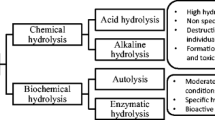
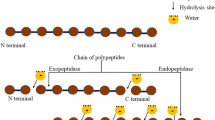
Abstract
Fish wastes offer tremendous unexploited potential for value adding for such materials, collagen, gelatin, and hydrolysate. Additionally, fish waste may be suitable for the production of bioactive peptides that exhibit strong antihypertensive, antioxidative, and anticancer activities. This paper reviews the production of purified bioactive peptides from various fish waste protein hydrolysates via enzymatic hydrolysis after enzyme screening and optimization. The purification of bioactive peptides is carried out using ultra- and gel filtration as well as the RP-HPLC method. Purified peptide characterizations in terms of molecular weight, amino acid sequence, and composition are also provided, illustrating that peptides with low molecular weight and short amino acid sequences are more potent as bioactive peptides. Hence, further studies are encouraged to examine the bioactivity and bioavailability of protein peptides derived from fish wastes, especially those with anticancer characteristics.

Similar content being viewed by others
References
Ahn, C. B., Je, J. Y., & Cho, Y. S. (2012). Antioxidant and anti-inflammatory peptide fraction from salmon byproduct protein hydrolysates by peptic hydrolysis. Food Research International, 49(1), 92–98.
Alemán, A., Gómez-Guillén, M. C., & Montero, P. (2013). Identification of ace-inhibitory peptides from squid skin collagen after in vitro gastrointestinal digestion. Food Research International, 54, 790–795.
Balti, R., Nedjar-Arroume, N., Bougatef, A., Guillochon, D., & Nasri, M. (2010). Three novel angiotensin I-converting enzyme (ACE) inhibitory peptides from cuttlefish (Sepiaofficinalis) using digestive proteases. Food Research International, 43, 1136–1143.
Barbana, C., & Boye, J. I. (2011). Angiotensin I-converting enzyme inhibitory properties of lentil protein hydrolysates: determination of the kinetics of inhibition. Food Chemistry, 127, 94–101.
Ben-Tal, A., & Nemirovski, A. (2002). Robust optimization—methodology and applications. Mathematical Programming, Ser. B, 92, 453–480.
Bhaskar, N., & Mahendrakar, N. S. (2008). Protein hydrolysate from visceral waste proteins of Catla (Catla catla): optimization of hydrolysis conditions for a commercial neutral protease. Bioresource Technology, 99(10), 4105–4111.
Brown, N. J., & Vaughan, D. E. (1998). Angiotensin-converting enzyme inhibitors. Circulation, 97(14), 1411–1420.
Cai, L., Wu, X., Zhang, Y., Li, X., Ma, S., & Li, J. (2015). Purification and characterization of three antioxidant peptides from protein hydrolysate of grass carp (Ctenopharyngodon idella) skin. Journal of Functional Food, 16, 234–242.
Cao, W., Zhang, C., Ji, H., & Hao, J. (2012). Optimization of peptic hydrolysis parameters for the production of angiotensin I-converting enzyme inhibitory hydrolysate from Acetes chinensis through Plackett–Burman and response surface methodological approaches. Journal of Science Food Agriculture, 92, 42–48.
Castro-Ceseña, A. B., Sánchez-Saavedra, M. D. P., & Márquez-Rocha, F. J. (2012). Characterisation and partial purification of proteolytic enzymes from sardine by-products to obtain concentrated hydrolysates. Food Chemistry, 135, 583–589.
Chalamaiah, M., Dinesh, B., Hemalatha, R., & Jyothirmayi, T. (2012). Fish protein hydrolysates: proximate composition, amino acid composition, antioxidant activities and applications: a review. Food Chemistry, 135(4), 3020–3038.
Chalamaiah, M., Hemalatha, M. D., Jyothirmayi, T., Diwan, P. V., Bhaskarachary, K., Vajreswari, A., Ramesh Kumar, R., & Dinesh Kumar, B. (2015). Chemical composition and immunomodulatory effects of enzymatic protein hydrolysates from common carp (Cyprinus carpio) egg. Nutrition, 31, 388–398.
