Abstract
Adsorption of copper using groundnut seed cake power, sesame seed cake powder and coconut cake powders as bioadsorbents was optimized at a pH of 5, temperature of 40 °C, initial metal concentration of 10 mg/L, contact time of 30 min and adsorbent dosage 0.75 g for groundnut seed cake powder and 1.0 g of sesame seed cake powder and coconut cake powder. From the results of kinetic studies, it was concluded that the adsorption process followed pseudo-second-order kinetics. Langmuir adsorption isotherm fit perfect for the adsorption of Cu(II) using the three adsorbents. Maximum adsorption capacity was found to be 4.24 mg/g. Artificial neural network modeling which was adopted for the predication of adsorption of Cu(II) using groundnut seed cake powder, sesame seed cake powder and coconut cake powder was carried out by using back-propagation algorithm. Correlation plot drawn for the experimental and ANN predicted values showed a strong correlation coefficient of 0.989, indicating that the network trained fit apt for the prediction of adsorption process.
Similar content being viewed by others
Introduction
Contamination of water by toxic heavy metals through the discharge of industrial wastewater is a global environmental concern (Rafatullah et al. 2012). Numerous metals such as Sb, Cr, Cd, Cu, Pb, Hg, etc., have toxic effects on mankind and environment (Taty-Costodess et al. 2003). Copper is one among the mentioned toxic heavy metals essential to human life and health. In minute quantities, the metal is essential in maintaining the health of an individual, whereas excess of the same is carcinogenic. Prolonged exposure to copper causes serious illness to human (Yu et al. 2000). The permissible limit of Cu in water is 2.5 mg/L (Prasad and Freitas 2000). Pulp and paper mills, fertilizers, petroleum refineries, basic steel work foundries, nonferrous metal works, motor vehicles, aircraft plating and finishing are the major contributors of copper into the environment. (Kadievelu et al. 2001; Chuah et al. 2005). Hence, the treatment of contaminated water is the need of the hour.
Chemical precipitation, membrane separations, ion exchange, solvent extraction, electrodialysis and reverse osmosis are the existing methods for the treatment of industrial waste water. Precipitation is the most widely used method for the removal of copper as its hydroxide or sulfide. However, major problem with precipitation is the disposal of precipitated cupric hydroxide (Aderonke et al. 2014). These existing methods that are generally expensive (Ferraz et al. 2015) lead to incomplete metal removal, high energy consumption and generation of toxic sludge. Use of low-cost adsorbents to remove toxic heavy metals was reported in recent years. These low-cost adsorbents include agricultural waste (Shobana et al. 2015), domestic waste (Jeyagowri and Yamuna 2015), industrial waste (Wang and Qin 2005), forest trees (Maheswari and Suresh 2015) and many more sources. These were found to be effective, efficient, abundant and cost-effective. The cost of these biomaterials is negligible compared with the cost of activated carbon and ion exchange resins that are in the range of approximately 2.0 US dollar to 4.0 US dollar/kg (Mrudula et al. 2016).
In recent years, there is growing interest in using low-cost commercially available materials for the adsorption of heavy metals. A wide variety of adsorbents such as rice husk (Rafatullah et al. 2012), modified cellulosic material (Okieimen et al. 1985), fly ash (Lita et al. 2017), wheat brean (Bulut and Tez 2006), modified bark (Jyothi et al. 2016), holly oak (Prasad and Freitas 2000), modified sawdust of walnut (Renu and Kailash 2016), green horse-chestnut shell (Parus 2017), oxidized multi-walled carbon nanotubes (Nyairo et al. 2018), animal manure-derived bio char (Idrees et al. 2018), pumpkin husk (Abuzer et al. 2017), eggplant peel (Darvanjooghi et al. 2018) and tea industry waste (Cay et al. 2004) are being used as low-cost alternatives to expensive adsorbents. Lignin content and other functional groups present on the surface of these adsorbents are responsible for the uptake of metals and nonmetals from aqueous solution.
The aim of this paper is to access the ability of low-cost adsorbents such as coconut seed cake powder, sesame seed cake powder, ground nut seed cake powder to adsorb Cu(II) from aqueous solution. The effect of the solution pH, temperature, contact time, initial adsorbate concentrations and adsorbent dosages on the removal of Cu(II) was studied. The kinetics and factors controlling the adsorption were also studied.
