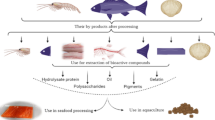
Abstract
Fish processing industry has experienced significant growth, playing an important role in the world economy. The increased exploration of marine resources contributes to the generation of considerable amounts of biowaste, which ends up as discards. In the face of the resultant disposal and environmental problems, many efforts have been made to deal with the fishery waste in more efficient ways. Nowadays, these by-products are regarded as important sources of high added value compounds, such as hydroxyapatite, collagen, gelatin, lipids, enzymes, hydrolysates and bioactive peptides, with great potential for human health applications. The present paper aims to review the current methods of extraction and characterization of added value products from fish by-products, as well as their actual and potential applications.
Graphic Abstract




Similar content being viewed by others
Notes
Is an obsolete term (IUPAC, 1997 “imino acids,”). In this review we will save the imino acid designation, for standardizing purposes.
References
FAO: The State of World Fisheries and Aquaculture—Opportunities and Challenges. FAO, Rome (2014)
Chalamaiah, M., Dinesh Kumar, B., Hemalatha, R., Jyothirmayi, T.: Fish protein hydrolysates: proximate composition, amino acid composition, antioxidant activities and applications: a review. Food Chem. 135, 3020–3038 (2012). https://doi.org/10.1016/j.foodchem.2012.06.100
Caruso, G.: Fishery wastes and by-products: a resource to be valorised. J. Fish. Sci. 9, 080–083 (2015)
Ferraro, V., Cruz, I.B., Jorge, R.F., Malcata, F.X., Pintado, M.E., Castro, P.M.L.: Valorisation of natural extracts from marine source focused on marine by-products: a review. Food Res. Int. 43, 2221–2233 (2010). https://doi.org/10.1016/j.foodres.2010.07.034
Hsu, K.-C.: Purification of antioxidative peptides prepared from enzymatic hydrolysates of tuna dark muscle by-product. Food Chem. 122, 42–48 (2010). https://doi.org/10.1016/J.FOODCHEM.2010.02.013
Schmidt, Dornelles, R.C.P., Mello, R.O., Kubota, E.H., Mazutti, M.A., Kempka, Demiate, I.M.: Collagen extraction process. Int. Food Res. J. 23, 913–922 (2016)
Liceaga-Gesualdo, A., Li-Chan, E.C.: Functional properties of fish protein hydrolysate from herring (Clupea harengus). J. Food Sci. 64, 1000–1004 (1999)
Chantachum, S., Benjakul, S., Sriwirat, N.: Separation and quality of fish oil from precooked and non-precooked tuna heads. Food Chem. 69, 289–294 (2000). https://doi.org/10.1016/S0308-8146(99)00266-6
Montero, P., Gomez-Guillen, M.C.: Extracting conditions for megrim (Lepidorhombus boscii) skin collagen affect functional properties of the resulting gelatin. J. Food Sci. 65, 434–438 (2000). https://doi.org/10.1111/j.1365-2621.2000.tb16022.x
Ozawa, M., Suzuki, S.: Microstructural development of natural hydroxyapatite originated from fish-bone waste through heat treatment. J. Argent. Chem. Soc. 17, 2000–2002 (2002). https://doi.org/10.1111/j.1151-2916.2002.tb00268.x
Kim, S.-K., Park, P.-J., Kim, J.-B., Shahidi, F.: Purification and characterization of a collagenolytic protease from the filefish, Novoden modestrus. J. Biochem. Mol. Biol. 35, 165–171 (2002)
Kim, S.K., Mendis, E.: Bioactive compounds from marine processing byproducts—a review. Food Res. Int. 39, 383–393 (2006). https://doi.org/10.1016/j.foodres.2005.10.010
Gildberg, A.: Enzymes and bioactive peptides from fish waste related to fish silage, fish Feed and fish Sauce production. J. Aquat. Food Prod. Technol. 13, 3–11 (2004). https://doi.org/10.1300/J030v13n02
Gildberg, A., Simpson, B.K., Haard, N.F.: Uses of enzymes from marine organisms. In: Haard, N.F., Simpson, B.K. (eds.) Seafood Enzymes: Utilization and Influence on Postharvest Seafood Quality, pp. 619–639. Marcel Dekker Inc, New York (2000)
Gildberg, A.: Digestive enzyme activities in starved pre-slaughter farmed and wild-captured, Atlantic cod (Gadus morhua). Aquaculture 238, 343–353 (2004). https://doi.org/10.1016/j.aquaculture.2004.03.021
Zhao, L., Budge, S.M., Ghaly, A.E., Brooks, S.M.: Extraction, purification and characterization of fish pepsin: a critical review. J. Food Process. Technol. (2011). https://doi.org/10.4172/2157-7110.1000126
Fernandes, P.: Enzymes in fish and seafood processing. Front. Bioeng. Biotechnol. 4, 1–14 (2016). https://doi.org/10.3389/fbioe.2016.00059
Shahidi, F., Janak Kamil, Y.V.A.: Enzymes from fish and aquatic invertebrates and their application in the food industry. Trends Food Sci. Technol. 12, 435–464 (2001). https://doi.org/10.1016/s0924-2244(02)00021-3
Souza, A.A.G., Amaral, I.P.G., Santo, A.R.E., Carvalho, L.B., Bezerra, R.S.: Trypsin-like enzyme from intestine and pyloric caeca of spotted goatfish (Pseudupeneus maculatus). Food Chem. 100, 1429–1434 (2007). https://doi.org/10.1016/j.foodchem.2005.12.016