Chen, J., Wang, Y., Zhong, Q., Wu, Y., & Xia, W. (2012). Purification and characterization of a novel angiotensin-I converting enzyme (ACE) inhibitory peptide derived from enzymatic hydrolysate of grass carp protein. Peptides, 33, 52–58.
Chen, J., Liu, S., Ye, R., Cai, G., Ji, B., & Wu, Y. (2013). Angiotensin-I converting enzyme (ACE) inhibitory tripeptides from rice protein hydrolysate: purification and characterization. Journal of Functional Foods, 5(4), 1684–1692.
Chi, C. F., Wang, B., Hu, F. Y., Wang, Y. M., Zhang, B., Deng, S. G., & Wu, C. W. (2015). Purification and identification of three novel antioxidant peptides from protein hydrolysate of bluefin leatherjacket (Navodon septentrionalis) skin. Food Research International, 73, 124–129.
Diniz, F. M., & Martin, A. M. (1997). Effects of the extent of enzymatic hydrolysis on functional properties of shark protein hydrolysate. LWT - Food Science and Technology, 30(3), 266–272.
Fahmi, A., Morimura, S., Guo, H. C., Shigematsu, T., Kida, K., & Uemura, Y. (2004). Production of angiotensin I converting enzyme inhibitory peptides from sea bream scales. Process Biochemistry, 39(10), 1195–1200.
Fang, X., Xie, N., Chen, X., Yu, H., & Chen, J. (2012). Optimization of antioxidant hydrolysate production from flying squid muscle protein using response surface methodology. Food and Bioproducts Processing, 90, 676–682.
FAO. (2014). The state of world fisheries and aquaculture. Rome: Food and Agriculture Organization of the United Nations http://www.fao.org/3/a-i3720e.pdf.
FAO (2016). Global aquaculture production (fish stat). http://www.fao.org/3/a-i5555e.pdf.
FitzGerald, R. J., & Meisel, H. (2000). Milk protein-derived peptide inhibitors of angiotensin-I-converting enzyme. The British Journal of Nutrition, 84(Suppl 1), 33–37.
Forghani, B., Ebrahimpour, A., Bakar, J., Hamid, A. A., Hassan, Z., & Saari, N. (2012). Enzyme hydrolysates from Stichopus horrens as a new source for angiotensin-converting enzyme inhibitory peptides. Corp. Evidence- Based Comp. Alt. Med., 1, 1–9.
Fujita, H., Yamagami, T., & Ohshima, K. (2001). Effects of an ace-inhibitory agent, katsuobushi oligopeptide, in the spontaneously hypertensive rat and in borderline and mildly hypertensive subjects. Nutrition Research, 21, 1149–1158.
Galla, N. R., Pamidighantam, P. R., Akula, S., & Karakala, B. (2012). Functional properties and in vitro antioxidant activity of roe protein hydrolysates of Channa striatus and Labeo rohita. Food Chemistry, 135, 1479–1484.
Gbogouri, G. A., Linder, M., Fanni, J., & Parmentier, M. (2004). Influence of hydrolysis degree on the functional properties of salmon byproduct hydrolysates. Journal of Food Science, 69, 615–622.
Ghassem, M., Arihara, K., Babji, A. S., Said, M., & Ibrahim, S. (2011). Purification and identification of ACE inhibitory peptides from Haruan (Channa striatus) myofibrillar protein hydrolysate using HPLC–ESI-TOF MS/MS. Food Chemistry, 129(4), 1770–1777.
Girgih, A. T., He, R., Hasan, F. M., Udenigwe, C. C., Gill, T. A., & Aluko, R. E. (2015). Evaluation of the in vitro antioxidant properties of a cod (Gadus morhua) protein hydrolysate and peptide fractions. Food Chemistry, 173, 652–659.
