Abstract
Introduction
Soft tissue sarcomas (STSs) are rare malignancies. Pre-therapeutic tumour grading and assessment are crucial in making treatment decisions. Radiomics is a high-throughput method for analysing imaging data, providing quantitative information beyond expert assessment. This review highlights the role of radiomic texture analysis in STSs evaluation.
Materials and methods
We conducted a systematic review according to the Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A comprehensive search was conducted in PubMed/MEDLINE and Scopus using the search terms: ‘radiomics [All Fields] AND ("soft tissue sarcoma" [All Fields] OR "soft tissue sarcomas" [All Fields])’. Only original articles, referring to humans, were included.
Results
A preliminary search conducted on PubMed/MEDLINE and Scopus provided 74 and 93 studies respectively. Based on the previously described criteria, 49 papers were selected, with a publication range from July 2015 to June 2023. The main domains of interest were risk stratification, histological grading prediction, technical feasibility/reproductive aspects, treatment response.
Conclusions
With an increasing interest over the last years, the use of radiomics appears to have potential for assessing STSs from initial diagnosis to predicting treatment response. However, additional and extensive research is necessary to validate the effectiveness of radiomics parameters and to integrate them into a comprehensive decision support system.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
Soft tissue sarcomas (STSs) are rare malignancies that arise from mesenchymal cells [1]. They account for about 1% of all adult cancers and have a wide range of histological subtypes. Pre-therapeutic tumour grading and assessment are crucial in making treatment decisions, as they provide prognostic information and guide the choice of the proper approach (surgery, chemotherapy, and radiotherapy) [2].
In diagnosing suspected STSs, essential imaging techniques such as ultrasound and Magnetic Resonance Imaging (MRI) are fundamental, with MRI being vital for comprehensive evaluation. Thoracic Computed Tomography (CT), or PET-CT scans are instrumental in identifying metastatic sites, while precise imaging is key for biopsy guidance to accurately localise the lesion [3].
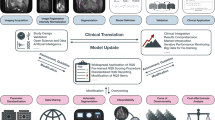
One of the most recenty introduced techniques in radiological science is Radiomics, a high-throughput approach for the analysis of imaging data; this method offers quantitative information that augments expert assessments [4, 5]. It involves the extraction of a large number of features from medical images that reflect the tumour characteristics such as shape, size, intensity, texture, and heterogeneity [6]. Radiomics has been applied to various types of cancers, including STSs, with promising results in terms of diagnosis, prognosis, and prediction [7, 8]. Figure 1 depicts an illustrative radiomics workflow applied to a left thigh myxoid fibrosarcoma, sourced from an open-source anonymized database (https://doi.org/10.7937/K9/TCIA.2015.7GO2GSKS) [9, 10].
This review aims to provide an overview of recent publications in the field of STS radiomics. It categorizes these studies into various domains of interest, highlighting the diverse applications and limitations of radiomic analysis in STS.
2 Materials and methods
We conducted a systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [11]. A comprehensive search was conducted in PubMed/MEDLINE and Scopus using the search terms: ‘radiomics [All Fields] AND ("soft tissue sarcoma" [All Fields] OR "soft tissue sarcomas" [All Fields])’. After removing duplicates, original published articles were included in the analysis.
All single, comparative studies, and primary studies that met the following PICO criteria were selected: P (patients): Patients with STSs; I (interventions): Radiomics; C (comparison): Conventional diagnostic imaging (including CT, MRI,PET/CT); O (outcome): The impact of radiomics on STSs on diagnosis, prognosis, risk stratification, genetic/histological prediction and technical feasibility aspects.
The following exclusion parameters were applied: (1) not original articles (e.g. letters, reviews, editorials, book chapters, congress communications); (2) papers not concerned radiomics topic; (3) researches not referred to humans (e.g. STSs in mice); (4) only articles in English, French, Spanish, Italian or German were included.
Two radiologists (RC, RDE) initially analysed all articles. An independent validation was performed by one other radiologist (MAB), by one resident in radiology (AM), and by one physicist (NC). The complete procedure, along with the results and any discussion regarding probable inconsistencies, was verified by one other independent radiologist (PS), expert in the field of musculo-skeletal oncological radiology.
