Abstract
Herein, indole derivatives namely 3-iodo-1-methyl-2-phenyl-1H-indole (4A), 3-iodo-1-methyl-2-(p-tolyl)-1H-indole (4B), and 2-(2,5-dimethylphenyl)-3-iodo-1-methyl-1H-indole (4C) prepared by Sonogashira coupling and electrophilic cyclization reactions with yield of 70%, 68%, and 67%, respectively. Organic catalysts were characterized by using Liquid Chromatography with tandem mass spectrometry (LC–MS–MS), Transmission Electron Microscopy (TEM), Fourier Transform Infrared Spektrofotometre (FT-IR), and Nuclear Magnetic Resonance (NMR). Cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), and chronoamperometry (CA) were utilized to observe the electrocatalytic behavior of 4A, 4B, and 4C. Specific activity for glucose electrooxidation of 4A was determined as 3.26 mA/cm2. Compound 4A exhibits long-term stability toward glucose electrooxidation. As a metal-free catalyst, Compound 4A may be a good candidate as an electrocatalyst for glucose electrooxidation.
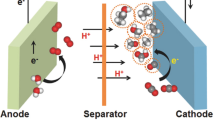
Graphical Abstract









Similar content being viewed by others
Data Availability
All data, models, and code generated or used during the study appear in the submitted article.
Abbreviations
- LC–MS/MS:
-
Liquid chromatography with tandem mass spectrometry
- FT-IR:
-
Fourier transform infrared spectroscopy
- NMR:
-
Nuclear magnetic resonance
- CV:
-
Cyclic voltammetry
- EIS:
-
Electrochemical impedance spectroscopy
- CA:
-
Chronoamperometry
- DGFC:
-
Direct glucose fuel cell
- PdCl2(PPh3)2 :
-
Bis(triphenylphosphine)palladium(II) dichloride
- Et3N:
-
Triethylamine
- THF:
-
Tetrahydrofuran
- CuI:
-
Copper(I) iodide
- MgSO4 :
-
Magnesium sulfate
- I2 :
-
Iodine
- TLC:
-
Thin-layer chromatography
- KOH:
-
Potassium hydroxide
- RT:
-
Room temperature
- J :
-
Coupling constant
- δ:
-
Chemical shift in parts per million
- CDCl3 :
-
Deuterochloroform
- µL:
-
Microliter
References
Yagizatli, Y., et al.: Improved fuel cell properties of Nano-TiO2 doped poly(vinylidene fluoride) and phosphonated poly(vinyl alcohol) composite blend membranes for PEM fuel cells. Int. J. Hydrogen Energy 45(60), 35130–35138 (2020)
Cali, A., et al.: Highly durable phosphonated graphene oxide doped polyvinylidene fluoride (PVDF) composite membranes. Int. J. Hydrogen Energy 45(60), 35171–35179 (2020)
Rusanen, A., et al.: Catalytic conversion of glucose to 5-hydroxymethylfurfural over biomass-based activated carbon catalyst. Catal Today 357, 94–101 (2020)
Maneeintr, K., et al.: Hydrothermal and enzymatic treatments of pineapple waste for energy production. Energy Procedia 152, 1260–1265 (2018)
Han, B., et al.: Catalytic conversion of glucose to 5-hydroxymethyfurfural over B2O3 supported solid acids catalysts. Waste Biomass Valorization 9(11), 2181–2190 (2018)
Chaudhuri, S.K., Lovley, D.R.J.N.B.: Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat. Biotechnol. 21(10), 1229–1232 (2003)
Xing, D., et al.: Change in microbial communities in acetate-and glucose-fed microbial fuel cells in the presence of light. Biosens. Bioelectron. 25(1), 105–111 (2009)
Xia, X., et al.: Electricity generation from glucose by a Klebsiella sp. in microbial fuel cells. Appl. Microbiol. Biotechnol. 87(1), 383–390 (2010)
Kuo, J., et al.: Dynamic changes in soil microbial communities with glucose enrichment in sediment microbial fuel cells. Indian J. Microbiol. 61(4), 497–505 (2021)
Shao, M., et al.: Optimization of a membraneless glucose/oxygen enzymatic fuel cell based on a bioanode with high coulombic efficiency and current density. ChemPhysChem 14(10), 2260–2269 (2013)
