Abstract
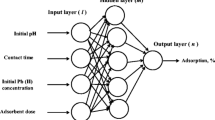
An artificial neural network (ANN) is used to model the static adsorption of copper, chromium, lead, and nickel by natural wastes, which are respectively charred cereal waste, Mediterranean biomass (Posidonia oceanica (L) DELILE), activated carbon as well as olive kernel and pulp. This intelligent model is used to predict and estimate the amount of adsorbed metal per mass unit of adsorbent or the yield percentage of the adsorption. The results obtained using multilayer neural network shows its effectiveness in predicting the experimental results. The relative error is 0.2 mg/g for charred cereal waste/Copper, Biomass/Chromium, and 1.9 % for the combinations activated carbon/Lead, olive kernel/Nickel, and olive pulp/Nickel, respectively. Furthermore, the same artificial neural network is exploited to predict the effect of some operating parameters (pH, temperature, initial metal concentration, contact time, agitation speed, ionic strength, and adsorbent weight) that affect the static adsorption of these metals by several types of adsorbents.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- AC:
-
Activated carbon
- ANN:
-
Artificial neural network
- b i :
-
Bias of the ith node
- BM:
-
Mediterranean biomass
- C in :
-
Initial concentration, mg/g
- Cu:
-
Copper
- I s :
-
Inonic strength, mol m3
- J :
-
Jacobian matrix of weights and biases derivate
- L :
-
ANN output dimension
- M :
-
Number of iterations
- Ni:
-
Nickel
- OK:
-
Olive kernel
- OP:
-
Olive pulp
- Pb:
-
Lead
- s :
-
Neuron inputs sum
- S a :
-
Agitation speed, round per minute (rpm)
- T :
-
Temperature, °C
- t c :
-
Contact time
- w ij :
-
Connection weight between node and node j
- WCC:
-
Waste charred cereal
- W d :
-
Adsorbent weight
- y i :
-
ith desired output
- z i :
-
ith neuron output
- μ :
-
Learning coefficient
- ε i :
-
Output error of the ith node
- i :
-
Node and output index
- j :
-
Node index
References
Mohand D, Pittman CU, Seele P (2007) Arsenic removal from water/wastewater using adsorbents—a critical review. J Hazard Mater 142(1–2):1–53
Nacim R, Tahir S (2001) Removal of Pb(II) from aqueous/acidic solutions by using bentonite as an adsorbent. Water Res 33(11):3982–3986
Boren A, Bowen DE, Santos FR (2003) Understanding biotechnology, 1st edn. Prentice Hall, Upper Saddle River
Ullmann (2002) Ullmann’s encyclopedia of industrial chemistry. Wiley, Weinheim
Vogel H (1997) Fermentation and biochemical engineering handbook principles, process design, and equipment, 2nd edn. Noyes, Westwood
Meinck M (1982) Les eaux résiduaires industrielles. Masson & Cie, Paris
Hendricks D (2010) Fundamentals of water treatment unit processes: physical, chemical, and biological. CRC Press, Boca Raton
Adebajo MO, Frost RL, Kloprogge JT, Carmody O, Kokot S (2003) Porous materials for oil spill cleanup: a review of synthesis and absorbing properties. J Porous Mater 1:159–170
Babel S, Kurniawan TA (2003) Low-cost adsorbents for heavy metals uptake from contaminated water: a review. J Hazard Mater B97:219–243
Bailey SE, Olin TJ, Bricka RM, Adrian DD (1999) A review of potentially low-cost sorbents for heavy metals. Water Res 33(11):2469–2479
Bal Y, Bal KE, Lallam A (2003) Removal of Bi (III) and Zn (II) by nonliving Streptomyces rimosus biomass from nitric solutions. Eur J Miner Process Environ Prot 3(1):42–48
Kong B, Tang B, Liu X, Zeng X, Duan H, Luo S, Wei W (2009) Kinetic and equilibrium studies for the adsorption process of cadmium (II) and copper (II) onto Pseudomonas aeruginosa using square wave anodic stripping voltammetry method. J Hazard Mater 167(1):455–460
Angelova N, Henkler D (1999) Rationalizing the design of polymeric biomaterials. Trends Biotechnol 17:409–420
Cardenas G, Orlando P, Edelio T (2001) Synthesis and applications of chitosan mercaptanes as heavy metal retention agent. Int J Biol Macromol 28:167–174
Chiron N, Guilet R, Deydier E (2003) Adsorption of Cu(II) and Pb(II) onto a grafted silica: isotherms and kinetic models. Water Res 37:3079–3086