Silva, J.F., Espósito, T.S., Marcuschi, M., Ribeiro, K., Cavalli, R.O., Oliveira, V., Bezerra, R.S.: Purification and partial characterisation of a trypsin from the processing waste of the silver mojarra (Diapterus rhombeus). Food Chem. 129, 777–782 (2011). https://doi.org/10.1016/j.foodchem.2011.05.019
Bougatef, A., Souissi, N., Fakhfakh, N., Ellouz-Triki, Y., Nasri, M.: Purification and characterization of trypsin from the viscera of sardine (Sardina pilchardus). Food Chem. 102, 343–350 (2007). https://doi.org/10.1016/j.foodchem.2006.05.050
Marcuschi, M., Espósito, T.S., Machado, M.F.M., Hirata, I.Y., Machado, M.F.M., Silva, M.V., Carvalho, L.B., Oliveira, V., Bezerra, R.S.: Purification, characterization and substrate specificity of a trypsin from the Amazonian fish tambaqui (Colossoma macropomum). Biochem. Biophys. Res. Commun. 396, 667–673 (2010). https://doi.org/10.1016/j.bbrc.2010.04.155
Khantaphant, S., Benjakul, S.: Purification and characterization of trypsin from the pyloric caeca of brownstripe red snapper (Lutjanus vitta). Food Chem. 120, 658–664 (2010). https://doi.org/10.1016/j.foodchem.2009.09.098
Fuchise, T., Kishimura, H., Sekizaki, H., Nonami, Y., Kanno, G., Klomklao, S., Benjakul, S., Chun, B.S.: Purification and characteristics of trypsins from cold-zone fish, Pacific cod (Gadus macrocephalus) and saffron cod (Eleginus gracilis). Food Chem. 116, 611–616 (2009). https://doi.org/10.1016/j.foodchem.2009.02.078
Lu, B.J., Zhou, L.G., Cai, Q.F., Hara, K., Maeda, A., Su, W.J., Cao, M.J.: Purification and characterisation of trypsins from the pyloric caeca of mandarin fish (Siniperca chuatsi). Food Chem. 110, 352–360 (2008). https://doi.org/10.1016/j.foodchem.2008.02.010
Nalinanon, S., Benjakul, S., Visessanguan, W., Kishimura, H.: Partitioning of protease from stomach of albacore tuna (Thunnus alalunga) by aqueous two-phase systems. Process Biochem. 44, 471–476 (2009). https://doi.org/10.1016/j.procbio.2008.12.018
Spelzini, D., Farruggia, B., Picó, G.: Features of the acid protease partition in aqueous two-phase systems of polyethylene glycol–phosphate: chymosin and pepsin. J. Chromatogr. B 821, 60–66 (2005). https://doi.org/10.1016/J.JCHROMB.2005.04.007
Tubío, G., Nerli, B., Picó, G.: Partitioning features of bovine trypsin and α-chymotrypsin in polyethyleneglycol-sodium citrate aqueous two-phase systems. J. Chromatogr. B 852, 244–249 (2007). https://doi.org/10.1016/J.JCHROMB.2007.01.025
Merz, M., Claaßen, W., Appel, D., Berends, P., Rabe, S., Blank, I., Stressler, T., Fischer, L.: Characterization of commercially available peptidases in respect of the production of protein hydrolysates with defined compositions using a three-step methodology. J. Mol. Catal. B Enzym. 127, 1–10 (2016)
Cao, M.J., Chen, W.Q., Du, C.H., Yoshida, A., Lan, W.G., Liu, G.M., Su, W.J.: Pepsinogens and pepsins from Japanese seabass (Lateolabrax japonicus). Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 158, 259–265 (2011). https://doi.org/10.1016/j.cbpb.2010.12.003
Klomklao, S., Kishimura, H., Yabe, M., Benjakul, S.: Purification and characterization of two pepsins from the stomach of pectoral rattail (Coryphaenoides pectoralis). Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 147, 682–689 (2007). https://doi.org/10.1016/j.cbpb.2007.04.008
Nalinanon, S., Benjakul, S., Kishimura, H.: Biochemical properties of pepsinogen and pepsin from the stomach of albacore tuna (Thunnus alalunga). Food Chem. 121, 49–55 (2010). https://doi.org/10.1016/J.FOODCHEM.2009.11.089
Tanji, M., Yakabe, E., Kageyama, T., Yokobori, S.I., Ichinose, M., Miki, K., Ito, H., Takahashi, K.: Purification and characterization of pepsinogens from the gastric mucosa of African coelacanth, Latimeria chalumnae, and properties of the major pepsins. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 146, 412–420 (2007). https://doi.org/10.1016/j.cbpb.2006.11.025
Wu, T., Sun, L.C., Du, C.H., Cai, Q.F., Zhang, Q.B., Su, W.J., Cao, M.J.: Identification of pepsinogens and pepsins from the stomach of European eel (Anguilla anguilla). Food Chem. 115, 137–142 (2009). https://doi.org/10.1016/j.foodchem.2008.11.077
Zhou, Q., Liu, G.M., Huang, Y.Y., Weng, L., Hara, K., Su, W.J., Cao, M.J.: Pepsinogens and pepsins from mandarin fish (Siniperca chuatsi). J. Agric. Food Chem. 56, 5401–5406 (2008). https://doi.org/10.1021/jf800458n
Candiotto, F.B., Freitas-Júnior, A.C.V., Neri, R.C.A., Bezerra, R.S., Rodrigues, R.V., Sampaio, L.A., Tesser, M.B.: Characterization of digestive enzymes from captive Brazilian flounder Paralichthys orbignyanus. Braz. J. Biol. 78, 281–288 (2017)
Kishimura, H., Klomklao, S., Benjakul, S., Chun, B.S.: Characteristics of trypsin from the pyloric ceca of walleye pollock (Theragra chalcogramma). Food Chem. 106, 194–199 (2008). https://doi.org/10.1016/j.foodchem.2007.05.056