Gu, R. Z., Li, C. Y., Liu, W. Y., Yi, W. X., & Cai, M. Y. (2011). Angiotensin I-converting enzyme inhibitory activity of low-molecular-weight peptides from Atlantic salmon (Salmo salar L.) skin. Food Research International, 44(5), 1536–1540.
Hamid, S. A., Halim, N. R. A., & Sarbon, N. M. (2015). Optimization of enzymatic hydrolysis conditions of Golden Apple snail (Pomacea canaliculata) protein by Alcalase. International Food Research Journal, 22(4), 1615–1623.
Harnedy, A., & Fitzgerald, R. J. (2012). Bioactive peptides from marine processing waste and shellfish: a review. Journal of Functional Foods, 4, 6–24.
Herman, R., Gao, Y., & Storer, N. (2006). Acid-induced unfolding kinetics in simulated gastric digestion of proteins. Regulatory Toxicology and Pharmacology, 46(1), 93–99.
Hernández-Ledesma, B., Contreras, M. M., & Recio, I. (2011). Antihypertensive peptides: production, bioavailability and incorporation into foods. Advances in Colloid and Interface Science., 165, 23–35.
Herpandi, N. H., Rosma, A., & Wan Nadiah, W. A. (2011). The tuna fishing industry: a new outlook on fish protein hydrolysates. Comprehensive Reviews in Food Science and Food Safety, 10(4), 195–207.
Himaya, S. W. A., Ngo, D. H., Ryu, B., & Kim, S. K. (2012). An active peptide purified from gastrointestinal enzyme hydrolysate of Pacific cod skin gelatin attenuates angiotensin-1 converting enzyme (ACE) activity and cellular oxidative stress. Food Chemistry, 132(4), 1872–1882.
Hou, H., Li, B., Zhao, X., Zhang, Z., & Li, P. (2011). Optimization of enzymatic hydrolysis of Alaska pollock frame for preparing protein hydrolysates with low-bitterness. LWT - Food Science and Technology, 44(2), 421–428.
Huang, F. F., Ding, G. F., Yang, Z. S., Yu, D., & Yang, Y. F. (2011). Anticancer activity of an oligopeptide isolated from hydrolysates of sepia ink. Chinese Journal of Natural Medicines, 9(2), 151–155.
Huo, J., & Zhao, Z. (2009). Study on enzymatic hydrolysis of Gadus morrhua skin collagen and molecular weight distribution of hydrolysates. Agricultural Sciences in China, 8(6), 723–729.
Intarasirisawat, R., Benjakul, S., Visessanguan, W., & Wu, J. (2012). Antioxidative and functional properties of protein hydrolysate from defatted skipjack (Katsuwonous pelamis) roe. Food Chemistry, 135(4), 3039–3048.
Intarasirisawat, R., Benjakul, S., Wu, J., & Visessanguan, W. (2013). Isolation of antioxidative and ACE inhibitory peptides from protein hydrolysate of skipjack (Katsuwana pelamis) roe. Journal of Functional Foods, 5(4), 1854–1862.
Ishak, N. H., & Sarbon, N. M. (2016). Optimization of the enzymatic hydrolysis conditions of waste from shortfin scad (Decapterus macrosoma) for the production of angiotensin I- converting enzyme (ACE) inhibitory peptide using response surface methodology. International Food Research Journal, in press.
Je, J. Y., Lee, K. H., Lee, M. H., & Ahn, C. B. (2009). Antioxidant and antihypertensive protein hydrolysates produced from tuna liver by enzymatic hydrolysis. Food Research International, 42(9), 1266–1272.
Jeon, Y. J., Byun, H. G., & Kim, S. K. (1999). Improvement of functional properties of cod frame protein hydrolysates using ultrafiltration membranes. Process Biochemistry, 35(5), 471–478.
Jumeri, & Kim, S. M. (2011). Antioxidant and anticancer activities of enzymatic hydrolysates of solitary tunicate (Styela clava). Food Science and Biotechnology, 20(4), 1075–1085.