Dupont L, Guillon E, Bouanda J, Dumonceau J, Laplincourt M (2002) EXAFS and XANES studies of retention of copper and lead by a lignocellulosic biomaterial. Environ Sci Technol 36:5062–5066
Dias MA, Castro HF, Pimentel F, Gomes NCM, Rosa CA, Linardi VR (2000) Removal of heavy metals from stainless steel effluents by waste biomass from Brazilian alcoholic beverage production. World J Microbiol Biotechnol 16:107–108
Fenga D, Aldrich C (2004) Adsorption of heavy metals by biomaterials derived from the marine alga Ecklonia maxima. Hydrometallurgy 73:1–10
Dakiky M, Khamis M, Manassra A, Mereb M (2002) Selective adsorption of chromium (VI) in industrial wastewater using low-cost abundantly available adsorbents. Adv Environ Res 6:533–540
Kadirvelu K, Thamaraiselvi K, Namasivayam C (2001) Removal of heavy metals from industrial wastewaters by adsorption onto activated carbon prepared from an agricultural solid waste. Bioresour Technol 76:63–65
Dong A, Xie J, Wang W, Yu L, Liu Q, Yin Y (2010) A novel method for amino starch preparation and its adsorption for Cu(II) and Cr(VI). J Hazard Mater 181(1–3):448–454
Meneses M, Llobet J, Granero S, Schuhmacher M, Domingo J (1999) Monitoring metals in the vicinity of a municipal waste incinerator: temporal variation in soils and vegetation. Sci Total Environ 226(2–3):157–164
Al-Othman Z, Ali R, Mu N (2012) Hexavalent chromium removal from aqueous medium by activated carbon prepared from peanut shell: adsorption kinetics, equilibrium and thermodynamic studies. Chem Eng J 184:238–247
Low KS, Lee CK, Leo AC (1995) Removal of metals from electroplating wastes using banana pith. Bioresour Technol 51(2–3):227–231
Ho YS, McKay G (2003) Sorption of dyes and copper ions onto biosorbents. Process Biochem 38(7):1047–1061
Marshall WE, Wartelle LH, Boler DE, Johns M, Toles CA (1999) Toles enhanced metal adsorption by soybean hulls modified with citric acid. Bioresour Technol 69(3):263–268
Aydın H, Bulut Y, Yerlikaya Ç (2008) Removal of copper (II) from aqueous solution by adsorption onto low-cost adsorbents. J Environ Manage 87(1):37–45
Zaggout FR (2001) Removal of copper from water by decaying Tamarix gallica leaves. Asian J Chem 13(2):639–650
Qiang Y, Xinshu Z, Qiong W, Wei Q, Xuesong T, Zhenhong Y (2012) Hydrolysis of sweet sorghum bagasse and eucalyptus wood chips with liquid hot water. Bioresour Technol 116:220–225
Darsey JA (2014) Neural networks in chemical and physical systems. World Scientific, Singapore
Schalkoff RJ (1997) Artificial neural networks. McGraw-Hill, New York
Hassoun M (2003) Fundamentals of artificial neural networks. A Bradford Book, MIT Press, Cambridge
Jeon C (2011) Removal of copper ion using rice hulls. J Ind Eng Chem 17(3):517–520
Ncibi MC (2008) Étude de la biosorption du chrome (VI) par une biomasse méditerranéenne: Posidonia oceanica (L.) delile. J Water Sci 21(4):441–449
Boulkrah H (2008) Etude comparative de l’adsorption des ions de plomb sur différents adsorbants. Théses et mémoires Faculté des sciences Mémoires de Magistère : Chimie Option:Pollution Chimique et Environnement, Skikda
Fiola N, Villaescusaa I, Martínezb M, Mirallesb N, Pochc J, Serarolsc J (2006) Sorption of Pb(II), Ni(II), Cu(II) and Cd(II) from aqueous solution by olive stone waste. Sep Purif Technol 50(1):132–140
Gharaibeh S, Abu-el-sha’r WY, Al-Kofahi M (1998) Removal of selected heavy metals from aqueous solutions using processed solid residue of olive mill products. Water Res 32(2):498–502
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Rebouh, S., Bouhedda, M., Hanini, S., Djellal, A. (2015). Neural Modeling Adsorption of Copper, Chromium, Nickel, and Lead from Aqueous Solution by Natural Wastes. In: Dincer, I., Colpan, C., Kizilkan, O., Ezan, M. (eds) Progress in Clean Energy, Volume 1. Springer, Cham. https://doi.org/10.1007/978-3-319-16709-1_24
Download citation
DOI: https://doi.org/10.1007/978-3-319-16709-1_24
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-16708-4
Online ISBN: 978-3-319-16709-1
eBook Packages: EnergyEnergy (R0)