Materials and methods
All the chemicals used were of analytical grade. Double-distilled water was used throughout the experiment. XRD analysis was carried out by using Bruker advanced D8 PXRD instrument. FTIR analysis was carried out by ATSON II FTIR instrument. Perkin Elmer high-sensitivity AAS was used to determine the concentration of metal ions.
Preparation of adsorbents
Groundnut seed cake, sesame seed cake and coconut seed cake were obtained from the local market. These are the substances remained after the extraction of oil from the respective seeds. This raw material was found to be a hard mass. Hence, the substance was first crushed and then ground into a fine powder using laboratory mill. The resulting powder was sieved to get a powder of homogenous particle size. The material was tested for the absence of any residual oil. Such powder samples were washed thoroughly with distilled water and dried in an oven at 100 °C. Such samples were stored for further experimentation.
Characterization of adsorbents
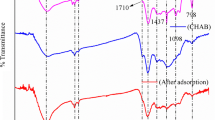
The surface area of groundnut seed cake, sesame seed cake and coconut seed cake was found to be 470, 456 and 480 m2/g using BET (Brunauer–Emmett–Teller) surface analysis. This value is higher on comparison with other carbons. The adsorption capacity of carbon is influenced by the chemical structure of its surface, which are of carbon–oxygen functional groups. Most often, the functional groups responsible for surface activity are carboxyl groups, phenolic groups, hydroxyl groups, carbonyl groups and lactones groups (Abdel Salam et al. 2011). The bulk chemical composition of the same was studied using XRD. X-ray diffraction studies were carried out for the adsorbents prior and after adsorption (Fig. 1a, b). FTIR spectrum of the adsorbents confirms the same (Fig. 2a, b).
Preparation of Cu(II) solution
Stock solution of copper sulfate was prepared by dissolving an adequate amount of the substance in distilled water to result solution of concentration 100 mg/L and standardized (Jeffery et al. 1988). Various concentrated solutions were obtained by diluting the stock solution.
Methodology
Batch adsorption experiments were carried out by agitating a series of bottles containing various amounts of the different adsorbents with the adsorbate (heavy metal ion) at optimum pH. The adsorbents were mixed with 50 ml of stock solution of the metal sulfate. The pH of the solution is adjusted by using 0.1 N HCl and 0.1 N NaOH until the pH was stabilized. Then, the resulting solution was agitated at room temperature for half an hour until equilibrium was attained. After the pre-determined time, the adsorbent particles were separated from the suspension through Whatmann No.1 filter paper. The residual concentration of heavy metals was determined by AAS(atomic absorption spectrophotometry).
Results and discussion
Effect of pH
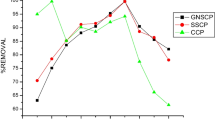
Uptake of heavy metals by adsorbents is significantly influenced by pH of the solution. Since, it determines the surface charge of the adsorbent and degree of ionization and speciation of adsorbate. The results obtained are shown in Fig. 3a and show the effect of pH on the removal of Cu+2 from aqueous solution on to the three adsorbents. As the pH of the solution increased, a significant increase in the equilibrium capacity of copper removal by the different adsorbents was observed from the results. At a higher initial pH (> 8), precipitation of copper ions was found. From the results, it was clear that the adsorption was effective at a pH value of 5 with the three adsorbents used; pH of 5 was fixed as optimum pH for the present study. These results were in agreement with those cited in literature (Pavan Kumar et al. 2018).
Effect of contact time
The effect of contact time on the removal efficiency of copper ions from the aqueous solution using different adsorbents was studied. The results are shown in Fig. 3b. The rate of uptake of metal ions was found to be quite rapid. A series of experiments were carried out to establish the equilibrium time for the effective removal of copper ions using the adsorbents. By fixing the adsorbent dosage, batch experiments were carried out at varying time intervals. From the results obtained, 99.7% of removal of copper ions was found at time of 30 min.