Jung, W., Mendis, E., Je, J., Park, P., Son, B. W., Kim, H. C., Choi, Y. K., & Kim, S. (2006). Angiotensin I-converting enzyme inhibitory peptide from yellowfin sole (Limanda aspera) frame protein and its antihypertensive effect in spontaneously hypertensive rats. Food Chemistry, 94, 26–32.
Karnjanapratum, S., & Benjakul, S. (2014). Glycyl endopeptidase from papaya latex: partial purification and use for production of fish gelatin hydrolysate. Food Chemistry, 165, 403–411.
Khiari, Z., Rico, D., Martin Diana, A. B., & Barry Ryan, C. (2013). Comparison between gelatines extracted from mackerel and blue whiting bones after different pre-treatments. Food Chemistry, 139(1–4), 347–354.
Kim, H. J., Park, K. H., Shin, J. H., Lee, J. S., Heu, M. S., Lee, D. H., & Kim, J. S. (2011). Antioxidant ACE inhibiting activities of the rockfish Sebastes hubbsi skin gelatin hydrolysates produced by sequential two-step enzymatic hydrolysis. Fisheries and Aquatic Science, 14, 1–10.
Kim, E. K., Kim, Y. S., Hwang, J. W., Lee, J. S., Moon, S. H., Jeon, B. T., & Park, P. J. (2013). Purification and characterization of a novel anticancer peptide derived from Ruditapes philippinarum. Process Biochemistry, 48(7), 1086–1090.
Kittiphattanabawon, P., Benjakul, S., Visessanguan, W., & Shahidi, F. (2012). Gelatin hydrolysate from blacktip shark skin prepared using papaya latex enzyme: Antioxidant activity and its potential in model systems. Food Chemistry, 135, 1118–1126.
Klompong, V., Benjakul, S., Kantachote, D., Hayes, K. D., & Shahidi, F. (2008). Comparative study on antioxidative activity of yellow stripe trevally protein hydrolysate produced from Alcalase and Flavourzyme. International Journal of Food Science and Technology, 43, 1019–1026.
Kristinsson, H. G., & Rasco, B. A. (2000). Fish protein hydrolysates: production, biochemical, and functional properties. Critical Reviews in Food Science and Nutrition, 40(1), 43–81.
Lee, J. K., Hong, S., Jeon, J. K., Kim, S. K., & Byun, H. G. (2009). Purification and characterization of angiotensin I converting enzyme inhibitory peptides from the rotifer, Brachionus rotundiformis. Bioresource Technology, 100(21), 5255–5259.
Lee, S. H., Qian, Z. J., & Kim, S. K. (2010). A novel angiotensin I converting enzyme inhibitory peptide from tuna frame protein hydrolysate and its antihypertensive effect in spontaneously hypertensive rats. Food Chemistry, 118, 96–102.
Lee, J. K., Jeon, J. K., & Byun, H. G. (2011). Effect of angiotensin I converting enzyme inhibitory peptide purified from skate skin hydrolysate. Food Chemistry, 125(2), 495–499.
Leng, B., Liu, X. D., & Chen, Q. X. (2005). Inhibitory effects of anticancer peptide from Mercenaria on the BGC-823 cells and several enzymes. Federation of European Biochemical Societies, 579, 1187–1190.
Li, Z., Wang, B., Chi, C., Gong, Y., Luo, H., & Ding, G. (2013). Influence of average molecular weight on antioxidant and functional properties of cartilage collagen hydrolysates from Sphyrna lewini, Dasyatis akajei and Raja porosa. Food Research International, 51, 283–293.
Li-Chan, E. C. (2015). Bioactive peptides and protein hydrolysates: Research trends and challenges for application as nutraceuticals and functional food ingredients. Current Opinion in Food Science, 1, 28–37.
Mahmoodani, F., Ghassem, M., Babji, A. S., Yusop, S. M., & Khosrokhavar, R. (2014). ACE inhibitory activity of pangasius catfish (Pangasius sutchi) skin and bone gelatin hydrolysate. Journal of Food Science and Technology, 51(9), 1847–1856.
