Abstract
In vitro and in vivo studies have reported the potential cardioprotective effects of fermented milks (FM). The aim of the present study was to evaluate the inhibitory activities of angiotensin converting enzyme (ACE), thrombin enzyme (TI) and micellar solubility of cholesterol of FM after 24 and 48 h of fermentation with Limosilactobacillus fermentum (J20, J23, J28 and J38), Lactiplantibacillus plantarum (J25) or Lactiplantibacillus pentosus (J34 and J37) exposed to simulated gastrointestinal digestion. Results showed that FM with J20 and J23 at 48 h of fermentation presented significantly (p < 0.05) higher degree of hydrolysis than other FM, and were not significantly different (p > 0.05) between them. Conversely, peptide relative abundance was significantly (p < 0.05) higher in FM with J20 than FM with J23. Moreover, IC50 (protein concentration necessary to inhibit enzyme activity by 50%) for ACE inhibition were 0.33 and 0.5 mg/mL for FM with J20 and J23, respectively. For TI inhibition, the IC50 were 0.3 and 0.24 mg/mL for FM with J20 and J23, respectively. Results exhibited 51 and 74% inhibition of micellar solubility cholesterol for FM with J20 and J23, respectively. Therefore, these results showed that not only peptide abundance, but also specific peptides might be responsible for these potential cardioprotective effects.
Similar content being viewed by others
Introduction
Cardiovascular diseases (CVD) are defined as a group of disorders of the heart and blood vessels that represent a serious public health problem worldwide, since they are the leading cause of death (WHO 2022). There are several risk factors associated with the development of CVD, including hypertension, hypercholesterolemia and thrombosis (Thomas and Lip 2017). Hypertension is a chronic degenerative disease in which blood pressure is persistently raised, with systolic blood pressure values above ≥ 130 mmHg and diastolic pressure ≥ 80 mmHg (Whelton et al. 2018). On the other hand, endothelial damage caused by hypertension and by the infiltration of low density lipoproteins and very low density lipoproteins due to hypercholesterolemia, promotes the development of an atherosclerotic plaque (Thomas and Lip 2017).
Thrombosis is commonly caused by platelet aggregation and/or abnormal blood clots in circulating blood. The development of these blood clots occurs when the coagulation cascade is activated; in this process the thrombin enzyme catalyzes the polymerization of fibrinogen to form fibrin. Moreover, thrombosis may further cause ischemia to the tissues and cells irrigated by the vessels. If the ischemia is prolonged, an irreversible cell injury occurs which may further affect other organs (Gaertner and Massberg 2016). Therefore, different pharmacological and non-pharmacological treatments have been developed to reduce CVD and their risk factors (Arnett et al. 2019).
In this regard, it has been reported that some functional foods contain bioactive components that may have a protective role against different metabolic diseases (Asgary et al. 2018). In particular, dairy products such as fermented milks (FM) and yogurts are a promising tool for the prevention and treatment of CVD (Companys et al. 2020; Bintsis and Papademas 2022). In this regard, regular consumption of yogurt (≥ 2 servings/week) along with a healthy diet was associated with a lower risk for developing CVD in hypertensive subjects (Buendia et al. 2018). Similarly, Gao et al. (2020) reported that a daily intake of 200 g per day of yogurt was associated with 5 and 8% reduction of all-cause and CVD mortalities, respectively. Also, Zhang et al. (2020) concluded that yogurt consumption significantly reduced the risk of CVD by 22%.
Furthermore, in vitro and in vivo studies have reported the potential antihypertensive, hypocholesterolemic and antithrombotic effect of FM, since they have the ability to inhibit the angiotensin-converting enzyme (ACE) (Beltrán-Barrientos et al. 2018), the thrombin enzyme and micellar solubility of cholesterol (Rendon-Rosales et al. 2019). Therefore, there is a growing interest in the search for new strains of LAB for the production of functional dairy foods with cardioprotective effects (Beltrán-Barrientos et al. 2016). Therefore, the evaluation of native LAB from artisanal dairy products may be promising due to their technological and functional capacities, which would allow them to compete with commercial bacteria (Beltrán-Barrientos et al. 2016).
In this regard, Heredia-Castro et al. (2015) isolated and characterized specific strains of the genus Limosilactobacillus spp. and Lactiplantibacillus spp. from artisanal Mexican Cocido cheese. Moreover, studies have also shown that FM with these strains may possess antimicrobial activity (Heredia-Castro et al. 2015), presented probiotic characteristics and possessed potential immunomodulatory (Santiago-López et al. 2018) and anti-inflammatory effects (Reyes-Díaz et al. 2018).
On the other hand, it is important to consider that bioactive peptides generated during milk fermentation, may be hydrolyzed by proteases during gastrointestinal digestion, and thus, their bioactivity may be affected (Toldrá et al. 2018). Therefore, when studying the role of food derived bioactive compounds, it is crucial to evaluate whether after gastrointestinal digestion process, bioaccessibility and bioavailability may affect bioactivity. Mainly, bioaccessibility is studied through in vitro gastrointestinal digestion studies, using gastrointestinal digestion simulations that partially but accurately mimic the complex physico-chemical and physiological conditions simultaneously (Amigo and Hernández-Ledesma 2020).
Therefore, the aim of this study was to evaluate the inhibitory activity of ACE, thrombin enzyme and micellar solubility of cholesterol in FM with specific Limosilactobacillus spp. and Lactiplantibacillus spp. strains, after being subjected to simulated gastrointestinal digestion (SGD).
Materials and methods
Substrates and chemicals
ACE (EC 3.4.15.1) from rabbit lung powder, human thrombin (EC: 3.4.21.5), cholesterol, α-amylase (EC: 3.2.1.1), lysozyme (EC: 3.2.1.17), pancreatin (EC: 232-468-9), pepsin (EC: 3.4.23.1), bile salts, bovine serum albumin (BSA), galactose, glucosamine, glucuronic acid, Hippuryl-L-histidyl-L-leucine, human fibrinogen, L-leucine, mucin, O-Phthaldialdehyde reagent (OPA), cholestyramine, linoleic acid, phosphatidylcholine, sodium taurocholate and trichloroacetic acid were purchased from Sigma-Aldrich Chemicals Co. (St. Louis, MO, USA). De Man, Rogosa and Sharpe Broth (MRS) BD were purchased from Difco™ (Le Pont de Claix, Francia). Cholesterol assay kit was purchased from RANDOX Laboratories (Crumlin, UK) and DC Lowry protein assay was purchased from Bio-Rad Laboratories (Hercules, CA, USA).