Klomklao, S., Kishimura, H., Nonami, Y., Benjakul, S.: Biochemical properties of two isoforms of trypsin purified from the Intestine of skipjack tuna (Katsuwonus pelamis). Food Chem. 115, 155–162 (2009). https://doi.org/10.1016/j.foodchem.2008.11.087
Gildberg, A.: Utilisation of male Arctic capelin and Atlantic cod intestines for fish sauce production—evaluation of fermentation conditions. Bioresour. Technol. 76, 119–123 (2001). https://doi.org/10.1016/S0960-8524(00)00095-X
Bougatef, A., Balti, R., Zaied, S.B., Souissi, N., Nasri, M.: Pepsinogen and pepsin from the stomach of smooth hound (Mustelus mustelus): purification, characterization and amino acid terminal sequences. Food Chem. 107, 777–784 (2008). https://doi.org/10.1016/j.foodchem.2007.08.077
El-Beltagy, A., El-Adawy, T., Rahma, E., El-Bedawey, A.: Purification and characterization of an acidic protease from the viscera of bolti fish (Tilapia nilotica). Food Chem. 86, 33–39 (2004). https://doi.org/10.1016/J.FOODCHEM.2003.08.009
Castillo-Yañez, F.J., Pacheco-Aguilar, R., Garcia-Carreño, F.L., Navarrete-Del Toro, M.D.L.A.: Characterization of acidic proteolytic enzymes from Monterey sardine (Sardinops sagax caerulea) viscera. Food Chem. 85, 343–350 (2004). https://doi.org/10.1016/j.foodchem.2003.07.008
Castillo-Yáñez, F.J., Pacheco-Aguilar, R., García-Carreño, F.L., Navarrete-Del Toro, M.D.L.Á.: Isolation and characterization of trypsin from pyloric caeca of Monterey sardine Sardinops sagax caerulea. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 140, 91–98 (2005). https://doi.org/10.1016/j.cbpc.2004.09.031
Wu, R., Wu, C., Liu, D., Yang, X., Huang, J., Zhang, J., Liao, B., He, H., Li, H.: Overview of antioxidant peptides derived from marine resources: the sources, characteristic, purification, and evaluation methods. Appl. Biochem. Biotechnol. 176, 1815–1833 (2015). https://doi.org/10.1007/s12010-015-1689-9
Guérard, F., Dufossé, L., De La Broise, D., Binet, A.: Enzymatic hydrolysis of proteins from yellowfin tuna (Thunnus albacares) wastes using Alcalase. J. Mol. Catal. B Enzym. 11, 1051–1059 (2001). https://doi.org/10.1016/S1381-1177(00)00031-X
Šližytė, R., Daukšas, E., Falch, E., Storrø, I., Rustad, T.: Characteristics of protein fractions generated from hydrolysed cod (Gadus morhua) by-products. Process Biochem. 40, 2021–2033 (2005). https://doi.org/10.1016/j.procbio.2004.07.016
Vázquez, J., Blanco, M., Massa, A., Amado, I., Pérez-Martín, R.: Production of fish protein hydrolysates from Scyliorhinus canicula discards with antihypertensive and antioxidant activities by enzymatic hydrolysis and mathematical optimization using response surface methodology. Mar. Drugs 15, 306 (2017). https://doi.org/10.3390/md15100306
Himonides, A.T., Taylor, A.K.D., Morris, A.J.: A study of the enzymatic hydrolysis of fish frames using model systems. Food Nutr. Sci. 2, 575–585 (2011). https://doi.org/10.4236/fns.2011.26081
Bhaskar, N., Benila, T., Radha, C., Lalitha, R.G.: Optimization of enzymatic hydrolysis of visceral waste proteins of Catla (Catla catla) for preparing protein hydrolysate using a commercial protease. Bioresour. Technol. 99, 335–343 (2008). https://doi.org/10.1016/j.biortech.2006.12.015
Wang, W., Li, Z., Liu, J., Wang, Y., Liu, S., Sun, M.: Comparison between thermal hydrolysis and enzymatic proteolysis processes for the preparation of tilapia skin. Czech J. Food Sci. 31, 1–4 (2013)
Murthy, P.S., Rai, A.K., Bhaskar, N.: Fermentative recovery of lipids and proteins from freshwater fish head waste with reference to antimicrobial and antioxidant properties of protein hydrolysate. J. Food Sci. Technol. 51, 1884–1892 (2014). https://doi.org/10.1007/s13197-012-0730-z
Villamil, O., Váquiro, H., Solanilla, J.F.: Fish viscera protein hydrolysates: production, potential applications and functional and bioactive properties. Food Chem. 224, 160–171 (2017). https://doi.org/10.1016/j.foodchem.2016.12.057
Najafian, L., Babji, A.S.S.: A review of fish-derived antioxidant and antimicrobial peptides: their production, assessment, and applications. Peptides 33, 178–185 (2012). https://doi.org/10.1016/j.peptides.2011.11.013
Wang, X., Yu, H., Xing, R., Li, P.: Characterization, preparation, and purification of marine bioactive peptides. Biomed. Res. Int. 2017, 1–16 (2017). https://doi.org/10.1155/2017/9746720
Ishak, N.H., Sarbon, N.M.: A review of protein hydrolysates and bioactive peptides deriving from wastes generated by fish processing. Food Bioprocess Technol. 11, 2–16 (2018). https://doi.org/10.1007/s11947-017-1940-1
Klompong, V., Benjakul, S., Kantachote, D., Shahidi, F.: Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 102, 1317–1327 (2007). https://doi.org/10.1016/J.FOODCHEM.2006.07.016
Elavarasan, K., Naveen Kumar, V., Shamasundar, B.A.: Antioxidant and functional properties of fish protein hydrolysates from fresh water Carp (Catla catla) as influenced by the nature of enzyme. J. Food Process. Preserv. 38, 1207–1214 (2014). https://doi.org/10.1111/jfpp.12081