Bollella, P., et al.: A glucose/oxygen enzymatic fuel cell based on gold nanoparticles modified graphene screen-printed electrode. Proof-of-concept in human saliva. Sens. Actuat. B Chem. 256, 921–930 (2018)
Sahin, O., et al.: Facile and rapid synthesis of microwave assisted Pd nanoparticles as non-enzymatic hydrogen peroxide sensor (vol 12, pg 762, 2017). Int. J. Electrochem. Sci. 13(2), 2186–2192 (2018)
Bona, D., et al.: Management of digestate and exhausts from solid oxide fuel cells produced in the dry anaerobic digestion pilot plant: microalgae cultivation approach. Waste Biomass Valorization 11(12), 6499–6514 (2020)
Brouzgou, A., et al.: Glucose electrooxidation over PdxRh/C electrocatalysts in alkaline medium. Appl. Catal. B 147, 481–489 (2014)
Prathiba, S., Kumar, P.S., Vo, D.-V.N.J.C.: Recent advancements in microbial fuel cells: A review on its electron transfer mechanisms, microbial community, types of substrates and design for bio-electrochemical treatment. Chemosphere 286, 131856 (2022)
Liu, J., Vipulanandan, C., Yang, M.: Biosurfactant production from used vegetable oil in the anode chamber of a microbial electrosynthesizing fuel cell. Waste Biomass Valorization 10(10), 2925–2931 (2019)
Sahin, O., et al.: Facile and rapid synthesis of microwave assisted Pd nanoparticles as non-enzymatic hydrogen peroxide sensor. Int. J. Electrochem. Sci. 12(1), 762–769 (2017)
Huang, J., et al.: Pt-catalyzed D-glucose oxidation reactions for glucose fuel cells. J. Electrochem. Soc. 168(6), 064511 (2021)
Zhu, G.D., et al.: Use of lignosulfonate from pulping industrial waste as a potential material for proton exchange membrane in fuel cells. Waste Biomass Valorization 13(6), 2861–2869 (2022)
Chen, J., et al.: An alkaline direct oxidation glucose fuel cell using three-dimensional structural Au/Ni-foam as catalytic electrodes. RSC Adv. 7(5), 3035–3042 (2017)
Pak, M., et al.: Nickel-gold bimetallic nanostructures with the improved electrochemical performance for non-enzymatic glucose determination. J. Electroanal. Chem. 900, 115729 (2021)
Hansu, T., et al.: Fabrication of novel palladium-platinum based graphene/ITO electrodes and third metal addition effect through the glucose electrooxidation. J. Electroanal. Chem. 918, 116505 (2022)
Carbas, B.B., et al.: Synthesis and electropolymerization of a new ion sensitive ethylenedioxy-substituted terthiophene monomer bearing a quinoxaline moiety. J. Electroanal. Chem. 677, 9–14 (2012)
Chai, D., et al.: Facile aqueous phase synthesis of Pd3Cu–B/C catalyst for enhanced glucose electrooxidation. J. Taiwan Inst. Chem. Eng. 95, 139–146 (2019)
Mello, G.A., et al.: Glucose electro-oxidation on Pt (100) in phosphate buffer solution (pH 7): A mechanistic study. Electrochim. Acta 354, 136765 (2020)
Xu, M., et al.: One-pot aqueous synthesis of icosahedral Au as bifunctional candidates for enhanced glucose electrooxidation and surface-enhanced Raman scattering. ACS Appl. Mater. Interfaces 12(10), 12186–12194 (2020)
Brouzgou, A., et al.: Glucose electrooxidation reaction in presence of dopamine and uric acid over ketjenblack carbon supported PdCo electrocatalyst. J. Electroanal. Chem. 855, 113610 (2019)
Eshghi, A., Kheirmand, M.J.S.E.: Electroplating of Pt–Ni–Cu nanoparticles on glassy carbon electrode for glucose electro-oxidation process. Surf. Eng. 35(2), 128–134 (2019)
