Abstract
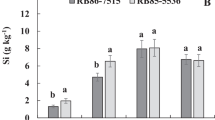
Silicon (Si) has been associated with minimizing water deficit damage in several plants, but its effects on physiological aspects and gas exchange responses for sugarcane during the ripening phase are still scarce. Therefore, this study aimed to determine whether Si fertilization enhances physiological and gas exchange responses, biomass and sugar production in response to water deficit during the ripening phase of sugarcane, and whether these responses are similar in drought-tolerant and drought-sensitive cultivars. A greenhouse experiment was conducted using two sugarcane cultivars (RB855536 is drought-sensitive; RB867515 is drought-tolerant) without and with Si (0 = zero and 1000 kg−1 Si; -Si and + Si) under well-watered or water deficit conditions at the ripening phase. The biometric, physiological, and gas exchange measurements were independently influenced by the water deficit, cultivar, and Si. The leaf Si concentration, chlorophyll a (Chla), carotenoids, net CO2 assimilation rate (A), electron transport rate (ETR), plant transpiration (E), stomatal conductance (gs), and sugar content in stalks increased with Si, and electrolyte leakage (EL), plant length, leaf area (LA), and stalk fresh biomass decreased. Water deficit decreased the leaf water potential (ψW), fresh biomass of leaves, and LA. RB867515 showed higher LA, plant length, and fresh leaves biomass, while RB855536 showed superior leaf Si, Chla, Chlb, carotenoids, A, ETR, E, sugar content, and EL and lower ψW. Si fertilization promotes enhancement in some physiological and gas exchange aspects and increased sugar contents in stalks, independent of the water deficit at ripening phase or the drought tolerance of sugarcane cultivars.
Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
Abbreviations
- Chl a :
-
Chlorophyll a
- Chl b :
-
Chlorophyll b
- LA :
-
Leaf area
- EL :
-
Electrolyte leakage
- FW :
-
Fresh weight
- RWC :
-
Relative water content
- TVD :
-
Top visible dewlap
- WD :
-
Water deficit
- WW :
-
Well-watered
- Ψw :
-
Leaf water potential
References
Azevedo RA, Carvalho RF, Cia MC, Gratão P (2011) Sugarcane under pressure: an overview of biochemical and physiological studies of abiotic stress. Tropical Plant Biol 4:42–51. https://doi.org/10.1007/s12042-011-9067-4
Boaretto LF, Carvalho G, Borgo L, Creste L, Landell MGA, Mazzafera P, Azevedo RA (2014) Water stress reveals differential antioxidant responses of tolerant and non-tolerant sugarcane genotypes. Plant Physiol Biochem 74:165–175. https://doi.org/10.1016/j.plaphy.2013.11.016
Ferreira THS, Tsunada MS, Bassi D, Araújo P, Mattiello L, Guidelli GV, Righetto GL, Gonçalves VR, Lakshmanan P, Menossi M (2017) Sugarcane water stress tolerance mechanisms and its implications on developing biotechnology solutions. Front Plant Sci 8:1–18. https://doi.org/10.3389/fpls.2017.01077
Bezerra BKL, Lima GPP, Reis AR, Silva MA, Camargo MS (2019) Physiological and biochemical impacts of silicon against water deficit in sugarcane. Acta Physiol Plant 41:189. https://doi.org/10.1007/s11738-019-2980-0
Rizwan M, Ali S, Ibrahim M, Farid M, Adrees M, Bharwana SA, Zia-Ur-Rehman M, Qayyum MF, Abbas F (2014) Mechanisms of silicon-mediated alleviation of drought and salt stress in plants: a review. Environ Sci Pollut Res 22:15416–15431. https://doi.org/10.1007/s11356-015-5305-x