The quality assessment of the eligible articles was evaluated using the Radiomics Quality Score (RQS) [12] by 2 evaluators (RC) and (SI). Each of the 16 essential criteria specified by the RQS was individually rated, yielding a composite score ranging from -8 to 36 points. These scores were subsequently transformed into RQS percentages, with a score of -8 to 0 points corresponding to 0% and a score of 36 points corresponding to 100% [12].
3 Results
A preliminary search conducted on PubMed/MEDLINE and Scopus provided 74 and 93 studies respectively. After removing duplicates and applying the aforementioned PICO criteria, a total of 94 papers were retained through evaluation of their titles and abstracts. Finally, after an extensive selection process (Fig. 2), 49 papers were eligible for analysis. In particular, 36 papers were excluded from consideration due to their non-original article status; 6 papers were omitted as they did not pertain to the subject of radiomics; 4 papers were excluded as they did not involve human subjects; 2 papers were disregarded based on their language of publication.
Among the retained papers, 46 studies were retrospective, and 3 studies were prospective, with a publication range from July 2015 to June 2023. The participant centres (Fig. 3) included China (n = 18; 36.7%), Italy (n = 6; 12.2%), France (n = 6; 12.2%), Germany (n = 5; 10.2%), USA (n = 4; 8.2%), Canada (n = 2; 4.1%), Netherlands (n = 2; 4.1%), Republic of Korea (n = 2; 4.1%), Spain (n = 1; 2%), United Kingdom (n = 1; 2%), Belgium (n = 1; 2%), Qatar (n = 1; 2%). MRI (n = 41; 77.4%), PET-CT (n = 5; 9.4%), CT (n = 5; 9.4%) or PET (n = 2; 3.8%) images were used for radiomics analysis (Fig. 4)—some of above-mentioned articles used more than one imaging techniques. The median number of patients involved in the analysis was 63 (range 11–540). The median number of radiomics features was 160 (range 30–2758).
Regarding the domain of interest (Fig. 5): 24 (36.4%) articles were focused on risk stratification, 19 (28.8%) articles on radiogenomics, 9 (13.6%) articles on technical feasibility/reproductive aspects, 9 (13.6%) articles on treatment response and 5 (7.6%) articles on diagnosis—some of aforementioned articles treated more than one domain of interest.
In total, 24 studies analysed the use of radiomics for risk stratification. 7 articles assessed the role of radiomics models for predicting lung metastasis [9, 13,14,15,16,17,18]; 3 articles analysed radiomics models for prediction of distant metastasis or metastatic relapse-free survival [19,20,21]. The ability to predict overall survival or free survival was evaluated in 6 studies [22,23,24,25,26,27]; in particular, according to Spraker et al. [26], texture features related to histogram_skewness, histogram_kurtosis, GLZSM_Small zone/low grey emphasis and GLZSM_Zone, obtained from T1-weighted contrast-enhanced images, were selected in the models for predicting overall survival. Fadli et al. [28] found that increase in heterogeneity (visually evaluated) and logarithmic change in radiomics features clusters, in contrast enhancement MRI T1-weighted images, were independent predictors for metastatic relapse-free and local relapse-free survival. One study developed a radiomics model for predicting disease-free survival in patients with STSs of the extremities and trunk who have undergone neoadjuvant radiotherapy [29]. Lee et al. [30] investigated the effectiveness of a radiomics model using T2-weighted Dixon sequence in differentiating the degree of STSs margin infiltration. Zhao et al. [31] evaluated the ability of various PET/MRI fusion methods to extract features for the prediction of recurrence/metastasis in patients with STSs. Tagliafico et al. [32] analysed MRI radiomics features in surveillance of local recurrence in patients with limb STSs. Liu et al. [33] evaluated the accuracy of two deep learning-radiomic nomogram models, in conjunction with clinical parameters, for predicting local recurrence in patients with STSs who underwent surgical resection. Lastly, one recent study presented a methodology employing MRI radiomic features for the prediction of metastasis and recurrence risk in patients with extremity STSs using formal logic models [34].