Ben Khaled, H., Ktari, N., Ghorbel-Bellaaj, O., Jridi, M., Lassoued, I., Nasri, M.: Composition, functional properties and in vitro antioxidant activity of protein hydrolysates prepared from sardinelle (Sardinella aurita) muscle. J. Food Sci. Technol. 51, 622–633 (2014). https://doi.org/10.1007/s13197-011-0544-4
Jamil, N.H., Halim, N.R.A., Sarbon, N.M.: Optimization of enzymatic hydrolysis condition and functional properties of eel (Monopterus sp.) protein using response surface methodology (RSM). Int. Food Res. J. 23, 1–9 (2016)
Jemil, I., Jridi, M., Nasri, R., Ktari, N., Salem, R.B.S.-B., Mehiri, M., Hajji, M., Nasri, M.: Functional, antioxidant and antibacterial properties of protein hydrolysates prepared from fish meat fermented by Bacillus subtilis A26. Process Biochem. 49, 963–972 (2014). https://doi.org/10.1016/j.procbio.2014.03.004
Ktari, N., Jridi, M., Bkhairia, I., Sayari, N., Ben Salah, R., Nasri, M.: Functionalities and antioxidant properties of protein hydrolysates from muscle of zebra blenny (Salaria basilisca) obtained with different crude protease extracts. Food Res. Int. 49, 747–756 (2012). https://doi.org/10.1016/J.FOODRES.2012.09.024
Liu, Y., Li, X., Chen, Z., Yu, J., Wang, F., Wang, J.: Characterization of structural and functional properties of fish protein hydrolysates from surimi processing by-products. Food Chem. 151, 459–465 (2014). https://doi.org/10.1016/J.FOODCHEM.2013.11.089
Taheri, A., Anvar, S.A.A., Ahari, H., Fogliano, V.: Comparison the functional properties of protein hydrolysates from poultry byproducts and rainbow trout. Iran. J. Fish. Sci. 12, 154–169 (2013)
Tanuja, S., Viji, P., Zynudheen, A., Joshy, C.: Composition, functional properties and antioxidative activity of hydrolysates prepared from the frame meat of Striped catfish (Pangasianodon hypophthalmus). Egypt. J. Biol. 14, 27–35 (2012). https://doi.org/10.4314/ejb.v14i1.3
Batista, I., Pires, C.: Utilization of Fish Waste Chapter 3: Functional Properties of Fish Protein Hydrolysates, pp. 59–75. CRC Press, Boca Raton (2013)
Kristinsson, H.G., Rasco, B.A.: Fish protein hydrolysates: production, biochemical, and functional properties. Crit. Rev. Food Sci. Nutr. 40, 43–81 (2000). https://doi.org/10.1080/10408690091189266
Zayas, J.F.: Functionality of Proteins in Food Chapter 4: Oil and Fat Binding Properties of Proteins, pp. 228–259. Springer, Berlin (1997)
Kaur, M., Singh, N.: Characterization of protein isolates from different Indian chickpea (Cicer arietinum L.) cultivars. Food Chem. 102, 366–374 (2007). https://doi.org/10.1016/j.foodchem.2006.05.029
Halim, N.R.A.R.A., Yusof, H.M.M., Sarbon, N.M.M.: Functional and bioactive properties of fish protein hydolysates and peptides: a comprehensive review. Trends Food Sci. Technol. 51, 24–33 (2016). https://doi.org/10.1016/j.tifs.2016.02.007
Cheng, X., Tang, X., Wang, Q., Mao, X.Y.: Antibacterial effect and hydrophobicity of yak κ-casein hydrolysate and its fractions. Int. Dairy J. 31, 111–116 (2013). https://doi.org/10.1016/J.IDAIRYJ.2012.12.004
Venkatesan, J., Anil, S., Kim, S.-K., Shim, M.: Marine fish proteins and peptides for cosmeceuticals: a review. Mar. Drugs 15, 143 (2017). https://doi.org/10.3390/md15050143
Silva, T.H., Moreira-Silva, J., Marques, A.L.P., Domingues, A., Bayon, Y., Reis, R.L.: Marine origin collagens and its potential applications. Mar. Drugs 12, 5881–5901 (2014). https://doi.org/10.3390/md12125881
Gómez-Guillén, M.C., Giménez, B., López-Caballero, M.E., Montero, M.P.: Functional and bioactive properties of collagen and gelatin from alternative sources: a review. Food Hydrocoll. 25, 1813–1827 (2011). https://doi.org/10.1016/j.foodhyd.2011.02.007
Karim, A.A., Bhat, R.: Fish gelatin: properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 23, 563–576 (2009). https://doi.org/10.1016/J.FOODHYD.2008.07.002
Karayannakidis, P.D., Zotos, A.: Fish processing by-products as a potential source of gelatin: a review. J. Aquat. Food Prod. Technol. 25, 65–92 (2016). https://doi.org/10.1080/10498850.2013.827767
Alfaro, A.T., Biluca, F.C., Marquetti, C., Tonial, I.B., de Souza, N.E.: African catfish (Clarias gariepinus) skin gelatin: extraction optimization and physical–chemical properties. Food Res. Int. 65, 416–422 (2014). https://doi.org/10.1016/J.FOODRES.2014.05.070
Chen, J., Li, L., Yi, R., Xu, N., Gao, R., Hong, B.: Extraction and characterization of acid-soluble collagen from scales and skin of tilapia (Oreochromis niloticus). LWT—Food Sci. Technol. 66, 453–459 (2016). https://doi.org/10.1016/j.lwt.2015.10.070
Chuaychan, S., Benjakul, S., Kishimura, H.: Characteristics of acid- and pepsin-soluble collagens from scale of seabass (Lates calcarifer). LWT Food Sci. Technol. 63, 71–76 (2015). https://doi.org/10.1016/J.LWT.2015.03.002