Bacterial strains and growth conditions
Bacterial strains were obtained from the culture collection of the Dairy Laboratory at Food Research and Development Center, A.C. (CIAD, A.C., Hermosillo, Sonora, México). Limosilactobacillus fermentum (J20, J23, J28 and J38), Lactiplantibacillus plantarum (J25), Lactiplantibacillus pentosus (J34 and J37) were previously isolated from artisanal Mexican Cocido cheese (Heredia-Castro et al. 2015). Bacterial strains were grown in MRS broth through three consecutive cultures (1% v/v inoculum) and incubated at 37 °C for 24, 18, and 12 h (Santiago-López et al. 2018).
Preparation of fermented milks
Nonfat dry milk was reconstituted (10% w/v) and subjected to a thermal treatment (110 °C, 10 min) (Beltrán-Barrientos et al. 2018); then, strains were subcultured (3% v/v) twice in milk and incubated for 24 h and 12 h at 37 °C to obtain fresh cultures for each strain (Santiago-López et al. 2018). Afterwards, pasteurized (80 °C, 30 min) nonfat reconstituted milk (10% w/v) was inoculated (3% v/v) with each of the fresh cultures and incubated at 37 °C for 24 and 48 h. The fermentation process was stopped by applying heat treatment (75 °C, 15 min), followed by cooling at 4 °C and stored at − 20 °C for further analysis. Unfermented milk as negative control was prepared as previously mentioned without LAB cultures.
Simulated gastrointestinal digestion model
FM were subjected to a simulated gastrointestinal digestion model as previously reported by Kopf-Bolanz et al. (2012), with some modifications. All the digestive fluids used were freshly prepared according to the concentrations previously described (Kopf-Bolanz et al. 2012), omitting the BSA and kept at physiological temperature (37 °C) prior to use. The digestion process consisted of three steps, simulating the mouth, the stomach and the small intestine. Water was used as a blank. First, 3 mL of samples (FM, unfermented milk or water) were mixed with 4 mL of artificial saliva solution incubated for 5 min (pH 6.8). Then, 8 mL of gastric juice was added adjusting the pH between 2 and 3 with HCl (1 M) and left to incubate for 2 h. Later, 8 mL of pancreatic juice and 4 mL of bile were simultaneously added to the mixtures adjusting the pH to 6.5-7 with NaOH (1 M) and incubated for 2 h. For the inhibition of micellar solubility of cholesterol assay, bile juices were omitted. All mixtures during the digestion process were incubated at 37 °C in a shaker water bath with 55 rpm (Rendon-Rosales et al. 2019). Once the simulated digestive process was completed, aliquots of 0.5 mL were taken to evaluate the degree of hydrolysis using the OPA method, as described below. Digested samples were centrifuged (4696 g, 4 °C, 45 min) and stored at − 20 °C for further analysis.
Determination of the degree of hydrolysis
The degree of hydrolysis of the fermentation and digestion process was evaluated by quantifying free-amino groups, with the OPA method (Church et al. 1983). An aliquot (0.5 mL) of the digested and undigested samples (from fermented or unfermented milks) was added with 1 mL of trichloroacetic acid (TCA, 0.75 N) and 0.1 mL of bidestillated water. The mixture was stirred for 1 min and rested for 10 min. Subsequently, the mixtures were centrifuged (10,000 xg, 4 °C, 45 min) (Thermo Scientific, Chelmsford, MA, USA) and the supernatant was collected and filtered with a 0.22 μm filter (Millex Millipore, Billerica, MA, USA). The reaction was performed in a 96 Well Black/Clear Bottom Plate, where 10 µL of the samples were mixed with 200 µL of the OPA reagent. The mixture was left to stand for 2 min at room temperature in complete darkness. Fluorescence was recorded using a spectrophotometer (SpectraMax M3, Molecular devices, Sunnyvale, CA, USA) at an excitation wavelength of 330 nm and emission of 436 nm. For the calculation of the degree of hydrolysis, a total hydrolysis sample of milk was prepared with HCl (6 M) (150 °C, 6 h) (Vázquez-Orttz et al. 1995). The hydrolysis percentage was calculated with the following equation:
Where h = hydrolysis of the sample; totalh = total hydrolysis.
Preparation of water-soluble fraction (WSF)
Digested FM, unfermented milk and water (blank), were centrifuged (4696 g, 4 °C, 45 min) and supernatants were collected. Subsequently, the crude extracts were fractionated using a stirred ultrafiltrator cell (Model 8050, Amicon, Bedford, MA, USA) with a molecular exclusion membrane (Ultracell 3 kDa, Millipore, Billerica, MA, USA). The WSF < 3 kDa were stored at − 20 °C until further analysis for inhibition of angiotensin converting enzyme and thrombin enzyme. For the inhibition of micellar solubility of cholesterol assay the WSF < 3 kDa were stored at − 80 °C and lyophilized with a freeze-dryer (Labconco, Kansas City, MO, USA). Protein content in the WSF was determined with the DC protein assay (Bio-Rad Laboratories Hercules, CA, USA) using bovine serum albumin as a standard protein (0–2 mg/mL).
Peptide profiles by reversed-phase HPLC
The peptide profiles of the < 3 kDa fraction of FM before and after digestion were analyzed by reversed-phase HPLC using a C18 Zorbax Eclipse AAA column (4.6 mm x 150 mm, 3.5 μm particle size, Agilent Technologies, Santa Clara, CA, USA) by injecting 20 µL of each sample. The conditions were a flow rate of 0.75 mL/min, mobile phase D was milli-Q water and trifluoroacetic acid (1000:0.4 v/v) and mobile phase B was acetonitrile and trifluoroacetic acid (1000:0.3 v/v). The peptides were eluted with a linear gradient of solvent B from 0.0 to 60% for 16 min, 60–95% for 17 min, 95–100% for 18 min and 100–0.0% for 18.5 min. Peptide profiles were detected at 214 nm (Reyes-Díaz et al. 2018).