19 radiogenomics articles aimed to establish a connection among image phenotype, gene expression, mutations, molecular, or pathological findings. In particular, 11 studies have been conducted to develop and evaluate MRI-based radiomics for the differentiation of grade in STS tumours [20, 25, 32, 35,36,37,38,39,40,41,42,43]; furthermore, a study among these aforementioned ones aimed to predict the grade and the Ki-67 expression level by utilising intravoxel incoherent motion MRI and diffusion kurtosis imaging parameter maps [43]. Corino et al. [42] discovered that the GLCM features related to dissimilarity and entropy showed higher values in the high-grade. Peeken et al. [44] further explored the potential of quantitative imaging features in CT radiotherapy planning for predicting the grading. In children, using T2-weighted MRI images, Giraudo et al. [45] discovered that lmc1 feature was associated with high-grade tumours and variance feature was associated with rhabdomyosarcomas histotype. One study [27] assessed the predictive value of FDG PET/CT conventional metrics and textural features in determining histopathological data; in particular, the FNCLCC score (representing a histologic surrogate for tumour aggressiveness) demonstrated a correlation with GCLM_dissimilarity, GLCM_contrast and an inverse correlation with GLCM_homogeneity. Crombé et al. [46] investigated the association between distinct patterns of natural evolution of STSs, based on MRI radiomics features, and differential gene expression. Timbergen et al. [47] assessed whether radiomics can differentiate between desmoid-type fibromatosis and STSs and can predict CTNNB1 mutation types in desmoid-type fibromatosis patients. One study [28] examined the associations between temporal alterations observed in MRI, based on qualitative/semi-quantitative features and radiomics features, and the survival outcomes and histopathological characteristics. Foreman et al. [48] developed radiogenomic models with the purpose of predicting the MDM2 gene amplification status and distinguishing between atypical lipomatous tumours and lipomas based on preoperative MRI scans. Nine studies assessed the role of radiomics features for evaluating the toxicity, management and treatment responses of patients with STSs treated with radiotherapy and/or chemotherapy [23, 44, 49,50,51,52,53,54,55].
There were 9 studies conducted to assess the technical feasibility and reproducibility of radiomics analysis [9, 16, 17, 31, 35, 55,56,57,58]. The influence of ComBatHarmonization on MRI-based radiomics models to differentiate between low-grade and high-grade STS tumours was analysed by Peeken et al. [35]. Thrussell et al. [58] evaluated the repeatability of radiomic features from Diffusion Weighted Imaging (DWI) and Apparent Diffusion Coefficient (ADC) maps in retroperitoneal STSs and compared their repeatability before and after radiotherapy. Vallières et al. [9, 16] assessed the influence of different extraction parameters and acquisition protocols on FDG-PET/MRI models to predict the risk of lung metastasis. Zhao et al. [31] evaluated the ability of various PET/MRI fusion methods to extract features for the prediction of recurrence/metastasis. Sheen et al. [17] analysed the efficacy of four segmentation methods in defining radiomics signatures and prediction models for lung metastases using PET-CT in STSs.
In 5 studies, there were observed associations between imaging features and the ability to diagnose and differentiate STSs from normal tissue or non-malignant lesions. Yue et al. [59] developed a clinical-MRI radiomics nomogram aimed at distinguishing between benign and malignant soft-tissue tumours. Tagliafico et al. [32] investigated the potential use of radiomics in MRI surveillance in patients with limb STSs to differentiate between normal tissue and local recurrence. In another study, Timbergen et al. [47] evaluated MRI radiomics models for distinguishing between desmoid-type fibromatosis and STSs. As previously mentioned, Foreman et al. [48] attempted to develop radiogenomic models with the goal of distinguishing between atypical lipomatous tumours and lipomas by analysing the MDM2 gene amplification status using preoperative MRI scans. Aouadi et al. [60] examined several datasets, in particular the LIPO dataset [61] for distinguishing between well-differentiated liposarcoma and lipoma, and the Desmoid dataset [61] for differentiating desmoid-type fibromatosis from extremity STSs.
Table 1 presents a comprehensive overview of all the aforementioned analysed studies, highlighting the primary objectives, outcomes, and various domains of interest.
The 49 articles showed a median RQS of 12 and the values varied from 2 to 23 (Table 2). Additionally, the median RQS expressed as a percentage was 33%, with the minimum recorded value being 6%, while the highest was 64%.