Effect of adsorbent dosage
A series of experiments were carried out to ascertain the adsorbent dose for the efficient removal of copper by the three different adsorbents. The experiments were conducted by taking 0.25, 0.5, 0.75, 1.0, 1.5 and 2.0 g of the adsorbents for batch studies. The results obtained are shown in Fig. 3c. From the results, it was found that removal of copper ions from aqueous solution was effective for 0.75 g of groundnut seed cake powder and 1.0 g of sesame seed cake and coconut seed cake powder. And the same was fixed as optimum adsorbent dosage.
Effect of initial metal ion concentration
A study was carried out to fix the initial metal ion concentration by varying the metal ion concentration and by fixing adsorbent dosage, pH and contact time. A series of copper ion solutions of concentration 10, 20, 30, 40, 50, 60, 70, 80 and 90 mg/L were mixed in separate flasks with 0.75 g of groundnut seed cake powder and 1.0 g of sesame seed cake and coconut seed cake powder. This mixture was agitated for 30 min at a pH of 5. After the pre-determined time, concentration of Cu+2 in the filtrate was determined. The results obtained are shown in Fig. 3d. From the results, it was clear that the removal of copper ion from aqueous solution decreases as the metal ion concentration increases. It is due to the absence of adsorption sites in the adsorbent. The maximum removal of copper using the adsorbents was found at a metal concentration of 10 mg/L.
Effect of temperature
The effect of temperature on the removal efficiency of copper ions from the aqueous solution using different adsorbents was studied. The results are shown in Fig. 3e. A series of experiments were carried out to establish the equilibrium temperature for the effective removal of copper ions using the adsorbents. By fixing the adsorbent dosage, contact time, pH and initial metal concentration, batch experiments were carried out at varying temperatures. From the results obtained, 99.7% of removal of copper ions was found at a temperature of 40 °C.
Optimized conditions for the removal of copper ions from aqueous solutions are presented in Table 1.
Adsorption isotherms
Among the many adsorption isotherms available, Langmuir, Freundlich and Temkin adsorption isotherms were used in the present study.
Langmuir adsorption isotherm
By assuming that maximum adsorption corresponds to a saturated monolayer of adsorbate on the adsorbent surface, that the energy of adsorption is constant Langmuir adsorption isotherms were studied. It was also assumed that there is no transmigration of adsorbate in the plane of the surface.
The Langmuir adsorption isotherm is defined as Ce
And in linearized form, the same is
where qm and K are Langmuir constants related to the sorption capacity and sorption energy, respectively. Ce is the equilibrium concentration in mg/L, and Qe is the amount of adsorbate adsorbed per unit weight of adsorbent. The plot of Ce/Qe against Ce is shown in Fig. 4. The adsorption of copper on different adsorbents gives a straight line. It is clear that a linear fit of the isotherm enables the Langmuir adsorption isotherm model’s applicability.
Freundlich adsorption isotherm
The Freundlich isotherm is defined as
And in linearized form
where Ce is the equilibrium concentration in mg/L, qe is the amount of adsorbate adsorbed per unit weight of adsorbent in mg/g, “KF” is parameter related to the temperature, and “n” is a characteristic constant for the adsorption. The plots for log qe against log Ce are presented in Fig. 5. The Freundlich isotherm constants and correlation data are presented in table.
Temkin isotherm
Temkin isotherm model can be represented as
This equation can also be represented as
KT is the equilibrium binding constant (L/mg); b is the Temkin isotherm constant and is the constant related to heat of sorption (J/mol); R is the universal gas constant; and T is the absolute temperature. The adsorption isotherms for the removal of copper using three adsorbents are graphically represented in Fig. 6. Adsorption parameters for the various isotherms are tabulated in Table 2.
Kinetic models of the present study
Kinetic models help in understanding the mechanism of metal adsorption and evaluate the performance of various adsorbents for the removal of metals. Among the many kinetic models developed, mostly used are the Lagergren’s pseudo-first-order kinetics and pseudo-second-order model. The sorption kinetics of pseudo-first-order was
where qe is the amount of solute adsorbed at equilibrium per unit weight of adsorbent, q is the amount of solute adsorbed at any time, and k is adsorption constant.
The pseudo-second-order kinetic model is described by the following equation
where qt and qe are the sorption quantity at time t and equilibrium, respectively, k is the rate constant. Thus, a plot of t/qt vs t gives the pseudo-second-order adsorption. Pseudo-second-order rate constant was determined from the respective plots.