ACE-Inhibitory activity assay
The ACE inhibitory activity was determined following the Cushman and Cheung method (1971) with some modifications. Hippuryl-L-histidyl-L-leucine (substrate) (5 mM) was dissolved in 0.1 M sodium borate buffer (pH 8.3) containing 0.3 M NaCl. Samples were diluted in the sodium borate buffer solution adjusting the protein concentration to 1 mg/mL, and ACE at 0.1 U/mL was used. The reaction was initiated by the addition of 100 µL of the substrate dissolved in the assay buffer, 20 µL of ACE and 40 µL of samples and incubated for 35 min at 37 °C. Reaction was stopped by the addition of 250 µL of 1 M HCl. The hippuric acid resulting from the reaction was extracted by incorporating 1 mL of ethyl acetate by vigorous agitation for 30 s and centrifuged (1500 g, 10 min, 4 °C). Subsequently, 750 µL of the organic phase was extracted and evaporated (75 °C, 15 min). The sample was resuspended in 1 mL of Milli-Q water and the absorbance at 228 nm was recorded in a Nanodrop 2000c spectrophotometer (Thermo Scientific, USA). Angiotensin converting enzyme-inhibition was calculated with the following equation:
Where: A = positive control (substrate + enzyme + Milli-Q water), B = test sample blank (substrate + Milli-Q water + sample), C = test sample (substrate + sample + enzyme), D = substrate blank (substrate + Milli-Q water).
Also, ACE inhibition was expressed as the IC50, which is the peptide content (mg/mL) necessary to inhibit ACE activity by 50%. ACEI activity from the water blank was subtracted from the ACE inhibition of unfermented and FM.
Thrombin inhibitory activity assay
The antithrombotic activity in vitro was determined using the turbidimetric method based on the polymerization of fibrin by thrombin enzyme reported by Rendón-Rosales et al. (2022), with some modifications. Samples were diluted in the buffer solution (50 mM TRIS-HCl, with 0.12 mM NaCl, pH 7.2) adjusting the protein concentration to 1 mg/mL. The microplate reader was adjusted to 37 °C. Then, 40 µL of sample or buffer solution and 140 µL of fibrinogen previously dissolved in the buffer (0.109% w/v) were added to the microplate wells. The microplate was stirred for 5 s and the mixture was incubated for 10 min; afterwards, the absorbance was recorded at 405 nm. To begin the fibrin polymerization reaction, 30 µL of thrombin (4.417 U/mL) was added to the mixture in each well. After 10 min of incubation, absorbances were recorded again. Thrombin inhibition was calculated with the following equation:
Where A = positive control (substrate + enzyme + buffer), B = negative control (substrate + buffer + buffer), C = test sample (substrate + enzyme + sample), and D = test sample blank (substrate + buffer + sample).
Thrombin inhibitory activity by samples was also expressed as the peptide content (mg/mL) necessary to inhibit thrombin activity by 50% (IC50). Thrombin enzyme inhibition activity from the water blank was subtracted from the thrombin enzyme inhibition of unfermented and FM.
Inhibition of micellar solubility of cholesterol
For the hypocholesterolemic activity, the inhibition of micellar solubility of cholesterol as previously reported by Kirana et al. (2005) and Rendon-Rosales et al. (2019) was determined. To prepare the artificial micelles, cholesterol 0.5 mM, phosphatidylcholine 2.4 mM and linoleic acid 1 mM were dissolved in methanol and dried under a stream of nitrogen. Then, they were suspended in a 15 mM sodium phosphate buffer (pH 7.4) with 132 mM NaCl and 6.6 mM sodium taurocholate salt, sonicated for 20 min using an ultrasonic bath system (Aquasonic 50D, VWR Ultrasonic cleaner, San Jose, CA, USA) and incubated for 2 h at 37 °C. Afterwards, 25 mg of samples or cholestyramine were added to 500 µL of micellar solution; the mixture was sonicated for 2 min and incubated for 2 h at 37 °C; subsequently, centrifuged (10,000 g, 10 min) and filtered with a 0.22 μm syringe filter. The remaining cholesterol content was quantified in a microplate well using a cholesterol assay from RANDOX Laboratories (Crumlin, UK). Inhibition of micellar solubility of cholesterol by cholestyramine was assigned a 100% value (Kahlon and Woodruff 2002) A patron cholesterol was used as a standard (0.04–2.61 mmol/L). The Inhibition of micellar solubility of cholesterol was calculated with the following equation:
Where C0 represents the cholesterol content of the micelles and CS represents the cholesterol content remaining in the micelles with the samples.
Statistical analysis
Each experiment was carried out in duplicate and all tests were run in triplicates. A completely randomized design was made and the normality of the data was verified. A one-way ANOVA was carried out and differences among means were compared with Tukey-Kramer test. A paired t-student test was performed to compare the differences between fermentation times (24 and 48 h). For all statistical analysis, a 95% confidence was used. The analysis was performed using NCSS 2022 (Statistical Software 2002, NCSS, LLC. Kaysville, UT, USA).
Results and discussion
Degree of hydrolysis
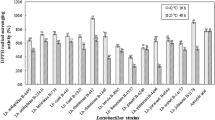
The degree of hydrolysis was determined in FM with specific Limosilactobacillus spp. and Lactiplantibacillus spp. strains (Fig. 1), and results showed a range of 0.40–0.54% for 24 h of fermentation and 0.47–1.14% for 48 h of fermentation. FM with J20, J23 and J25 at 24 h of fermentation presented a significantly higher (p < 0.05) degree of hydrolysis. Also, for the 48 h of fermentation, milk fermented with J20 and J23, followed by J25 and J28, presented a significantly higher degree of hydrolysis (p < 0.05). Furthermore, FM at 48 h of fermentation presented a significantly (p < 0.05) higher degree of hydrolysis than 24 h of fermentation. These results indicated differences between fermentation times and the ability for each bacterium to hydrolyze milk proteins, which were strain-dependent. It has been widely reported that during the milk fermentation, the proteolytic system of LAB hydrolyzes milk proteins, since during bacterial growth proteases are released into the extracellular medium, leading to the breakdown of larger to smaller peptides; and thus, releasing new potential bioactive peptides (Lim et al. 2019).
Degree of hydrolysis (%) of fermented milks (FM) by Limosilactobacillus spp. and Lactiplantibacillus spp. strains. Data are presented as means (n = 2) ± SD. Different letters indicate significant differences (p < 0.05) between FM for 24 h (lowercase) or 48 h (uppercase) of fermentation. *Represents differences (p < 0.05) between fermentation times for each FM.