4 Discussion
Imaging has a prominent role in diagnosis and in treatment decision making in patients with STS. When an STS is clinically suspected, the role of diagnostic imaging and multidisciplinary discussions are essential. Ultrasound is often the first-approach modality, but MRI is mandatory to characterize the lesion, assess its anatomical limits and therefore guide treatment decision [3]. Also, some STS features seen on MRI may predict the grade of malignancy of the lesions [62], recurrences [63, 64], post-treatment oedema and seroma [65]. Thoracic CT or PET-CT are used to assess secondary lesions. Imaging is also important to guide a biopsy, which may be necessary to confirm the exact histological nature of the lesion. Recently, the development of new imaging techniques, such as whole-body MRI, hybrid PET/MRI, diffusion weighted MRI, dynamic contrast MRI and advances in artificial intelligence have greatly enhanced the radiologist role in tumour grading and staging assessment.
However, the only use of imaging has still a very limited accuracy and therefore a histological confirmation is often needed. In order to improve imaging accuracy, several new imaging techniques were proposed in latest years. One of these techniques, radiomics, has shown promising results. It may be associated with the notion of radiogenomics, positing that imaging characteristics correlate with genetic signatures [6]. This method surpasses conventional imaging by being non-invasive, objective, and cost-effective. Importantly, radiomics could provide complementary information to histopathology and molecular biomarkers, enhancing tumour evaluation and aiding in personalised treatment strategies.
In recent advancements within oncologic imaging, radiomics has extended its utility to the field of STSs. Our review elucidates the versatile applications of radiomic analysis in STSs, showcasing interesting results across various critical domains. Notably, radiomics has demonstrated significant potential in aiding with diagnosis, facilitating risk stratification, predicting grading or genomic status, and evaluating treatment responses in STSs.
Our review underscores the potential of radiomics to complement and augment conventional imaging approaches. We have observed that radiomics, with its high-throughput data analysis capabilities, can provide deeper insights into the imaging characteristics of STSs. This integration of radiomic techniques with traditional imaging approaches marks a significant advancement in the field.
Albeit radiomics discoveries have shown promises, more work is needed to ensure these results can be replicated with a satisfactory degree of proof by enrolling larger patient datasets. The heterogeneity of features, the variability of acquisition protocols and multiple image modalities further complicate the generalizability of radiomics observations. Moreover, independent validation of proposed predictive models often is lacking, making extensive radiomics studies in STSs challenging. Additionally, the complexities inherent in radiomics may challenge its integration into routine clinical practice, suggesting a need for tailored training and resources to facilitate its use among clinicians and radiologists. An improved implementation of the latest guidelines [12, 56, 66,67,68] and increased knowledge of radiomics will be necessary to enhance the quality of sarcoma radiomics studies and to enable their implementation in the clinical setting.
5 Conclusion
Our review highlights radiomics as a potential adjunct to conventional STS methodologies, enhancing diagnostic and treatment strategies. Yet, the limitations of the current approach necessitate further studies. Additionally, the complexities inherent in radiomics may challenge its integration into routine clinical practice. Future investigations should focus on validating radiomics' clinical application and establishing its role in STS management practices.
Data availability
All data are available on PubMed/MEDLINE and Scopus.
Abbreviations
- STS:
-
Soft tissue sarcoma
- CT:
-
Computed tomography
- PET:
-
Positron Emission Tomography
- MRI:
-
Magnetic Resonance Imaging
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RQS:
-
Radiomics Quality Score
- DWI:
-
Diffusion Weighted Imaging
- ADC:
-
Apparent Diffusion Coefficient
- T2FS:
-
Fat-Suppressed T2-weighted
- ROC:
-
Receiver Operating Characteristic
- AUC:
-
Area Cnder the receiver operator characteristic curve
- LASSO:
-
Least Absolute Shrinkage and Selection Operator
- SVM:
-
Support Vector Machine
- RF:
-
Random Forest
References
Gutierrez JC, Perez EA, Franceschi D, Moffat FL, Livingstone AS, Koniaris LG. Outcomes for soft-tissue sarcoma in 8249 cases from a large state cancer registry. J Surg Res. 2007;141:105–14.
Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. AJCC cancer staging manual. Springer International Publishing; 2018.
Vibhakar AM, Cassels JA, Botchu R, Rennie WJ, Shah A. Imaging update on soft tissue sarcoma. J Clin Orthop Trauma. 2021;22: 101568.