The results obtained are tabulated in Table 3. Graphical representation for the pseudo-first-order and pseudo-second-order kinetic models is presented in Fig. 7.
Adsorption modeling using ANN
Artificial neural networks (ANN) tool was adopted to predict the adsorption of Cu(II) ions in aqueous solutions. Back-propagation methodology with L–M algorithm was used for the present study. MATLAB software was used to compute ANN tool. Parameters such as pH, contact time, adsorbent dosage, temperature and type of adsorbent were given as inputs to the network. Percent removal of copper ions was given as output. A set of parameters data was used for simulation. The network was trained until less number of epochs was achieved. Thereafter, simulation of the network with the set of data was processed. The results obtained were compared with the experimental results. The schematic representation of the network trained is presented in Fig. 8a. Correlation plots between validation, testing and simulation with target are presented in Fig. 8b. From the results, it was found that an error of 0.4% was obtained between the experimental and ANN predicted values, and a comparative plot is presented in Fig. 8c. It was concluded that the chosen algorithm and methodology is apt for the prediction.
Conclusions
Artificial neural network modeling which was adopted for the predication of adsorption of Cu(II) using groundnut seed cake powder, sesame seed cake powder and coconut cake powder was carried out by using back-propagation algorithm. Adsorption of copper using the chosen bioadsorbents was optimized at a pH of 5, temperature of 40 °C, initial metal concentration of 10 mg/L, contact time of 30 min and adsorbent dosage 0.75 g for groundnut seed cake powder and 1.0 g of sesame seed cake powder and coconut cake powder. From the results of kinetic studies, it was concluded that the adsorption process followed pseudo-second-order kinetics. Langmuir adsorption isotherm fit perfect for the adsorption of Cu(II) using the three adsorbents. Maximum adsorption capacity was found to be 4.24 mg/g. Correlation plot drawn for the experimental and ANN predicted values showed a strong correlation coefficient of 0.989, indicating that the network trained fit apt for the prediction of adsorption process.
References
Abdel Salam OE, Reiad NA, Elshafei MM (2011) A study of the removal characteristics of heavy metals from waste water by low cost adsorbents. J Adv Res 2:297–303
Abuzer Ç, Bayram B, Hüseyin B (2017) Development of a new adsorbent from pumpkin husk by KOH-modification to remove copper ions. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-017-1160-2
Aderonke AO, Abimbola BA, Ifeanye EO, Omotayo SA, Oluwagbemiga SA, Oladotoun WM (2014) Adsorption of heavy metal ions onto chitosan grafted cocoa husk char. Afr J Pure Appl Chem 8(10):147–161
Bulut Y, Tez Z (2006) Removal of heavy metals by modified sawdust of walnut. Fresenius Environ Bull 12(12):1499–1504
Cay S, Uyanik A, Ozisik A (2004) Single and Binary component adsorption of copper (II) and cadmium (II) from aqueous solutions using tea industry waste. Sep Purif Technol 38(3):273–280
Chuah TG, Jumasiah IA, Katayon S, Choong SYT (2005) Rice husk as a potentially low-cost biosorbent for heavy metal and dye removal: an overview. Desalination 175:305–316
Darvanjooghi MHK, Davodi SM, Dursu AY, Ehsani MR, Imani K, Elham A (2018) Application of treated eggplant peel as a low cost adsorbent for water treatment toward elimination of Pb+2: kinetic modeling and isotherm study. Adsorpt Sci Technol 36:1112–1143. https://doi.org/10.1177/0263617417753784
Ferraz AI, Amorium C, Javares T, Teixeria JA (2015) Chromium (III) biosorption onto spent grains residual from brewing industry: equilibrium, kinetics and column studies. Int J Environ Sci Technol 12:1591–1602