On the other hand, the degree of hydrolysis of FM after simulated gastrointestinal digestion was within a range of 55–60%. In fact, these values were significantly higher (p < 0.05) than the digested unfermented milk, which presented a degree of hydrolysis of 50.34 ± 1.04%. These differences may be attributed to pre-hydrolysis caused by LAB during fermentation (Lim et al. 2019). In fact, during the digestion of FM, the bioavailability of the peptides may be modified, due to the action of gastrointestinal proteases such as pepsin, trypsin and chymotrypsin. As a result, peptide concentration is generally increased; nevertheless, the aforementioned may also affect the bioactivity of peptides (Toldrá et al. 2018). Thus, it is important to determine potential physiological effects after gastrointestinal digestion (Amigo and Hernández-Ledesma 2020).
Indeed, in vitro gastrointestinal digestion models have been used preferably over in vivo studies in order to overcome significant ethical restrictions, besides their high cost and long duration. Thus, several in vitro gastrointestinal digestion models have been developed in order to evaluate peptide digestibility and bioactivity. Since static models are inexpensive, easy to use and do not require special equipment, they are the most widespread used digestive systems (Amigo and Hernández-ledesma 2020). Thus, in this study, the in vitro static model of Kopf-Bolanz et al. (2012) validated for milk digestion was used in order to further evaluate potential fermented milk bioactivities.
Angiotensin converting enzyme inhibitory activity
ACE inhibitory activity of FM after being subjected to simulated gastrointestinal digestion conditions are depicted in Fig. 2. All FM showed ACE inhibiotry activity in a range of 41–83% and 35–90% for 24 and 48 h of fermentation; respectively. Moreover, ACE inhibitions were significantly higher (p < 0.05) for FM with J20, J28 and J38 after 48 h of fermentation than the rest of the FM. Interestingly, although it is widely reported that higher degree of hydrolysis results in more peptide release, it does not necessarily indicate higher bioactivity. In this regard, FM with J23 and FM with J28 presented significantly (p < 0.05) higher ACE inhibitory activity at 24 h of fermentation than at 48 h of fermentation. Thus, this may be due to the specific peptides released after fermentation and digestion. On the other hand, unfermented milk presented significantly (p < 0.05) lower ACE inhibition (21.62 ± 5.73%) than FM. Although unfermented milk presented a high degree of hydrolysis (50%) after gastrointestinal digestion, this result showed that the released peptides did not present the specific amino acid sequences needed to exhibit high ACE inhibition.
Angiotensin-converting enzyme inhibitory (ACEI) activity (%) of fermented milks (FM) by specific Limosilactobacillus spp. and Lactiplantibacillus spp. strains. Data are presented as means (n = 2) ± SD. Different letters indicate significant differences (p < 0.05) between FM for 24 h (lowercase) or 48 h (uppercase) of fermentation. *Represents differences (p < 0.05) between fermentation times for each FM.
The IC50 values for ACE inhibitory activity presented for FM (Table 1) were in a range of 0.27–0.95 mg/mL at 24 h of fermentation and 0.27–0.84 mg/mL at 48 h of fermentation. The lowest (p < 0.05) IC50 values were obtained for FM with J20, J23, J28 and J38 at 24 h of fermentation and J20, J28 and J38 at 48 h of fermentation. For FM with J38, the IC50 was inversely related to fermentation time. Contrary to this, FM with J23 presented an IC50 value significantly (p < 0.05) higher at 48 h of fermentation. Overall, from all analyzed FM only these four FM (J20, J23, J28 and J38) presented potential antihypertensive activity since they required the least peptidic nitrogen to inhibit ACE by 50%.
Also, it has been widely reported that the peptide amino acid sequence plays an important role in the ACE inhibitory activity. In this sense, peptides containing hydrophobic amino acids in the C-terminal such as proline, tyrosine and phenylalanine, and isoleucine and valine at the N-terminal have significant ACE inhibition (Shi et al. 2020). Indeed, several peptide sequences containing these amino acids were previously identified in FM with J28 (Reyes-Díaz et al. 2018). The structural and the chemical characteristics of food proteins and peptides released during food fermentation may influence the physiological properties of released peptides (Amigo and Hernández-ledesma 2020). Additionally, after digestion new released sequences containing these specific amino acids may be providing these ACE inhibitory activities. In this regard, different gastrointestinal enzymes may present specificity at the site of digestion that will determine the type of peptides released. In this sense, pepsin hydrolyzes peptide bonds next to aromatic amino acids, such as phenylalanine, tryptophan and tyrosine. Moreover, trypsin hydrolyzes next to the basic amino acids, such as arginine and lysine (Amigo and Hernández-ledesma 2020).
Several in vitro studies have reported differences on the physicochemical properties of ACE among species, mainly because of the substrate used and the source of the enzyme (Mansurah et al. 2013). Although it has been reported that there is a limitation on extrapolating in vitro models using ACE from animals rather than humans (Balcells et al. 1997), ACE isolated from rabbit’s lung has shown to be reliable and reproducible during ACE inhibition analysis (Mansurah et al. 2013); thus, it was selected for the present study.
Thrombin enzyme inhibition
In this study, the antithrombotic potential of fermented milks with specific Limosilactobacillus spp. and Lactiplantibacillus spp. strains were evaluated by thrombin-induced fibrin polymerization inhibition. FM with J20, J23, J28 and J38 at 24 and 48 h of fermentation showed thrombin enzyme inhibition (Fig. 3). In fact, FM with J28 and J38 at 24 and 48 h fermentation presented significantly higher (p < 0.05) thrombin enzyme inhibition and were not significantly (p > 0.05) different between fermentation times and strains. Moreover, for unfermented milk 9.59 ± 0.62% of inhibition was observed.
Thrombin enzyme inhibition (%) of fermented milks (FM) by specific Limosilactobacillus spp. and Lactiplantibacillus spp. strains. Data are presented as means (n = 2) ± SD. Different letters indicate significant differences (p < 0.05) between FM for 24 h (lowercase) or 48 h (uppercase) of fermentation. *Represents differences (p < 0.05) between fermentation times for each FM. ND: Not detected
Table 1 shows IC50 for thrombin enzyme inhibition of FM. Similarly, results showed that FM with J20, J23, J28 and J38 presented thrombin enzyme inhibitory activity, as well as ACE inhibitory activity. Interestingly, at 48 h of fermentation IC50 were not significantly (p > 0.05) different between FM with J20, J23, J28 and J38. The results obtained in the present study were similar to those reported by Rendon-Rosales et al. (2019), who evaluated the inhibition of thrombin-induced fibrin polymerization of fermented milks with specific strains of Lactococcus lactis spp. after being subjected to a simulated gastrointestinal digestion model. Authors reported IC50 values within a range of 0.045–0.91 mg/mL and 0.049–0.98 mg/mL at 24 and 48 h of fermentation; respectively. Peptides NAVPITPTLN, QEPVLGPVRGPFIIV, DVENLHLPLL and HIQKEDVPS obtained from fermented milks with Lactoccocus lactis NRRL B-50,572 have been reported to be efficient inhibitors of the thrombin enzyme. Specifically, sequences NA, PITPTL, QEPV, GPV, GPF, IIV, IQK, EDV, PS, DV, EN and PLP released after in silico digestion might be responsible for thrombin inhibition. Also, authors reported that negative charge and uncharged peptides with hydrophobic amino acids appeared to be associated with higher inhibition activity (Rendón-Rosales et al. 2022). Additionally, Ren et al. (2016) reported that peptides with negatively charged amino acids in the C-terminal and Lys in their sequence may possess high antithrombotic effect.