Peeken JC, Nüsslin F, Combs SE. Radio-oncomics. Strahlenther Onkol. 2017;193:767–79.
Peeken JC, Bernhofer M, Wiestler B, Goldberg T, Cremers D, Rost B, et al. Radiomics in radiooncology—challenging the medical physicist. Phys Med Eur J Med Phys. 2018;48:27–36.
Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RGPM, Granton P, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012;48:441–6.
Fanciullo C, Gitto S, Carlicchi E, Albano D, Messina C, Sconfienza LM. Radiomics of musculoskeletal sarcomas: a narrative review. J Imaging Sci Technol. 2022. https://doi.org/10.3390/jimaging8020045.
Crombé A, Roulleau-Dugage M, Italiano A. The diagnosis, classification, and treatment of sarcoma in this era of artificial intelligence and immunotherapy. Cancer Commun. 2022;42:1288–313.
Vallières M, Freeman CR, Skamene SR, El Naqa I. A radiomics model from joint FDG-PET and MRI texture features for the prediction of lung metastases in soft-tissue sarcomas of the extremities. Phys Med Biol. 2015;60:5471–96.
Clark K, Vendt B, Smith K, Freymann J, Kirby J, Koppel P, et al. The Cancer Imaging Archive (TCIA): maintaining and operating a public information repository. J Digit Imaging. 2013;26:1045–57.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.
Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14:749–62.
Liang H-Y, Yang S-F, Zou H-M, Hou F, Duan L-S, Huang C-C, et al. Deep learning radiomics nomogram to predict lung metastasis in soft-tissue sarcoma: a multi-center study. Front Oncol. 2022;12: 897676.
Hu Y, Wang H, Yue Z, Wang X, Wang Y, Luo Y, et al. A contrast-enhanced MRI-based nomogram to identify lung metastasis in soft-tissue sarcoma: A multi-centre study. Med Phys. 2022. https://doi.org/10.1002/mp.16136.
Escobar T, Vauclin S, Orlhac F, Nioche C, Pineau P, Champion L, et al. Voxel-wise supervised analysis of tumors with multimodal engineered features to highlight interpretable biological patterns. Med Phys. 2022;49:3816–29.
Vallières M, Laberge S, Diamant A, El Naqa I. Enhancement of multimodality texture-based prediction models via optimization of PET and MR image acquisition protocols: a proof of concept. Phys Med Biol. 2017;62:8536–65.
Sheen H, Shin H-B, Kim JY. Comparison of radiomics prediction models for lung metastases according to four semiautomatic segmentation methods in soft-tissue sarcomas of the extremities. J Korean Phys Soc. 2022;80:247–56.
Deng J, Zeng W, Shi Y, Kong W, Guo S. Fusion of FDG-PET image and clinical features for prediction of lung metastasis in soft tissue sarcomas. Comput Math Methods Med. 2020;2020:8153295.
Tian L, Zhang D, Bao S, Nie P, Hao D, Liu Y, et al. Radiomics-based machine-learning method for prediction of distant metastasis from soft-tissue sarcomas. Clin Radiol. 2021;76(158):e19-158.e25.
Giraudo C, Fichera G, Del Fiore P, Mocellin S, Brunello A, Rastrelli M, et al. Tumor cellularity beyond the visible in soft tissue sarcomas: Results of an ADC-based, single center, and preliminary radiomics study. Front Oncol. 2022;12: 879553.
Crombé A, Fadli D, Buy X, Italiano A, Saut O, Kind M. High-grade soft-tissue sarcomas: can optimizing dynamic contrast-enhanced mri postprocessing improve prognostic radiomics models? J Magn Reson Imaging. 2020;52:282–97.
Yang Y, Ma X, Wang Y, Ding X. Prognosis prediction of extremity and trunk wall soft-tissue sarcomas treated with surgical resection with radiomic analysis based on random survival forest. Updates Surg. 2022;74:355–65.
González-Viguera J, Reynés-Llompart G, Lozano A. Outcomes and computed tomography radiomic features extraction in soft tissue sarcomas treated with neoadjuvant radiation therapy. Rep Pract Oncol Radiother. 2021;26:804–13.
Peeken JC, Neumann J, Asadpour R, Leonhardt Y, Moreira JR, Hippe DS, et al. Prognostic assessment in high-grade soft-tissue sarcoma patients: a comparison of semantic image analysis and radiomics. Cancers. 2021. https://doi.org/10.3390/cancers13081929.
