwater users in Iran: a cultural environmental bias. Hydrogeol J 26:285–295. https://doi.org/10.1007/s10040-017-1634-9
Saxena V, Ahmed S (2003) Inferring the chemical parameters for the dissolution of fluoride in groundwater. Environ Geol 43:731–736. https://doi.org/10.1007/s00254-002-0672-2
Shah T (2007) The groundwater economy of South Asia: an assessment of size, significance and socio-ecological impacts. The agricultural groundwater revolution: opportunities and threats to development. CABI, Wallingford, pp 7–36. https://doi.org/10.1079/9781845931728.0007
Sharma D, Kansal A (2011) Water quality analysis of River Yamuna using water quality index in the national capital territory, India (2000–2009). Appl Water Sci 1:147–157
Srinivasamoorthy K, Vasanthavigar M, Chidambaram S, Anandhan P, Manivannan R, Rajivgandhi R (2012) Hydrochemistry of groundwater from Sarabanga minor basin, Tamilnadu, India. Proc Int Acad Ecol Environ Sci 2:193
Subramani T, Elango L, Damodarasamy S (2005) Groundwater quality and its suitability for drinking and agricultural use in Chithar River Basin, Tamil Nadu, India. Environ Geol 47:1099–1110
Udeshani W, Dissanayake H, Gunatilake S, Chandrajith R (2020) Assessment of groundwater quality using water quality index (WQI): a case study of a hard rock terrain in Sri Lanka. Groundw Sustain Dev 11:100421. https://doi.org/10.1016/j.gsd.2020.100421
UPC (2019) Five year vision oriented integrated sustainable development plan of Uva Province, Sri Lanka.
Villholth KG, Rajasooriyar LD (2010) Groundwater resources and management challenges in Sri Lanka–an overview. Water Resour Manag 24:1489–1513. https://doi.org/10.1007/s11269-009-9510-6
Walsh P, Wheeler W (2012) Water quality index aggregation and cost benefit analysis. J Benefit Cost Anal. https://doi.org/10.1515/jbca-2012-0005
Wanasinghe W, Gunarathna M, Herath H, Jayasinghe G (2018) Drinking water quality on chronic kidney disease of unknown aetiology (CKDu) in Ulagalla Cascade, Sri Lanka. Sabaragamuwa Univ J 16:17–27. https://doi.org/10.4038/suslj.v16i1.7714
Wanigasuriya KP, Peiris-John RJ, Wickremasinghe R (2011) Chronic kidney disease of unknown aetiology in Sri Lanka: is cadmium a likely cause? BMC Nephrol 12:32. https://doi.org/10.1186/1471-2369-12-32
WHO (2004) Guidelines for drinking water quality: training pack. World Health Organization, Geneva
Wilson M, Deer W, Howie R, Zussman J (2013) Rock-forming minerals, volume 3C, sheet silicates: clay minerals. Geological Society, London
Young SM, Pitawala A, Ishiga H (2011) Factors controlling fluoride contents of groundwater in north-central and northwestern Sri Lanka. Environ Earth Sci 63:1333–1342. https://doi.org/10.1007/s12665-010-0804-z
Acknowledgements
The authors would like to acknowledge the Accelerating Higher Education Expansion and Development (AHEAD) World Bank funded Sri Lankan government project (AHEAD/DOR/STEM/33) for providing the financial support for the research. Furthermore, the authors would like to thank Sabaragamuwa University of Sri Lanka for providing advanced laboratory facilities to carry out this research.
Funding
This study was funded by the Accelerating Higher Education Expansion and Development (AHEAD) World Bank funded Sri Lankan government project (AHEAD/DOR/STEM/ Grant number 33).
Author information
Authors and Affiliations
Contributions
Study conception and design and Critical revision: Prof. S.K. Gunatilake, Dr. L.V. Ranaweera and Dr. E.P.N. Udayakumara. Manuscript editing and modification after revising: Prof. C.B.Dissanayake. Acquisition of data, Analysis and interpretation of data and Drafting of manuscript: I.D.U.H. Piyathilake.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Piyathilake, I.D.U.H., Ranaweera, L.V., Udayakumara, E.P.N. et al. Assessing groundwater quality using the Water Quality Index (WQI) and GIS in the Uva Province, Sri Lanka. Appl Water Sci 12, 72 (2022). https://doi.org/10.1007/s13201-022-01600-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-022-01600-y