On the other hand, it has been reported that casein hydrolysates with trypsin have a thrombin enzyme inhibition of 45.47% at a concentration of 3.5 mg/mL (Tu et al. 2017). Therefore, in the present study fermented milks with Limosilactobacillus spp. and Lactiplantibacillus spp. were more efficient to inhibit thrombin enzyme, since IC50 values were lower than those with hydrolyzed casein with trypsin. Also, peptides from other several food sources have shown antithrombotic activity at different concentrations, such as egg white (90 mg/mL) (Yang et al. 2007), rapeseed (30–40 mg/mL) (Zhang et al. 2008), amaranth (80 µg/mL) (Sabbione et al. 2015), Whitmania pigra (0.1 mg/mL) (Ren et al. 2016), blue mussel (Mytilus edulis) (5 mg/mL) (Qiao et al. 2018) and Tenebrio molitor larvae (8 mg/mL) (Chen et al. 2019). Nevertheless, these studies did not consider determining antithrombotic activity after simulated gastrointestinal digestion; thus, their potential effect may be affected (Toldrá et al. 2018).
Inhibition of micellar solubility of cholesterol
Cholesterol is an important molecule within the body and a component of cell membranes and precursor of different compounds, such as steroid hormones, bile salts and vitamin D. However, the altered synthesis regulation, absorption and excretion of cholesterol leads to hypercholesterolemia. The micellar solubilization is an indispensable mechanism for cholesterol absorption throughout the intestinal mucosal barrier. Therefore, various hypocholesterolemic treatments focus on the inhibition of micellar solubility of cholesterol (Ko et al. 2020).
In the present study, the inhibition of micellar solubility of cholesterol for FM after being subjected to a simulated gastrointestinal digestion was evaluated (Fig. 4). Cholestyramine inhibition percentages from FM were in the range of 29–55% and 51–74% at 24 and 48 h of fermentation; respectively. Significant differences (p < 0.05) for inhibition of micellar solubility of cholesterol were observed between fermentation times for FM with J23, J25 and J34. In addition, the highest (p < 0.05) inhibition was obtained for FM with the strain J20 at 24 h of fermentation. As for the FM at 48 h of fermentation, J23, followed by J25 presented significantly higher (p < 0.05) inhibition activity.
Inhibition of micellar solubility of cholesterol (%) of fermented milks (FM) by specific Limosilactobacillus spp. and Lactiplantibacillus spp. strains. Data are presented as means (n = 2) ± SD. Different letters indicate significant differences (p < 0.05) between FM for 24 h (lowercase) or 48 h (uppercase) of fermentation. *Represents differences (p < 0.05) between fermentation times for each FM. ND: Not detected
Some food protein-derived peptides have various cholesterol-lowering mechanisms, including the disruption of cholesterol micelles in the gastrointestinal tract. In this sense, hypocholesterolemic peptides such as VLPVPQ, VAPFPE, TDVEN and LQPE obtained from milk casein hydrolyzed with neutrase presented hydrophobic or amphipathic properties (Jiang et al. 2020). Moreover, peptide IAEKK, has been reported with hypocholesterolemic effect (Nagaoka et al. 2001). Interestingly, a previous study reported the presence of AIAEKKA peptide in FM with J28 before simulated gastrointestinal digestion (Reyes-Díaz et al. 2018), which presents IAEKK in its sequence. Nevertheless, in this study, FM with J28 did not present inhibition of micellar solubility of cholesterol. Therefore, this fact also highlights the importance of determining in vitro peptide bioactivity after simulated gastrointestinal digestion.
On the other hand, other peptide food sources have also shown inhibition of micellar solubility of cholesterol, such as soybean (81.3%) (Zhong et al. 2007), freshwater clam meat (18.5%) (Lin et al. 2010, 2017), chickpea (50%) (Yust et al. 2012) and fermented sea bass byproduct (42.1%) (Chen et al. 2021); among others. Interestingly, Lin et al. (2010) reported that peptidic digestion significantly decreased (0.7 times) inhibition of micellar solubility of cholesterol and was attributed to the generation of new specific peptides. Additionally, Chen et al. (2021) observed a decrease of inhibition of micellar solubility of cholesterol from 88.4 to 42.1%. Hence, the importance of determining the stability of potential bioactive peptides after simulated gastrointestinal digestion.
Peptide profiles of fermented milks before and after simulated gastrointestinal digestion
Since fermented milks with Limosilactobacillus fermentum J20 and J23 at 48 h of fermentation were those that presented inhibition of ACE, thrombin and micellar solubility of cholesterol, we analyzed the chromatographic peptide profile of these fermented milks before and after SGD (Fig. 5). The peptide profile of FM with J20 at 48 h of fermentation showed a total area of 32,639.55 mAU. Moreover, after being subjected to SGD the total area significantly (p < 0.05) increased to 185,907.5 mAU. On the other hand, for FM with J23 at 48 h of fermentation, the peptide profile showed a total area of 22,092.2 mAU. Then, after being subjected to SGD, the total area significantly (p < 0.05) increased to 98,095.8 mAU. Interestingly, although the degree of hydrolysis was not significantly (p > 0.05) different between FM with J20 and FM with J23, results showed that FM with J20 presented more peptide abundance than FM with J23.
In general, it has been reported that more peptide abundance is related to a greater bioactivity (Peredo-Lovillo et al. 2022). In this sense, ACE inhibition for FM with J20 was significantly higher (p < 0.05) than that for FM with J23. Contrary to this, thrombin enzyme and micellar cholesterol inhibitions were significantly (p < 0.05) higher for FM with J23 than that for FM with J20. Thus, these results showed that not only peptide abundance but also specific peptides released might be responsible for these effects (Peredo-Lovillo et al. 2022).