Kaewdang, O., Benjakul, S., Kaewmanee, T., Kishimura, H.: Characteristics of collagens from the swim bladders of yellowfin tuna (Thunnus albacares). Food Chem. 155, 264–270 (2014). https://doi.org/10.1016/j.foodchem.2014.01.076
Liu, D., Liang, L., Regenstein, J.M., Zhou, P.: Extraction and characterisation of pepsin-solubilised collagen from fins, scales, skins, bones and swim bladders of bighead carp (Hypophthalmichthys nobilis). Food Chem. 133, 1441–1448 (2012). https://doi.org/10.1016/J.FOODCHEM.2012.02.032
Mahboob, S.: Isolation and characterization of collagen from fish waste material- skin, scales and fins of Catla catla and Cirrhinus mrigala. J. Food Sci. Technol. (2014). https://doi.org/10.1007/s13197-014-1520-6
Ramanathan, G., Muthukumar, T., Tirichurapalli Sivagnanam, U.: In vivo efficiency of the collagen coated nanofibrous scaffold and their effect on growth factors and pro-inflammatory cytokines in wound healing. Eur. J. Pharmacol. 814, 45–55 (2017). https://doi.org/10.1016/J.EJPHAR.2017.08.003
Li, Q., Mu, L., Zhang, F., Sun, Y., Chen, Q., Xie, C., Wang, H.: A novel fish collagen scaffold as dural substitute. Mater. Sci. Eng., C 80, 346–351 (2017). https://doi.org/10.1016/J.MSEC.2017.05.102
Bhagwat, P.K., Dandge, P.B.: Isolation, characterization and valorizable applications of fish scale collagen in food and agriculture industries. Biocatal. Agric. Biotechnol. 7, 234–240 (2016). https://doi.org/10.1016/J.BCAB.2016.06.010
Ahmad, T., Ismail, A., Ahmad, S.A., Khalil, K.A., Kumar, Y., Adeyemi, K.D., Sazili, A.Q.: Recent advances on the role of process variables affecting gelatin yield and characteristics with special reference to enzymatic extraction: a review. Food Hydrocoll. 63, 85–96 (2017). https://doi.org/10.1016/J.FOODHYD.2016.08.007
Pal, G.K., Suresh, P.V.: Comparative assessment of physico-chemical characteristics and fibril formation capacity of thermostable carp scales collagen. Mater. Sci. Eng., C 70, 32–40 (2017). https://doi.org/10.1016/j.msec.2016.08.047
Wang, J.K., Yeo, K.P., Chun, Y.Y., Tan, T.T.Y., Tan, N.S., Angeli, V., Choong, C.: Fish scale-derived collagen patch promotes growth of blood and lymphatic vessels in vivo. Acta Biomater. 63, 246–260 (2017). https://doi.org/10.1016/J.ACTBIO.2017.09.001
Gildberg, A., Arnesen, J.A., Carlehög, M.: Utilisation of cod backbone by biochemical fractionation. Process Biochem. 38, 475–480 (2002). https://doi.org/10.1016/S0032-9592(02)00103-6
Regenstein, J.M., Zhou, P.: Collagen and gelatin from marine by-products. Maximising Value Mar. By-Prod. (2007). https://doi.org/10.1533/9781845692087.2.279
Sinthusamran, S., Benjakul, S., Kishimura, H.: Comparative study on molecular characteristics of acid soluble collagens from skin and swim bladder of seabass (Lates calcarifer). Food Chem. 138, 2435–2441 (2013). https://doi.org/10.1016/j.foodchem.2012.11.136
Coelho, R.C.G., Marques, A.L.P., Oliveira, S.M., Diogo, G.S., Pirraco, R.P., Moreira-Silva, J., Xavier, J.C., Reis, R.L., Silva, T.H., Mano, J.F.: Extraction and characterization of collagen from Antarctic and Sub-Antarctic squid and its potential application in hybrid scaffolds for tissue engineering. Mater. Sci. Eng., C 78, 787–795 (2017). https://doi.org/10.1016/j.msec.2017.04.122
Ali, A.M.M., Kishimura, H., Benjakul, S.: Extraction efficiency and characteristics of acid and pepsin soluble collagens from the skin of golden carp (Probarbus Jullieni) as affected by ultrasonication. Process Biochem. 66, 237–244 (2018). https://doi.org/10.1016/J.PROCBIO.2018.01.003
Muhammed, A., Ali, M., Kishimura, H., Benjakul, S.: Physicochemical and molecular properties of gelatin from skin of golden carp (Probarbus Jullieni) as influenced by acid pretreatment and prior-ultrasonication. Food Hydrocoll. (2018). https://doi.org/10.1016/j.foodhyd.2018.03.052
Khong, N.M.H., Yusoff, F.M., Jamilah, B., Basri, M., Maznah, I., Chan, K.W., Armania, N., Nishikawa, J.: Improved collagen extraction from jellyfish (Acromitus hardenbergi) with increased physical-induced solubilization processes. Food Chem. 251, 41–50 (2018). https://doi.org/10.1016/J.FOODCHEM.2017.12.083
Sila, A., Martinez-Alvarez, O., Haddar, A., Carmen Gómez-Guillén, M., Nasri, M., Montero, M.P., Bougatef, A.: Recovery, viscoelastic and functional properties of Barbel skin gelatine: investigation of anti-DPP-IV and anti-prolyl endopeptidase activities of generated gelatine polypeptides. Food Chem. 168, 478–486 (2015). https://doi.org/10.1016/j.foodchem.2014.07.086
Abdelmalek, B.E., Gómez-Estaca, J., Sila, A., Martinez-Alvarez, O., Gómez-Guillén, M.C., Chaabouni-Ellouz, S., Ayadi, M.A., Bougatef, A.: Characteristics and functional properties of gelatin extracted from squid (Loligo vulgaris) skin. LWT—Food Sci. Technol. 65, 924–931 (2016). https://doi.org/10.1016/j.lwt.2015.09.024
Zhang, Y., Dutilleul, P., Li, C., Simpson, B.K.: Alcalase-assisted production of fish skin gelatin rich in high molecular weight (HMW) polypeptide chains and their characterization for film forming capacity. LWT (2018). https://doi.org/10.1016/j.lwt.2018.12.012