Mendis, E., Rajapakse, N., & Kim, S. K. (2005). Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. Journal of Agricultural and Food Chemistry, 53, 581–587.
Najafian, L., & Babji, A. S. (2012). A review of fish-derived antioxidant and antimicrobial peptides: their production, assessment, and applications. Peptides, 33(1), 178–185.
Ngo, D. H., Qian, Z. J., Ryu, B., Park, J. W., & Kim, S. K. (2010). In vitro antioxidant activity of a peptide isolated from Nile tilapia (Oreochromis niloticus) scale gelatin in free radical-mediated oxidative systems. Journal of Functional Foods, 2, 107–117.
Ngo, D. H., Ryu, B., & Kim, S. K. (2014). Active peptides from skate (Okamejei kenojei) skin gelatin diminish angiotensin-I converting enzyme activity and intracellular free radical-mediated oxidation. Food Chemistry, 143, 246–255.
Ovissipour, M., Abedian, A., Motamedzadegan, A., Rasco, B., Safari, R., & Shahiri, H. (2009). The effect of enzymatic hydrolysis time and temperature on the properties of protein hydrolysates from Persian sturgeon (Acipenser persicus) viscera. Food Chemistry, 115(1), 238–242.
Phanturat, P., Benjakul, S., Visessanguan, W., & Roytrakul, S. (2010). Use of pyloric caeca extract from bigeye snapper (Priacanthus macracanthus) for the production of gelatin hydrolysate with antioxidative activity. LWT - Food Science and Technology, 43(1), 86–97.
Picot, L., Ravallec, R., Fouchereau-Perón, M., Vandanjon, L., Jaouen, P., Chaplain-Derouiniot, M., Guérard, F., Chabeaud, A., Legal, Y., Alvarez, O. M., Bergé, J. P., Piot, J. M., Batista, I., Pires, C., Thorkelsson, G., Delannoy, C., Jakobsen, G., Johansson, I., & Bourseau, P. (2010). Impact of ultrafiltration and nanofiltration of an industrial fish protein hydrolysate on its bioactive properties. Journal of the Science of Food and Agriculture, 90(11), 1819–1826.
Rai, C. (2013). Purification and characterization of ace inhibitory peptide from aquatic resources: a review. Internatioanal Journal of Plant, Animal and Enviromental Sciences, 3(1), 220–233.
Razali, A. N., Amin, A. M., & Sarbon, N. M. (2015). Antioxidant activity and functional properties of fractionated cobia skin gelatin hydrolysate at different molecular weight. International Food Research Journal, 22(2), 651–660.
Ren, J. Y., Zhao, M. M., Shi, J., Wang, J. S., Jiang, Y. M., Cui, C., Kakuda, Y., & Xue, S. J. (2008). Optimization of antioxidant peptide production from grass carp sarcoplasmic protein using response surface methodology. Food Science and Technology, 41, 1624–1632.
Roslan, J., Mustapa Kamal, S. M., Yunos, K. F., & Abdullah, N. (2015). Optimization of enzymatic hydrolysis of tilapia (Oreochromis niloticus) by-product using response surface methodology. International Food Research Journal, 22(3), 1117–1123.
Saidi, S., Deratani, A., Belleville, M. P., & Ben Amar, R. (2014). Production and fractionation of tuna by-product protein hydrolysate by ultrafiltration and nanofiltration: impact on interesting peptides fractions and nutritional properties. Food Research International, 65, 453–461.
Sampath Kumar, N. S., Nazeer, R. A., & Jaiganesh, R. (2012). Purification and identification of antioxidant peptides from the skin protein hydrolysate of two marine fishes, horse mackerel (Magalaspis cordyla) and croaker (Otolithes ruber). Amino Acids, 42(5), 1641–1649.
Sarmadi, B. H., & Ismail, A. (2010). Antioxidative peptides from food proteins: a review. Peptides, 31(10), 1949–1956.