In this regard, the peptide profile after digestion showed a greater abundance of peptides in both fermented milks. Several studies have reported that molecular interaction between ACE and inhibitory peptides are related to their amino acids sequence. ACE-inhibitory peptides generally have hydrophobic amino acids such proline, isoleucine and leucine; as well as positively charged amino acids such as lysine and arginine; and aromatic or cyclic amino acid residues such as tryptophan, tyrosine and proline at the C-terminal (Fan et al. 2019). These peptides interact in the catalytic site of the ACE in the presence of the cofactor Zn of the enzyme through hydrogen bonds and hydrophobic interactions (Pina and Roque 2009).
To date, only few studies have described the mechanism by which peptides may inhibit the thrombin enzyme. Studies with molecular docking suggest that these peptides may interact with the active site of the enzyme and at exosite I through hydrogen bonding, hydrophobic interactions and van der Waals forces (Tu et al. 2017; Chen and Huang 2020). Specifically, thrombin-inhibitory peptides have amino acids negatively charged in their sequence, these might interact with the positively charged thrombin residues at the active site or exosite of the enzyme (Ren et al. 2016; Feng et al. 2018; Chen and Huang 2020). Therefore, thrombin-inhibitory peptides require negatively charged amino acids, such as aspartic and glutamic acids, and also hydrophobic amino acids such as proline, valine and leucine in their sequence (Rendón-Rosales et al. 2022).
Hypocholesterolemic peptides have mainly hydrophobic or amphipathic properties which allows them to disrupt the formation of cholesterol micelles. These peptides due to their hydrophobic properties may compete with cholesterol by the incorporation in micelles and may also interact with bile salts making them unavailable for their solubilization (Howard and Udenigwe 2013). Also, studies have reported that some hydrophilic peptides with asparagine, glutamic acid, glutamine, aspartic acid and threonine in their sequence were able to bind with salts and disintegrate cholesterol micelles (Lapphanichayakool and Sutheerawattananonda 2017; Jiang et al. 2020).
Additionally, it is noteworthy to mention that recent reports have suggested that specific food groups may regulate the pathogenesis of infectious diseases, such as SARS-CoV-2. In this regard, negative correlations were observed between symptom severity of positive tested COVID-19 patients and the intake of milk and milk products (Salazar-Robles et al. 2021). In fact, in European countries where fermented food products with high ACE inhibitory activity were consumed, a reduced COVID-19 severity was reported (Avery 2021).
Conclusion
From all fermented milks evaluated in the present study, those with Limosilactobacillus J20 and J23 that were the most proteolytic strains, presented angiotensin converting enzyme, thrombin enzyme and micellar solubility of cholesterol inhibitory activities. Nevertheless, inhibitory activities were not necessarily related to peptide abundance, but rather may be associated with specific peptides. Overall, fermented milks with Limosilactobacillus J20 and J23 presented potential cardioprotective effects. However, in vivo studies are necessary in order to determine these beneficial effects.
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Abbreviations
- ACE:
-
Angiotensin-converting enzyme
- ANOVA:
-
One-way analysis of variance
- CVD:
-
Cardiovascular diseases
- FM:
-
Fermented milks
- IC50 :
-
Protein concentration necessary to inhibit enzyme activity by 50%
- LAB:
-
Lactic acid bacteria
- OPA:
-
O-Phthaldialdehyde
- SGD:
-
Simulated gastrointestinal digestion
- TI:
-
Thrombin enzyme inhibition
- WSF:
-
Water-soluble fraction
References
Amigo L, Hernández-Ledesma B (2020) Current evidence on the bioavailability of food bioactive peptides. Molecules 25:4479. https://doi.org/10.3390/molecules25194479
Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Muñoz D, Smith SC, Virani SS, Williams KA, Yeboah J, Ziaeian B (2019) 2019 ACC/AHA Guideline on the primary prevention of cardiovascular disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation 140:e596–e646. https://doi.org/10.1161/CIR.0000000000000678
Asgary S, Rastqar A, Keshvari M (2018) Functional food and cardiovascular disease prevention and treatment: a review. J Am Coll Nutr 37:429–455. https://doi.org/10.1080/07315724.2017.1410867
Avery A (2021) Can diet influence the COVID-19 mortality rate? Komp Nutr Diet 1:16–18. https://doi.org/10.1159/000512841
Balcells E, Meng QC, Johnson Jr WH, Oparil S, Dellitalia LJ (1997) Angiotensin II formation from ACE and chymase in human and animal hearts: methods and species considerations. Am J Physiol 273(4):H1769–H1774. https://doi.org/10.1152/ajpheart.1997.273.4.H1769
Beltrán-Barrientos LM, Hernández-Mendoza A, Torres-Llanez MJ, González-Córdova AF, Vallejo-Córdoba B (2016) Invited review: fermented milk as antihypertensive functional food. J Dairy Sci 99:4099–4110. https://doi.org/10.3168/jds.2015-10054
Beltrán-Barrientos LM, González-Córdova AF, Hernández-Mendoza A, Torres-Inguanzo EH, Astiazarán-García H, Esparza-Romero J, Vallejo-Cordoba B (2018) Randomized double-blind controlled clinical trial of the blood pressure–lowering effect of fermented milk with Lactococcus lactis: a pilot study. J Dairy Sci 101:2819–2825. https://doi.org/10.3168/jds.2017-13189
Bintsis T, Papademas P (2022) The evolution of fermented milks, from Artisanal to Industrial Products: a critical review. Fermentation 8:1–21. https://doi.org/10.3390/fermentation8120679
Buendia JR, Li Y, Hu FB, Cabral HJ, Bradlee ML, Quatromoni PA, Singer MR, Curhan GC, Moore LL (2018) Regular yogurt intake and risk of cardiovascular disease among hypertensive adults. Am J Hypertens 31:557–565. https://doi.org/10.1093/ajh/hpx220