Yang Y, Zhou Y, Zhou C, Zhang X, Ma X. MRI-based computer-aided diagnostic model to predict tumor grading and clinical outcomes in patients with soft tissue sarcoma. J Magn Reson Imaging. 2022;56:1733–45.
Spraker MB, Wootton LS, Hippe DS, Ball KC, Peeken JC, Macomber MW, et al. MRI radiomic features are independently associated with overall survival in soft tissue sarcoma. Adv Radiat Oncol. 2019;4:413–21.
Annovazzi A, Ferraresi V, Covello R, Ascione A, Vari S, Petrongari MG, et al. Prognostic value of pre-treatment [18F]FDG PET/CT texture analysis in undifferentiated soft-tissue sarcoma. J Clin Med Res. 2022. https://doi.org/10.3390/jcm12010279.
Fadli D, Kind M, Michot A, Le Loarer F, Crombé A. Natural changes in radiological and radiomics features on mris of soft-tissue sarcomas naïve of treatment: correlations with histology and patients’ outcomes. J Magn Reson Imaging. 2022;56:77–96.
Chen S, Li N, Tang Y, Chen B, Fang H, Qi S, et al. Radiomics analysis of fat-saturated T2-weighted MRI sequences for the prediction of prognosis in soft tissue sarcoma of the extremities and trunk treated with neoadjuvant radiotherapy. Front Oncol. 2021;11: 710649.
Lee S, Jung J-Y, Nam Y, Jung C-K, Lee S-Y, Lee J, et al. Diagnosis of marginal infiltration in soft tissue sarcoma by radiomics approach using T2-weighted dixon sequence. J Magn Reson Imaging. 2023;57:752–60.
Zhao W, Huang X, Wang G, Guo J. PET/MR fusion texture analysis for the clinical outcome prediction in soft-tissue sarcoma. Cancer Imaging. 2022;22:7.
Tagliafico AS, Bignotti B, Rossi F, Valdora F, Martinoli C. Local recurrence of soft tissue sarcoma: a radiomic analysis. Radiol Oncol. 2019;53:300–6.
Liu S, Sun W, Yang S, Duan L, Huang C, Xu J, et al. Deep learning radiomic nomogram to predict recurrence in soft tissue sarcoma: a multi-institutional study. Eur Radiol. 2022;32:793–805.
Casale R, Varriano G, Santone A, Messina C, Casale C, Gitto S, et al. Predicting risk of metastases and recurrence in soft-tissue sarcomas via Radiomics and Formal Methods. JAMIA Open. 2023;6:ooad025.
Peeken JC, Spraker MB, Knebel C, Dapper H, Pfeiffer D, Devecka M, et al. Tumor grading of soft tissue sarcomas using MRI-based radiomics. EBioMedicine. 2019;48:332–40.
Zhang Y, Zhu Y, Shi X, Tao J, Cui J, Dai Y, et al. Soft tissue sarcomas: preoperative predictive histopathological grading based on radiomics of MRI. Acad Radiol. 2019;26:1262–8.
Xu W, Hao D, Hou F, Zhang D, Wang H. Soft tissue sarcoma: preoperative mri-based radiomics and machine learning may be accurate predictors of histopathologic grade. AJR Am J Roentgenol. 2020;215:963–9.
Yang Y, Zhang L, Wang T, Jiang Z, Li Q, Wu Y, et al. MRI fat-saturated T2-weighted radiomics model for identifying the Ki-67 index of soft tissue sarcomas. J Magn Reson Imaging. 2022. https://doi.org/10.1002/jmri.28518.
Yan R, Hao D, Li J, Liu J, Hou F, Chen H, et al. Magnetic resonance imaging-based radiomics nomogram for prediction of the histopathological grade of soft tissue sarcomas: a two-center study. J Magn Reson Imaging. 2021;53:1683–96.
Wang H, Chen H, Duan S, Hao D, Liu J. Radiomics and machine learning with multiparametric preoperative mri may accurately predict the histopathological grades of soft tissue sarcomas. J Magn Reson Imaging. 2020;51:791–7.