Byler, D.M., Susi, H.: Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers 25, 469–487 (1986). https://doi.org/10.1002/bip.360250307
Muyonga, J., Cole, C.G., Duodu, K.: Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult Nile perch (Lates niloticus). Food Chem. 86, 325–332 (2004). https://doi.org/10.1016/J.FOODCHEM.2003.09.038
Zhang, Q., Wang, Q., Lv, S., Lu, J., Jiang, S., Regenstein, J.M., Lin, L.: Comparison of collagen and gelatin extracted from the skins of Nile tilapia (Oreochromis niloticus) and channel catfish (Ictalurus punctatus). Food Biosci. 13, 41–48 (2016)
Qi, P., Zhou, Y., Wang, D., He, Z., Li, Z.: A new collagen solution with high concentration and collagen native structure perfectly preserved. RSC Adv. 5, 87180–87186 (2015). https://doi.org/10.1039/C5RA14423J
Júnior, Z.S.S., Botta, S.B., Ana, P.A., França, C.M., Fernandes, K.P.S., Mesquita-Ferrari, R.A., Deana, A., Bussadori, S.K.: Effect of papain-based gel on type I collagen—spectroscopy applied for microstructural analysis. Sci. Rep. 5, 11448 (2015). https://doi.org/10.1038/srep11448
Díaz-Calderón, P., Flores, E., González-Muñoz, A., Pepczynska, M., Quero, F., Enrione, J.: Influence of extraction variables on the structure and physical properties of salmon gelatin. Food Hydrocoll. 71, 118–128 (2017). https://doi.org/10.1016/J.FOODHYD.2017.05.004
Sun, L., Hou, H., Li, B., Zhang, Y.: Characterization of acid- and pepsin-soluble collagen extracted from the skin of Nile tilapia (Oreochromis niloticus). Int. J. Biol. Macromol. 99, 8–14 (2017). https://doi.org/10.1016/J.IJBIOMAC.2017.02.057
Feng, Y., Melacini, G., Taulane, J.P., Goodman, M.: Acetyl-terminated and template-assembled collagen-based polypeptides composed of Gly-Pro-Hyp sequences. 2. Synthesis and conformational analysis by circular dichroism, ultraviolet absorbance, and optical rotation. J. Am. Chem. Soc. 118, 10351–10358 (1996). https://doi.org/10.1021/ja961260c
Kozlowska, J., Sionkowska, A., Skopinska-Wisniewska, J., Piechowicz, K.: Northern pike (Esox lucius) collagen: extraction, characterization and potential application. Int. J. Biol. Macromol. 81, 220–227 (2015). https://doi.org/10.1016/j.ijbiomac.2015.08.002
Li, J., Wang, M., Qiao, Y., Tian, Y., Liu, J., Qin, S., Wu, W.: Extraction and characterization of type I collagen from skin of tilapia (Oreochromis niloticus) and its potential application in biomedical scaffold material for tissue engineering. Process Biochem. 74, 156–163 (2018). https://doi.org/10.1016/J.PROCBIO.2018.07.009
da Silva, E.V.C., Lourenço, L.D.F.H., Pena, R.S.: Optimization and characterization of gelatin from kumakuma (Brachyplatystoma filamentosum) skin. CyTA—J. Food. 15, 361–368 (2017). https://doi.org/10.1080/19476337.2016.1266391
Dorozhkin, V.S.: Bioceramics of calcium orthophosphates. Biomaterials 31, 1465–1485 (2010). https://doi.org/10.1016/j.biomaterials.2009.11.050
Boutinguiza, M., Pou, J., Comesaña, R., Lusquiños, F., De Carlos, A., León, B.: Biological hydroxyapatite obtained from fish bones. Mater. Sci. Eng., C 32, 478–486 (2012). https://doi.org/10.1016/j.msec.2011.11.021
Goto, T., Sasaki, K.: Effects of trace elements in fish bones on crystal characteristics of hydroxyapatite obtained by calcination. Ceram. Int. 40, 10777–10785 (2014). https://doi.org/10.1016/j.ceramint.2014.03.067
Huang, Y.C., Hsiao, P.C., Chai, H.J.: Hydroxyapatite extracted from fish scale: effects on MG63 osteoblast-like cells. Ceram. Int. 37, 1825–1831 (2011). https://doi.org/10.1016/j.ceramint.2011.01.018
Venkatesan, J., Kim, S.K.: Effect of temperature on isolation and characterization of hydroxyapatite from tuna (Thunnus obesus) bone. Materials 3, 4761–4772 (2010). https://doi.org/10.3390/ma3104761
Piccirillo, C., Pullar, R.C., Tobaldi, D.M., Castro, L.P.M., Pintado, E.M.M.: Hydroxyapatite and chloroapatite derived from sardine by-products. Ceram. Int. 40, 13231–13240 (2014). https://doi.org/10.1016/j.ceramint.2014.05.030
Prabakaran, K., Rajeswari, S.: Development of hydroxyapatite from natural fish bone through heat treatment. Trends Biomater. Artif. Organs 20, 20–23 (2006)
Sunil, B.R., Jagannatham, M.: Producing hydroxyapatite from fish bones by heat treatment. Mater. Lett. 185, 411–414 (2016). https://doi.org/10.1016/j.matlet.2016.09.039
Venkatesan, J., Qian, Z.J., Ryu, B., Thomas, N.V., Kim, S.K.: A comparative study of thermal calcination and an alkaline hydrolysis method in the isolation of hydroxyapatite from Thunnus obesus bone. Biomed. Mater. 6, 035003 (2011). https://doi.org/10.1088/1748-6041/6/3/035003
Mondal, S., Mahata, S., Kundu, S., Mondal, B.: Processing of natural resourced hydroxyapatite ceramics from fish scale. Adv. Appl. Ceram. 109, 234–239 (2010). https://doi.org/10.1179/174367613X13789812714425