Chen F, Huang G (2020) Mechanism and inhibition kinetics of peptide P13 as thrombin inhibitor. Int J Biol Macromol 150:1046–1052. https://doi.org/10.1016/j.ijbiomac.2019.10.109
Chen F, Jiang H, Lu Y, Chen W, Huang G (2019) Identification and in silico analysis of antithrombotic peptides from the enzymatic hydrolysates of Tenebrio molitor larvae. Eur Food Res Technol 245:2687–2695
Chen G-W, Lin H-TV, Huang L-W, Lin C-H, Lin Y-H (2021) Purification and identification of cholesterol micelle formation inhibitory peptides of hydrolysate from high hydrostatic pressure-assisted protease hydrolysis of fermented seabass byproduct. Int J Mol Sci 22(10):5295. https://doi.org/10.3390/ijms22105295
Church FC, Swaisgood HE, Porter DH, Catignani GL (1983) Spectrophotometric assay using o-Phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J Dairy Sci 66:1219–1227. https://doi.org/10.3168/jds.S0022-0302(83)81926-2
Companys J, Pla-Pagà L, Calderón-Pérez L, Llauradó E, Solà R, Pedret A, Valls RM (2020) Fermented dairy products, probiotic supplementation, and cardiometabolic diseases: a systematic review and meta-analysis. Adv nutr 11:834–863. https://doi.org/10.1093/advances/nmaa030
Cushman DW, Cheung HS (1971) Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem Pharmacol 20:1637–1648. https://doi.org/10.1016/0006-2952(71)90292-9
Fan H, Liao W, Wu J (2019) Molecular interactions, bioavailability, and cellular mechanisms of angiotensin-converting enzyme inhibitory peptides. J Food Biochem 43:1–8. https://doi.org/10.1111/jfbc.12572
Feng L, Tu M, Qiao M et al (2018) Thrombin inhibitory peptides derived from mytilus edulis proteins: identification, molecular docking and in silico prediction of toxicity. Eur Food Res Technol 244:207–217. https://doi.org/10.1007/s00217-017-2946-7
Gaertner F, Massberg S (2016) Blood coagulation in immunothrombosis: at the frontline of intravascular immunity. Semin Immunol 28:561–569. https://doi.org/10.1016/j.smim.2016.10.010
Gao X, Jia Hy, Chen GC, Li CY, Hao M (2020) Yogurt intake reduces all-cause and cardiovascular disease mortality: a meta-analysis of eight prospective cohort studies. Chin J Integr Med 26:462–468. https://doi.org/10.1007/s11655-020-3085-8
Heredia-Castro PY, Méndez-Romero JI, Hernández-Mendoza A, Acedo-Félix E, González-Córdova AF, Vallejo-Cordoba B (2015) Antimicrobial activity and partial characterization of bacteriocin-like inhibitory substances produced by Lactobacillus spp. isolated from artisanal mexican cheese. J Dairy Sci 98:8285–8293. https://doi.org/10.3168/jds.2015-10104
Howard A, Udenigwe CC (2013) Mechanisms and prospects of food protein hydrolysates and peptide-induced hypolipidaemia. Food Funct 4:40–51. https://doi.org/10.1039/c2fo30216k
Jiang X, Pan D, Zhang T, Liu C, Zhang J, Su M, Wu Z, Zeng X, Sun Y, Guo Y (2020) Novel milk casein: derived peptides decrease cholesterol micellar solubility and cholesterol intestinal absorption in Caco-2 cells. J Dairy Sci 103:3924–3936. https://doi.org/10.3168/jds.2019-17586
Kahlon TS, Woodruff CL (2002) In vitro binding of bile acids by soy protein, pinto beans, black beans and wheat gluten. Food Chem 79:425–429. https://doi.org/10.1016/S0308-8146(02)00192-9
Kirana C, Rogers PF, Bennett LE, Abeywardena MY, Patten GS (2005) Naturally derived micelles for rapid in vitro screening of potential cholesterol-lowering bioactives. J Agric Food Chem 53:4623–4627. https://doi.org/10.1021/jf050447x
Ko CW, Qu J, Black DD, Tso P (2020) Regulation of intestinal lipid metabolism: current concepts and relevance to disease. Nat Rev Gastroenterol Hepatol 17:169–183. https://doi.org/10.1038/s41575-019-0250-7
Kopf-Bolanz KA, Schwander F, Gijs M, Vergéres G, Portmann R, Egger L (2012) Validation of an in vitro digestive system for studying macronutrient decomposition in humans. J Nutr 142:245–250. https://doi.org/10.3945/jn.111.148635
Lapphanichayakool P, Sutheerawattananonda M (2017) Hypocholesterolemic effect of sericin-derived oligopeptides in high-cholesterol fed rats. J Nat Med 71:208–215. https://doi.org/10.1007/s11418-016-1050-9
Lim YH, Foo HL, Loh TC, Mohamad R, Abdullah N (2019) Comparative studies of versatile extracellular proteolytic activities of lactic acid bacteria and their potential for extracellular amino acid productions as feed supplements. J Anim Sci Biotechnol 10:1–13. https://doi.org/10.1186/s40104-019-0323-z
Lin YH, Tsai JS, Hung LB, Pan BS (2010) Hypochlesterolemic effect of compounded freshwater clam protein hydrolysate and Gracilaria. Food Chem 123:395–399
Lin YH, Tsai JS, Chen GW (2017) Purification and identification of hypocholesterolemic peptides from freshwater clam hydrolysate with in vitro gastrointestinal digestion. J Food Biochem 41:e12385
Mansura AA, Aimola IA, Annette RO, Abdullahi S (2013) Isolation, partial purification and characterization of Angiotensin converting enzyme (ACE) from rabbit (Oryctolagus ciniculus) lungs. Am J Drug Discov Dev 3(3):120–129. https://doi.org/10.3923/ajdd.2013.120.129
Nagaoka S, Futamura Y, Miwa K, Awano T, Yamauchi K, Kanamaru Y, Tadashi K, Kuwata T (2001) Identification of novel hypocholesterolemic peptides derived from bovine milkβ-lactoglobulin. Biochem Biophys Res Commun 281:11–17. https://doi.org/10.1006/bbrc.2001.4298
Peredo-Lovillo A, Hernández-Mendoza A, Vallejo-Cordoba B, Romero-Luna HE (2022) Conventional and in silico approaches to select promising food-derived bioactive peptides: a review. Food Chem 13:100183. https://doi.org/10.1016/J.FOCHX.2021.100183
Pina AS, Roque ACA (2009) Studies on the molecular recognition between bioactive peptides and angiotensin- converting enzyme. J Mol Recognit 2008:162–168. https://doi.org/10.1002/jmr.905
Qiao M, Tu M, Wang Z, Mao F, Chen H, Qin L, Du M (2018) Identification and antithrombotic activity of peptides from Blue Mussel (Mytilus edulis). Protein Int J Mol Sci 19:138. https://doi.org/10.3390/ijms19010138