Liu X, Guo L, Wang H, Guo J, Yang S, Duan L. Research on imbalance machine learning methods for MR[Formula: see text]WI soft tissue sarcoma data. BMC Med Imaging. 2022;22:149.
Corino VDA, Montin E, Messina A, Casali PG, Gronchi A, Marchianò A, et al. Radiomic analysis of soft tissues sarcomas can distinguish intermediate from high-grade lesions. J Magn Reson Imaging. 2018;47:829–40.
Zhu Y-F, Li Y-S, Zhang Y, Liu Y-J, Zhang Y-N, Tao J, et al. Radiomics model based on intravoxel incoherent motion and diffusion kurtosis imaging for predicting histopathological grade and Ki-67 expression level of soft tissue sarcomas. Acta Radiol. 2023; 2841851231179933.
Peeken JC, Bernhofer M, Spraker MB, Pfeiffer D, Devecka M, Thamer A, et al. CT-based radiomic features predict tumor grading and have prognostic value in patients with soft tissue sarcomas treated with neoadjuvant radiation therapy. Radiother Oncol. 2019;135:187–96.
Giraudo C, Fichera G, Stramare R, Bisogno G, Motta R, Evangelista L, et al. Radiomic features as biomarkers of soft tissue paediatric sarcomas: preliminary results of a PET/MR study. Radiol Oncol. 2022;56:138–41.
Crombé A, Bertolo F, Fadli D, Kind M, Le Loarer F, Perret R, et al. Distinct patterns of the natural evolution of soft tissue sarcomas on pre-treatment MRIs captured with delta-radiomics correlate with gene expression profiles. Eur Radiol. 2023;33:1205–18.
Timbergen MJM, Starmans MPA, Padmos GA, Grünhagen DJ, van Leenders GJLH, Hanff DF, et al. Differential diagnosis and mutation stratification of desmoid-type fibromatosis on MRI using radiomics. Eur J Radiol. 2020;131: 109266.
Foreman SC, Llorián-Salvador O, David DE, Rösner VKN, Rischewski JF, Feuerriegel GC, et al. Development and evaluation of MR-based radiogenomic models to differentiate atypical lipomatous tumors from lipomas. Cancers. 2023. https://doi.org/10.3390/cancers15072150.
Miao L, Ma S-T, Jiang X, Zhang H-H, Wang Y-M, Li M. Prediction of the therapeutic efficacy of epirubicin combined with ifosfamide in patients with lung metastases from soft tissue sarcoma based on contrast-enhanced CT radiomics features. BMC Med Imaging. 2022;22:131.
Peeken JC, Asadpour R, Specht K, Chen EY, Klymenko O, Akinkuoroye V, et al. MRI-based delta-radiomics predicts pathologic complete response in high-grade soft-tissue sarcoma patients treated with neoadjuvant therapy. Radiother Oncol. 2021;164:73–82.
Fields BKK, Demirjian NL, Cen SY, Varghese BA, Hwang DH, Lei X, et al. Predicting soft tissue sarcoma response to neoadjuvant chemotherapy using an mri-based delta-radiomics approach. Mol Imaging Biol. 2023. https://doi.org/10.1007/s11307-023-01803-y.
Crombé A, Périer C, Kind M, De Senneville BD, Le Loarer F, Italiano A, et al. T -based MRI Delta-radiomics improve response prediction in soft-tissue sarcomas treated by neoadjuvant chemotherapy. J Magn Reson Imaging. 2019;50:497–510.
Tomaszewski MR, Fan S, Garcia A, Qi J, Kim Y, Gatenby RA, et al. AI-radiomics can improve inclusion criteria and clinical trial performance. Tomography. 2022;8:341–55.
Gao Y, Kalbasi A, Hsu W, Ruan D, Fu J, Shao J, et al. Treatment effect prediction for sarcoma patients treated with preoperative radiotherapy using radiomics features from longitudinal diffusion-weighted MRIs. Phys Med Biol. 2020;65: 175006.
Crombé A, Saut O, Guigui J, Italiano A, Buy X, Kind M. Influence of temporal parameters of DCE-MRI on the quantification of heterogeneity in tumor vascularization. J Magn Reson Imaging. 2019;50:1773–88.
Zwanenburg A, Vallières M, Abdalah MA, Aerts HJWL, Andrearczyk V, Apte A, et al. The image biomarker standardization initiative: standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology. 2020;295:328–38.