Medeiros DB, Silva EC, Nogueira RJMC, Teixeira MM, Buckeridge MS (2013) Physiological limitations in two sugarcane varieties under water suppression and after recovering. Theor Exp Plant Physiol 25:213–222. https://doi.org/10.1590/S2197-00252013000300006
Vital CE, Giordano A, Almeida Soares E, Rhys Williams TC, Mesquita RO, Vidigal PMP, Santana Lopes A, Pacheco TG, Rogalski M, Oliveira Ramos HJ, Loureiro ME (2017) An integrative overview of the molecular and physiological responses of sugarcane under drought conditions. Plant Mol Biol 94:577–594. https://doi.org/10.1007/s11103-017-0611-y
Ramesh P (2000) Effect of different levels of drought during the formative phase on growth parameters and its relationship with dry matter accumulation in sugarcane. J Agron Crop Sci 185:83–89. https://doi.org/10.1046/j.1439-037x.2000.00404.x
Inman-Bamber NG, Smith DM (2005) Water relations in sugarcane and response to water deficits. Field Crops Res 92:185–202. https://doi.org/10.1016/j.fcr.2005.01.02312. Inman-Bamber NG (2004) Sugarcane water stress criteria for irrigation and drying off. Field Crops Res 89:107–122
Machado RS, Ribeiro RV, Marchiori PER, Machado DFSP, Machado EC, Landell MGA (2009) Biometric and physiological responses to water deficit in sugarcane at different phenological stages. Pesq Agropec Bras 44:1575–1582. https://doi.org/10.1590/S0100-204X2009001200003
Graça JP, Rodrigues FA, Farias JRB, Oliveira CN, Hoffmann-Campo CB, Zingaretti SM (2010) Physiological parameters in sugarcane cultivars submitted to water deficit. Braz J Plant Physiol 22:189–197. https://doi.org/10.1590/S1677-04202010000300006
Inman-Bamber NG (2004) Sugarcane water stress criteria for irrigation and drying off. Field Crops Res 89:107–122
Ribeiro RV, Machado RS, Machado EC, Machado DFSP, Magalhães Filho JR (2013) Landell MGA (2013) Revealing drought resistance and productive patterns in sugarcane genotypes by evaluating both physiological responses and stalk yield. Exp Agric 49:212–224. https://doi.org/10.1017/S0014479712001263
Sales CR, Ribeiro RV, Silveira JA, Machado EC, Martins MO, Lagôa AM (2013) Superoxide dismutase and ascorbate peroxidase improve the recovery of photosynthesis in sugarcane plants subjected to water deficit and low substrate temperature. Plant Physiol Biochem 73:326–336. https://doi.org/10.1016/j.plaphy.2013.10.012
Sales CRG, Marchiori PER, Machado RS, Fontenele AV, Machado EC, Silveira JAG, Ribeiro RV (2008) Photosynthetic and antioxidant responses to drought during sugarcane ripening. Photosynth 53:547–554
Epstein E (2009) Silicon: its manifold roles in plants. Ann Appl Biol 155:155–160. https://doi.org/10.1111/j.1744-7348.2009.00343.x
Chen W, Yao X, Cai K, Chem J (2011) Silicon alleviates drought stress of rice plants by improving plant water status, photosynthesis and mineral nutrient absorption. Biol Trace Elem Res 142:67–76
Ming DF, Pei ZF, Naeem MS, Gong HJ, Zhou WJ (2012) Silicon alleviates PEG-induced water deficit stress in upland rice seedlings. J Agr Crop Sci 198:14–26. https://doi.org/10.1111/j.1439-037X.2011.00486.x
Geng A, Wang X, Wu L, Wang F, Wu Z, Yang H, Chen Y, Wen D, Liu X (2018) Silicon improves growth and alleviates oxidative stress in rice seedlings (Oryza sativa L.) by strengthening antioxidant defense and enhancing protein metabolism under arsanilic acid exposure. Ecotoxicol Environ Saf 158:266–273. https://doi.org/10.1016/j.ecoenv.2018.03.050