See, S. F., Hoo, L. L., & Babji, A. S. (2011). Optimization of enzymatic hydrolysis of Salmon (Salmo salar) skin by Alcalase. International Food Research Journal, 18(4), 1359–1365.
Senphan, T., & Benjakul, S. (2014). Antioxidative activities of hydrolysates from seabass skin prepared using protease from hepatopancreas of Pacific white shrimp. Journal of Functional Foods, 6, 147–156.
Shim, Y. S., Yoon, W. J., Ha, J., Seo, D., Lee, K. W., Lee, W. Y., et al. (2013). Method validation of 16 types of structural amino acids using an automated amino acid analyzer. Food Science and Biotechnology, 22(6), 1567–1571.
Udenigwe, C. C., & Aluko, R. E. (2012). Food protein-derived bioactive peptides: production, processing, and potential health benefits. Journal of Food Science, 77(1), 11–24.
Umayaparvathi, S., Meenakshi, S., Vimalraj, V., Arumugam, M., Sivagami, G., & Balasubramanian, T. (2014). Antioxidant activity and anticancer effect of bioactive peptide from enzymatic hydrolysate of oyster (Saccostrea cucullata). Biomedicine and Preventive Nutrition, 4, 343–353.
Vohra, A., & Satyanarayana, T. (2002). Statistical optimization of the medium components by response surface methodology to enhance phytase production by Pichia anomala. Process Biochemistry, 37(9), 999–1004.
Wang, G. G., & Shan, S. (2011). Review of metamodeling techniques for product design with computation intensive processes. Proc. Can. Eng., 1–11.
Wang, B., Li, L., Chi, C. F., Ma, J. H., Luo, H. Y., & Xu, Y. F. (2013). Purification and characterisation of a novel antioxidant peptide derived from blue mussel (Mytilus edulis) protein hydrolysate. Food Chemistry, 138, 1713–1719.
Weng, W., Tang, L., Wang, B., Chen, J., Su, W., Osako, K., & Tanaka, M. (2014). Antioxidant properties of fractions isolated from blue shark (Prionace glauca) skin gelatin hydrolysates. Journal of Functional Foods, 11, 342–351.
Wiriyaphan, C., Chitsomboon, B., & Yongsawadigul, J. (2012). Antioxidant activity of protein hydrolysates derived from threadfin bream surimi byproducts. Food Chemistry, 132(1), 104–111.
Wisuthiphaet, N., & Kongruang, S. (2015). Production of fish protein hydrolysate by acid and enzymatic hydrolysis. Journal of Medical and Bioengineering, 4(6), 466–470.
Wu, H., He, H. L., Chen, X. L., Sun, C. Y., Zhang, Y. Z., & Zhou, B. C. (2008). Purification and identification of novel angiotensin-I-converting enzyme inhibitory peptides from shark meat hydrolysate. Process Biochemistry, 43(4), 457–461.
Zhang, Y., Duan, X., & Zhuang, Y. (2012). Purification and characterization of novel antioxidant peptides from enzymatic hydrolysates of tilapia (Oreochromis niloticus) skin gelatin. Peptides, 38(1), 13–21.
Zhao, Y., Li, B., Dong, S., Liu, Z., Zhao, X., Wang, J., & Zeng, M. (2009). A novel ACE inhibitory peptide isolated from Acaudina molpadioidea hydrolysate. Peptides, 30, 1028–1033.
Acknowledgments
With sincere appreciation, the authors gratefully acknowledge the Ministry of Higher Education for its financial support through the Fundamental Research Grant Scheme (FRGS/1/2014/59337), as well as the Universiti Malaysia Terengganu for its laboratory facilities and technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ishak, N., Sarbon, N. A Review of Protein Hydrolysates and Bioactive Peptides Deriving from Wastes Generated by Fish Processing. Food Bioprocess Technol 11, 2–16 (2018). https://doi.org/10.1007/s11947-017-1940-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-017-1940-1