Ren Y, Yang Y, Wu W, Zhang M, Wu H, Li X (2016) Identification and characterization of novel anticoagulant peptide with thrombolytic effect and nutrient oligopeptides with high branched chain amino acid from Whitmania pigra protein. Amino Acids 48:2657–2670. https://doi.org/10.1007/s00726-016-2299-8
Rendon-Rosales M, Torres-Llanez MJ, González-Córdova AF, Hernández-Mendoza A, Mazorra-Manzano MA, Vallejo-Cordoba B (2019) In vitro antithrombotic and hypocholesterolemic activities of milk fermented with specific strains of Lactococcus lactis. Nutrients 11:2150. https://doi.org/10.3390/nu11092150
Rendón-Rosales M, Torres-Llanez MJ, Mazorra-Manzano MA, González-Córdova AF, Hernández-Mendoza A, Vallejo-Cordoba B (2022) In vitro and in silico evaluation of multifunctional properties of bioactive synthetic peptides identified in milk fermented with Lactococcus lactis NRRL B-50571 and NRRL B-50572. LWT- Food Sci Technol 154:112581. https://doi.org/10.1016/J.LWT.2021.112581
Reyes-Díaz A, Mata-Haro V, Hernández J, González-Córdova AF, Hernández-Mendoza A, Reyes-Díaz R, Torres-Llanez MJ, Beltrán-Barrientos LM, Vallejo-Cordoba B (2018) Milk fermented by specific Lactobacillus strains regulates the serum levels of IL-6, TNF-α and IL-10 cytokines in a LPS-stimulated murine model. Nutrients 10:691. https://doi.org/10.3390/nu10060691
Sabbione AC, Scilingo A, Añón MC (2015) Potential antithrombotic activity detected in amaranth proteins and its hydrolysates. LWT - Food Sci Technol 60:171–177. https://doi.org/10.1016/j.lwt.2014.07.015
Salazar-Robles E, Kalantar-Zadeh K, Badillo H, Calderón-Juárez M, García-Bárcenas CA, Ledesma-Pérez PD, Lerma A, Lerma C (2021) Association between severity of COVID-19 symptoms and habitual food intake in adult outpatients. BMJ Nutr Prev Health 4:e000348. https://doi.org/10.1136/bmjnph-2021-000348
Santiago-López L, Hernández-Mendoza A, Mata-Haro V, Vallejo-Cordoba B, González-Córdova AF (2018) Immune response induced by fermented milk with potential probiotic strains isolated from artisanal cocido cheese. Food Agric Immunol 29:911–929. https://doi.org/10.1080/09540105.2018.1485632
Shi J, Su R-Q, Zhang W-T, Chen J (2020) Purification and the secondary structure of a novel angiotensin I-converting enzyme (ACE) inhibitory peptide from the alcalase hydrolysate of seahorse protein. J Food Sci Technol 57:3927–3934. https://doi.org/10.1007/s13197-020-04427-0
Thomas MR, Lip GYH (2017) Novel risk markers and risk assessments for cardiovascular disease. Circ Res 120:133–149. https://doi.org/10.1161/CIRCRESAHA.116.309955
Toldrá F, Reig M, Aristoy MC, Mora L (2018) Generation of bioactive peptides during food processing. Food Chem 267:395–404. https://doi.org/10.1016/j.foodchem.2017.06.119
Tu M, Feng L, Wang Z, Qiao M, Shahidi F, Lu W, Du M (2017) Sequence analysis and molecular docking of antithrombotic peptides from casein hydrolysate by trypsin digestion. J Funct Foods 32:313–323. https://doi.org/10.1016/j.jff.2017.03.015
Vázquez-Orttz FA, Caire G, Higuera-Ciapara I, Hernández G (1995) High performance Liquid Chromatographic determination of free amino acids in shrimp. J Liq Chromatogr Relat Technol 18:2059–2068. https://doi.org/10.1080/10826079508013960
Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Williamson JD, Wright JT 2017, ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH (2018) /ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol 71:e127–e248. https://doi.org/10.1016/j.jacc.2017.11.006
World Health Organization (WHO) (2022) Cardiovascular diseases. https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1. Accessed June 2, 2022
Yang WG, Wang Z, Xu SY (2007) A new method for determination of antithrombotic activity of egg white protein hydrolysate by microplate reader. Chin Chem Letter 18:449–451
Yust MDM, Millán-Linares MDC, Alcaide-Hidalgo JM, Millán F, Pedroche J (2012) Hypocholesterolaemic and antioxidant activities of chickpea (Cicer arietinum L.) protein hydrolysates. J Sci Food Agric 92:1994–2001
Zhang SB, Wang Z, Xu SY (2008) Antioxidant and antithrombotic activities of rapeseed peptides. J Am Oil Chem Soc 85:521–527
Zhang K, Chen X, Zhang L, Deng Z (2020) Fermented dairy foods intake and risk of cardiovascular diseases: a meta-analysis of cohort studies. Crit Rev Food Sci Nutr 60:1189–1194. https://doi.org/10.1080/10408398.2018.1564019
Zhong F, Zhang X, Ma J, Shoemaker CF (2007) Fractionation and identification of a novel hypocholesterolemic peptide derived from soy protein alcalase hydrolysates. Food Res Int 40:756–762
Acknowledgements
The authors express their gratitude to the Mexican Council of Science and Technology (CONACYT) for the graduate scholarship granted to author Miriam Zambrano-Cervantes.
Funding
This study was supported by the Mexican Council of Science and Technology (CONACYT; México City, México) research project 240338 and 2757 CONACYT.
Author information
Authors and Affiliations
Contributions
MZC Writing – original draft, Investigation; AFGC Supervision, Resources; AHM Validation, Visualization; LMBB Validation, Writing - review & editing. MÁRR Methodology, Supervision; CGMQ Methodology; MJTL Methodology; BVC Conceptualization, Writing – review & editing, Project administration.
Corresponding author
Ethics declarations
Conflict of interest
/Competing Interests The authors declare no conflict of interests. The authors declare that they have no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zambrano-Cervantes, M., González-Córdova, A.F., Hernández-Mendoza, A. et al. Fermented milks with specific Lactobacillus spp. with potential cardioprotective effects. J Food Sci Technol 60, 1749–1760 (2023). https://doi.org/10.1007/s13197-023-05715-1
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-023-05715-1