Venkatesan, J., Lowe, B., Manivasagan, P., Kang, K.H., Chalisserry, E.P., Anil, S., Kim, D.G., Kim, S.K.: Isolation and characterization of nano-hydroxyapatite from salmon fish bone. Materials 8, 5426–5439 (2015). https://doi.org/10.3390/ma8085253
Pon-On, W., Suntornsaratoon, P., Charoenphandhu, N., Thongbunchoo, J., Krishnamra, N., Tang, I.M.: Hydroxyapatite from fish scale for potential use as bone scaffold or regenerative material. Mater. Sci. Eng., C 62, 183–189 (2016). https://doi.org/10.1016/j.msec.2016.01.051
Muhammad, N., Gao, Y., Iqbal, F., Ahmad, P., Ge, R., Nishan, U., Rahim, A., Gonfa, G., Ullah, Z.: Extraction of biocompatible hydroxyapatite from fish scales using novel approach of ionic liquid pretreatment. Sep. Purif. Technol. 161, 129–135 (2016). https://doi.org/10.1016/j.seppur.2016.01.047
Kongsri, S., Janpradit, K., Buapa, K., Techawongstien, S., Chanthai, S.: Nanocrystalline hydroxyapatite from fish scale waste: preparation, characterization and application for selenium adsorption in aqueous solution. Chem. Eng. J. 215–216, 522–532 (2013). https://doi.org/10.1016/j.cej.2012.11.054
Mondal, S., Mondal, B., Dey, A., Mukhopadhyay, S.S.: Studies on processing and characterization of hydroxyapatite biomaterials from different bio wastes. J. Miner. Mater. Charact. Eng. 11, 55–67 (2012). https://doi.org/10.4236/jmmce.2012.111005
Panda, N.N., Pramanik, K., Sukla, L.B.: Extraction and characterization of biocompatible hydroxyapatite from fresh water fish scales for tissue engineering scaffold. Bioprocess Biosyst. Eng. 37, 433–440 (2014). https://doi.org/10.1007/s00449-013-1009-0
Haider, A., Haider, S., Han, S.S., Kang, I.K.: Recent advances in the synthesis, functionalization and biomedical applications of hydroxyapatite: a review. RSC Adv. 7, 7442–7458 (2017)
Adeoti, I.A., Hawboldt, K.: A review of lipid extraction from fish processing by-product for use as a biofuel. Biomass Bioenergy 63, 330–340 (2014). https://doi.org/10.1016/j.biombioe.2014.02.011
Rubio-Rodríguez, N., Beltrán, S., Jaime, I., de Diego, S.M., Sanz, M.T., Carballido, J.R.: Production of omega-3 polyunsaturated fatty acid concentrates: a review. Innov. Food Sci. Emerg. Technol. 11, 1–12 (2010). https://doi.org/10.1016/j.ifset.2009.10.006
Khoddami, A.: Quality and fatty acid profile of the oil extracted from fish waste (head, intestine and liver) (Euthynnus affinis). Afr. J. Biotechnol. (2012). https://doi.org/10.5897/ajb10.1699
Rodríguez, C., Acosta, C., Badía, P., Cejas, J.R., Santamaría, F.J., Lorenzo, A.: Assessment of lipid and essential fatty acids requirements of black seabream (Spondyliosoma cantharus) by comparison of lipid composition in muscle and liver of wild and captive adult fish. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 139, 619–629 (2004)
Bonilla, J.R., Hoyos Concha, J.L.: Métodos de extracción, refinación y concentración de aceite de pescado como fuente de ácidos grasos omega-3. Cienc. y Tecnol. Agropecu. 19, 645–668 (2018). https://doi.org/10.21930/rcta.vol19_num2_art:684
Food and Agriculture Organization of the United Nations: The Production of Fish Meal and Oil. Food and Agriculture Organization of the United Nations, Rome (1986)
Thomas, A., Matthäus, B., Fiebig, H.: Ullmann’s Encyclopedia of Industrial Chemistry, Vol. 14, pp. 1–70. Fats and fatty oils, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim (2015)
Lee, J.Y., Yoo, C., Jun, S.Y., Ahn, C.Y., Oh, H.M.: Comparison of several methods for effective lipid extraction from microalgae. Bioresour. Technol. 101, S75–S77 (2010). https://doi.org/10.1016/j.biortech.2009.03.058
Halim, R., Danquah, M.K., Webley, P.A.: Extraction of oil from microalgae for biodiesel production: a review. Biotechnol. Adv. 30, 709–732 (2012). https://doi.org/10.1016/j.biotechadv.2012.01.001
Mouahid, A., Crampon, C., Toudji, S.-A.A., Badens, E.: Supercritical CO2 extraction of neutral lipids from microalgae: experiments and modelling. J. Supercrit. Fluids 77, 7–16 (2013). https://doi.org/10.1016/j.supflu.2013.01.024
Hathwar, S.C., Bijinu, B., Rai, A.K., Narayan, B.: Simultaneous recovery of lipids and proteins by enzymatic hydrolysis of fish industry waste using different commercial proteases. Appl. Biochem. Biotechnol. 164, 115–124 (2011). https://doi.org/10.1007/s12010-010-9119-5
Qi-Yuan, L., Jun-Qing, Q., Xiao-Ge, W.: Optimization of enzymatic fish oil extraction from mackerel viscera by response surface methodology. Int. Food Res. J. 23, 992–997 (2016)
Fernández-Lorente, G., Betancor, L., Carrascosa, A.V., Guisán, J.M.: Release of omega-3 fatty acids by the hydrolysis of fish oil catalyzed by lipases immobilized on hydrophobic supports. JAOCS 88, 1173–1178 (2011). https://doi.org/10.1007/s11746-011-1776-1
Beheshti, S.: A study on the fatty acid composition of fish liver oil from two marine fish. Turk. J. Chem. 27, 251–258 (2003)
Christie, W.W., Han, X.: Lipid Analysis-Isolation, Separation, Identification and Lipidomic Analysis [Book Review]. Woodhead Publishing, Cambridge (2010)