Zhiani, M., Abedini, A., Majidi, S.J.E.: Comparison of electro-catalytic activity of Fe-Ni-Co/C and Pd/C nanoparticles for glucose electro-oxidation in alkaline half-cell and direct glucose fuel cell. Electrocatalysis 9(6), 735–743 (2018)
Rafaïdeen, T., Baranton, S., Coutanceau, C.J.F.I.C.: Pd-shaped nanoparticles modified by gold ad-atoms: effects on surface structure and activity toward glucose electrooxidation. Front. Chem. 7, 453 (2019)
Er, Ö.F., Ulaş, B., Kivrak, H.D.J.T.J.O.C.: Remarkable bismuth-gold alloy decorated on MWCNT for glucose electrooxidation: the effect of bismuth promotion and optimization via response surface methodology. Turk. J. Chem. 45(4), 1173–1188 (2021)
Abuzaied, M.M., et al.: Enhanced glucose electrooxidation at Ni–Cu binary oxide nanocatalyst. Int. J. Electrochem. Sci. 15(3), 2449–2457 (2020)
Er, O.F., et al.: Enhanced electrochemical glucose oxidation in alkaline solution over indium decorated carbon supported palladium nanoparticles. Mater. Chem. Phys. 254, 123318 (2020)
Escalona-Villalpando, R., et al.: Electrodeposition of gold on oxidized and reduced graphite surfaces and its influence on glucose oxidation. J. Electroanal. Chem. 816, 92–98 (2018)
Caglar, A., et al.: Fabrication of carbon-doped titanium dioxide nanotubes as anode materials for photocatalytic glucose fuel cells. J. Electron. Mater. 50(4), 2242–2253 (2021)
Ozok-Arici, O., et al.: Glucose electrooxidation study on 3-iodo-2-(aryl/alkyl)benzo[b]thiophene organic catalyst. J. Electron. Mater. 51, 1653–1662 (2022)
Algso, M.A.S., et al.: Synthesis and biological evaluation of novel benzothiophene derivatives. J. Chem. Sci. (2018). https://doi.org/10.1007/s12039-018-1523-3
Hamad, A.R., et al.: Indole-based novel organic anode catalyst for glucose electrooxidation. Int. J. Energy Res. 46(2), 1659–1671 (2022)
Ozok, O., et al.: Novel benzothiophene based catalyst with enhanced activity for glucose electrooxidation. Int. J. Hydrogen Energy 45(53), 28706–28715 (2020)
Brouzgou, A., Tsiakaras, P.: Electrocatalysts for glucose electrooxidation reaction: a review. Top. Catal. 58(18), 1311–1327 (2015)
Kaya, S., et al.: Highly active RuPd bimetallic catalysts for sodium borohydride electrooxidation and hydrolysis. J. Electron. Mater. 51(1), 403–411 (2022)
Cao, M., et al.: Nickel-copper oxide nanoflowers for highly efficient glucose electrooxidation. Int. J. Hydrogen Energy 46(56), 28527–28536 (2021)
Neha, N., et al.: Remarkably efficient carbon-supported nanostructured platinum-bismuth catalysts for the selective electrooxidation of glucose and methyl-glucoside. Electrocatalysis 12(1), 1–14 (2021)
Er, O.F., Kivrak, H.J.E.S.: Highly active carbon nanotube supported iridium, copper, ruthenium catalysts for glucose electrooxidation. Energy Storage 3(6), e271 (2021)
Neto, N.F.A., et al.: Evaluation of ITO/TiO2/Co3O4 as a non-enzymatic heterojunction electrode to glucose electrooxidation. Ionics 27(4), 1597–1609 (2021)
Ulas, B., et al.: Disentangling the enhanced catalytic activity on Ga modified Ru surfaces for sodium borohydride electrooxidation. Surf. Interfaces 23, 100999 (2021)
Acknowledgements
We acknowledge with gratitude the support provided by the TUBİTAK (120Z928) for chemicals.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Calis, H., Ulas, B., Yilmaz, Y. et al. Synthesis of 3-Iodoindoles and Their Glucose Electrooxidation Performance as an Anode Catalyst. Waste Biomass Valor 14, 3285–3295 (2023). https://doi.org/10.1007/s12649-023-02163-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-023-02163-y