Idrees M, Saima B, Tanzila K, Summera Y, Amna K, Sadaf R, Qjang Z, Jie K (2018) Animal Manure-derived biochars via fast pyrolysis for the removal of divalent copper from aqueous media. J Environ Manag 213:109–118
Jeffery GH, Bassett J, Mendham J, Denny RC (1988) Vogel’s quantitative inorganic analysis, 5th edn. ELBS, Berlin
Jeyagowri B, Yamuna RT (2015) Biosorption of methylene blue from aqueous solution by modified mesoporous simarouba glauca seed shell powder. Glob NEST J 17(4):701–715
Jyothi S, Kumar Sanjeev, Sudha S, Rajeev S, Rupinder S (2016) Removal of nickel from aqueous solutions using low cost adsorbents a review. Int J Sci Eng Appl Sci 2(7):48–73
Kadievelu K, Thamaraiselvi K, Namasivayam C (2001) Removal of heavy metals from industrial waste water by adsorption onto activated carbon prepared from an agriculture solid waste. Biores Technol 76:63–65
Lita D, Suprihanto N, Enri D (2017) Removal of copper (II) ions in aqueous solutions by sorption onto fly ash. J Eng Technol Sci 49(4):546–559
Maheswari U, Suresh G (2015) Removal of Cr(VI) from waste water using activated neem bark in a fixed bed column: interference of other ions and kinetic modeling studies. Desalin Water Treat 57:1–12. https://doi.org/10.1080/19443994.2015.1030709
Mrudula V, Vijaya T, Chnadra Mouli K, Naga Jyothi U, Aishwarya S, Reddy Vasudeva (2016) Novel method for removal of heavy metals by using low cost adsorbents. Indo Am J Pharm Res 6(5):5472–5480
Nyairo WN, Eker YR, Kowenje C, Akin I, Bingol H, Tor A, Ongeri DM (2018) Efficient adsorption of lead (II) and copper (II) from aqueous phase using oxidized multiwalled carbon nanotubes/polypyrrole composite. Sep Sci Technol 53:1498–1510. https://doi.org/10.1080/01496395.2018.1424203
Okieimen FE, Ogbeifn DE, Nwala GN, Kumsah GA (1985) Binding of cadmium, copper, and lead ions by modified cellulosic materials. Bull Environ Contam Toxicol 34:866–870
Parus A (2017) Copper(II) ions’ removal from aqueous solution using green horse-chestnut shell as a low-cost adsorbent. Chem Ecol 34:56–69. https://doi.org/10.1080/02757540.2017.1396452
Pavan Kumar GVSR, Srinivasa Rao K, Yadav Arunendra, Lakshman Kumar M, Partha Sarathi TVN (2018) Biosorption of copper (II) and manganese (II) from waste water using low cost bio adsorbents. J Indian Chem Soc 93:4
Prasad MNV, Freitas H (2000) Removal of toxic metals from solution by leaf, stem and root phytomass of Quercus ilex L. (holly oak). Environ Pollut 110:277–283
Rafatullah M, Sulaiman O, Hashim R, Ahmed A (2012) Removal of cadmium (II) from aqueous solutions using meranti wood. Wood Sci Technol 46:221–241
Renu Madhu A, Kailash S (2016) A survey of modified agriculture waste for heavy metal removal from waste water. Int J Eng Sci Res Technol 5(12):1014–1018
Shobana R, Arockia Sahayaraj P, Dharmalingam V, Souruba R, Angelin Prema A (2015) Isothermal, kinetic and thermodynamic study of removal of Cu(II) removal from aqueous solution using activated kigelia pinnata fruit carbon. Int J Nano Corros Sci Eng 2(5):429–446
Taty-Costodess VC, Fauduet H, Porte C, Delacroixs A (2003) Removal of Cd(II) and P(II) ions from aqueous solutions by adsorption onto sawdust of pinus sylvestris. J Hazard Mater 105(1–3):121–142
Wang XS, Qin Y (2005) Equilibrium sorption isotherms for Cu +2 on rice bran. Process Biochem 40:677–680
Yu B, Zhang Y, Shukla A, Shukla SS, Dorris KL (2000) The removal of heavy metal from aqueous solutions by saw dust adsorption-removal of copper. J Hazard Mater 80:33–42
Acknowledgements
The authors thank the management and principal of MVGR College of Engineering (A), Vizianagaram-535005, India, for the facilities provided and for their constant support and encouragement.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pavan Kumar, G.V.S.R., Malla, K., Yerra, B. et al. Removal of Cu(II) using three low-cost adsorbents and prediction of adsorption using artificial neural networks. Appl Water Sci 9, 44 (2019). https://doi.org/10.1007/s13201-019-0924-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-019-0924-x