Antolín, E.M., Delange, D.M., Canavaciolo, V.G.: Evaluation of five methods for derivatization and GC determination of a mixture of very long chain fatty acids (C24:0-C36:0). J. Pharm. Biomed. Anal. 46, 194–199 (2008). https://doi.org/10.1016/j.jpba.2007.09.015
Guil-Guerrero, J.L., Venegas-Venegas, E., Rincón-Cervera, M.Á., Suárez, M.D.: Fatty acid profiles of livers from selected marine fish species. J. Food Compos. Anal. 24, 217–222 (2011). https://doi.org/10.1016/j.jfca.2010.07.011
Van Wychen, S., Laurens, L.M.L.: Determination of total lipids as fatty acid methyl esters (FAME) by in situ transesterification. Contract 303, 275–3000 (2013)
Suganya, T., Kasirajan, R., Renganathan, S.: Ultrasound-enhanced rapid in situ transesterification of marine macroalgae Enteromorpha compressa for biodiesel production. Bioresour. Technol. 156, 283–290 (2014). https://doi.org/10.1016/j.biortech.2014.01.050
Dodds, E.D., McCoy, M.R., Rea, L.D., Kennish, J.M.: Gas chromatographic quantification of fatty acid methyl esters: flame ionization detection vs. electron impact mass spectrometry. Lipids 40, 419–428 (2005). https://doi.org/10.1007/s11745-006-1399-8
Aursand, M., Gribbestad, I.S., Martinez, I.: Omega-3 fatty acid content of intact muscle of farmed Atlantic Salmon (Salmo salar) examined by 1 H MAS NMR spectroscopy. Mod. Magn. Reson. 1, 941–945 (2008). https://doi.org/10.1007/978-3-319-28275-6_80-1.pdf
Nilsang, S., Lertsiri, S., Suphantharika, M., Assavanig, A.: Optimization of enzymatic hydrolysis of fish soluble concentrate by commercial proteases. J. Food Eng. 70, 571–578 (2005). https://doi.org/10.1016/j.jfoodeng.2004.10.011
Samaranayaka, A.G.P., Li-Chan, E.C.Y.: Autolysis-assisted production of fish protein hydrolysates with antioxidant properties from Pacific hake (Merluccius productus). Food Chem. 107, 768–776 (2008). https://doi.org/10.1016/j.foodchem.2007.08.076
Prabha, J., Narikimelli, A., Sajini, M.I., Vincent, S.: Optimization for autolysis assisted production of fish protein hydrolysate from underutilized fish Pellona ditchela. Int. J. Sci. Eng. Res. 4, 1863–1869 (2013)
dos Santos, S.D., Martins, V.G., Salas-Mellado, M., Prentice, C.: Evaluation of functional properties in protein hydrolysates from bluewing searobin (Prionotus punctatus) obtained with different microbial enzymes. Food Bioprocess Technol. 4, 1399–1406 (2011). https://doi.org/10.1007/s11947-009-0301-0
Galla, N.R., Karakala, B., Akula, S., Pamidighantam, P.R.: Physico-chemical, amino acid composition, functional and antioxidant properties of roe protein concentrates obtained from Channa striatus and Lates calcarifer. Food Chem. 132, 1171–1176 (2012). https://doi.org/10.1016/J.FOODCHEM.2011.11.055
Jemil, I., Abdelhedi, O., Mora, L., Nasri, R., Aristoy, M.-C., Jridi, M., Hajji, M., Toldrá, F., Nasri, M.: Peptidomic analysis of bioactive peptides in zebra blenny (Salaria basilisca) muscle protein hydrolysate exhibiting antimicrobial activity obtained by fermentation with Bacillus mojavensis A21. Process Biochem. 51, 2186–2197 (2016). https://doi.org/10.1016/J.PROCBIO.2016.08.021
Wald, M., Schwarz, K., Rehbein, H., Bußmann, B., Beermann, C.: Detection of antibacterial activity of an enzymatic hydrolysate generated by processing rainbow trout by-products with trout pepsin. Food Chem. 205, 221–228 (2016). https://doi.org/10.1016/J.FOODCHEM.2016.03.002
Egerton, S., Culloty, S., Whooley, J., Stanton, C., Ross, R.P.: Characterization of protein hydrolysates from blue whiting (Micromesistius poutassou) and their application in beverage fortification. Food Chem. 245, 698–706 (2018). https://doi.org/10.1016/J.FOODCHEM.2017.10.107
Acknowledgements
This work was supported by FCT-Fundação para a Ciência e a Tecnologia (projectPEst-OE/QUI/UI0674/2019, CQM, Portuguese Government funds), and through Madeira 14–20 Program, project PROEQUIPRAM—Reforço do Investimento em Equipamentos e Infraestruturas Científicas na RAM (M1420-01-0145-FEDER-000008) and by ARDITI-Agência Regional para o Desenvolvimento da Investigação Tecnologia e Inovação, through the project M1420-01-0145-FEDER-000005—Centro de Química da Madeira—CQM+ (Madeira 14-20). The work was also performed in the frame of project MarineBlueRefine PROCiência2020 (Portaria nº 371/2015, de 16/12), M1420-01-0247-FEDER-000006; Pedro Ideia is the recipient of a PhD Grant under the project M1420-09-5369-FSE-000001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The author declares no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ideia, P., Pinto, J., Ferreira, R. et al. Fish Processing Industry Residues: A Review of Valuable Products Extraction and Characterization Methods. Waste Biomass Valor 11, 3223–3246 (2020). https://doi.org/10.1007/s12649-019-00739-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-019-00739-1