Bologna M, Corino VDA, Montin E, Messina A, Calareso G, Greco FG, et al. Assessment of stability and discrimination capacity of radiomic features on apparent diffusion coefficient images. J Digit Imaging. 2018;31:879–94.
Thrussell I, Winfield JM, Orton MR, Miah AB, Zaidi SH, Arthur A, et al. Radiomic features from diffusion-weighted MRI of retroperitoneal soft-tissue sarcomas are repeatable and exhibit change after radiotherapy. Front Oncol. 2022;12: 899180.
Yue Z, Wang X, Wang Y, Wang H, Jiang W. Clinical-radiomics nomogram from T1W, T1CE, and T2FS MRI for improving diagnosis of soft-tissue sarcoma. Mol Imaging Biol. 2022;24:995–1006.
Aouadi S, Torfeh T, Arunachalam Y, Paloor S, Riyas M, Hammoud R, et al. Investigation of radiomics and deep convolutional neural networks approaches for glioma grading. Biomed Phys Eng Express. 2023. https://doi.org/10.1088/2057-1976/acc33a.
Starmans MPA, Timbergen MJM, Vos M, Padmos GA, Grünhagen DJ, Verhoef C, et al. The WORC database: MRI and CT scans, segmentations, and clinical labels for 930 patients from six radiomics studies. BioRxiv. 2021. https://doi.org/10.1101/2021.08.19.21262238.
Sedaghat S, Ravesh MS, Sedaghat M, Both M, Jansen O. Configuration of soft-tissue sarcoma on MRI correlates with grade of malignancy. Radiol Oncol. 2021;55:158–63.
Sedaghat S, Salehi Ravesh M, Sedaghat M, Meschede J, Jansen O, Both M. Does the primary soft-tissue sarcoma configuration predict configuration of recurrent tumors on magnetic resonance imaging? Acta Radiol. 2022;63:642–51.
Sedaghat S, Sedaghat M, Meschede J, Jansen O, Both M. Diagnostic value of MRI for detecting recurrent soft-tissue sarcoma in a long-term analysis at a multidisciplinary sarcoma center. BMC Cancer. 2021. https://doi.org/10.1186/s12885-021-08113-y.
Sedaghat S, Schmitz F, Meschede J, Sedaghat M. Systematic analysis of post-treatment soft-tissue edema and seroma on MRI in 177 sarcoma patients. Surg Oncol. 2020;35:218–23.
Zhong J, Hu Y, Si L, Jia G, Xing Y, Zhang H, et al. A systematic review of radiomics in osteosarcoma: utilizing radiomics quality score as a tool promoting clinical translation. Eur Radiol. 2021;31:1526–35.
Spadarella G, Stanzione A, Akinci D’Antonoli T, Andreychenko A, Fanni SC, Ugga L, et al. Systematic review of the radiomics quality score applications: an EuSoMII Radiomics Auditing Group Initiative. Eur Radiol. 2023;33:1884–94.
Guiot J, Vaidyanathan A, Deprez L, Zerka F, Danthine D, Frix A-N, et al. A review in radiomics: making personalized medicine a reality via routine imaging. Med Res Rev. 2022;42:426–40.
Acknowledgements
We extend our gratitude to the authors and the Jules Bordet Institute for their valuable contributions and collaboration, which greatly enriched the completion of this review. Our sincere acknowledgment goes to the PRISMA guidelines, providing a well-defined framework that structured our systematic review process. We express our appreciation for the indispensable resources and databases offered by PubMed/MEDLINE and Scopus, which significantly facilitated our research endeavours. The authors would like to thank “la Fondation Rose et Jean Hoguet” for providing a grant for a research fellowship.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
DAR & CR: Conceptualization, Methodology, Formal analysis, Resources, Writing—original draft. CN: Methodology, Formal analysis, Investigation. IS & MA: Methodology, Visualization, Formal analysis, Writing—review. SP & BMA: Supervision, Methodology, Writing—review & editing.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All the authors consent for the publication of this paper.
Competing interests
No conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
De Angelis, R., Casale, R., Coquelet, N. et al. The impact of radiomics in the management of soft tissue sarcoma. Discov Onc 15, 62 (2024). https://doi.org/10.1007/s12672-024-00908-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-024-00908-2