Dorairaj D, Ismail MR, Sinniah UR, Tan KB (2020) Silicon mediated improvement in agronomic traits, physiological parameters and fiber content in Oryza sativa. Acta Physiol Plant 42:38. https://doi.org/10.1007/s11738-020-3024-5
Hattori T, Inanaga S, Araki H, Morita S, Luxová M, Lux A (2005) Application of silicon enhanced drought tolerance in Sorghum bicolor. Physiol Plantarum 123:459–466. https://doi.org/10.1111/j.1399-3054.2005.00481.x
Ahmed M, Qadeer U, Aslam MA (2014) Silicon application and drought tolerance mechanism of sorghum. Afr J Agr Res 6:594–607
Gong H, Zhu X, Chen K, Wang S, Zhang C (2005) Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci 169:313–321. https://doi.org/10.1016/j.plantsci.2005.02.023
Ahmed M, Qadeer U, Ahmed ZI, Hassan F (2016) Improvement of wheat (Triticum aestivum) drought tolerance by seed priming with silicon. Arch Agron Soil Sci 62:299–335. https://doi.org/10.1080/03650340.2015.1048235
Crusciol CAC, Pulz AL, Lemos LB, Soratto RP, Lima GPP (2009) Effects of silicon and drought stress on tuber yield and leaf biochemical characteristics in potato. Crop Sci 49:949–954. https://doi.org/10.2135/cropsci2008.04.0233
Shen X, Zhou Y, Duan L, Eneji AE, Li J (2010) Silicon effects on photosynthesis and antioxidant parameters of soybean seedlings under drought and ultraviolet-B radiation. J Plant Physiol 167:1248–1252. https://doi.org/10.1016/j.jplph.2010.04.011
Majumdar S, Prakash NB (2020) An overview on the potencial of silicon in promoting defence against biotic and abiotic stresses in sugarcane. J Soil Sci Plant Anal. https://doi.org/10.1007/s42729-020-00269-z
Oliveira CMR, Passos R, Andrade FV, Reis ED, Sturm GM, Souza RB (2010) Corrective of the acidity of the soil and levels of humidity in the development and nutrition of the sugarcane. R Bras Ci Agrarias 5:25–31
De Camargo MS, Bezerra BKL, Vitti AC, Silva MA, Oliveira AL (2017) Silicon fertilization reduces the deleterious effects of water deficit in sugarcane. J Soil Sci Plant Nutr 17:99–111. https://doi.org/10.4067/S0718-95162017005000008
De Camargo MS, Bezerra BKL, Holanda LA, Oliveira AL, Vitti AC, Silva MA (2019) Silicon fertilization improves physiological responses in sugarcane cultivars grown under water deficit. J Soil Sci Plant Nutr 19:81–91. https://doi.org/10.1007/s42729-019-0012-1
Verma KK, Liu XH, Wu KC, Singh RK, Song QQ, Malviya MK, Song XP, Singh P, Verma CL, Li YR (2020) The impact of silicon on photosynthetic and biochemical responses of sugarcane under different soil moisture levels. SILICON 12:1355–1367. https://doi.org/10.1007/s12633-019-00228-z
Bokhtiar SM, Huang HR, Li YR, Dalvi A (2012) Effects of silicon on yield contributing parameters and its accumulation in abaxial epidermis of sugarcane leaf blades using energy dispersive x-ray analysis. JPlantNutr 35:1255–1275. https://doi.org/10.1080/01904167.2012.676379
Camargo MS, Fernández Honaine M, Osterrieth M, Bozza NG, Silva VM, Benvenutto ML, Silva MA (2021) Silicon fertilization increases gas-exchange and biomass by silicophytolith deposition in the leaves of contrasting drought-tolerant sugarcane cultivars under well-watered conditions. Plant Soil 466:581–595. https://doi.org/10.1007/s11104-021-05063-z
De Camargo MS, Bozza NG, Pereira HS, Silva VM (2020) Silva MA (2020) Increase in silicate fertilization improves the biomass of drought-tolerant contrasting cultivars without prejudicial effects in nutrient uptake in sugarcane. J Soil Sci Plant Nutr 20:2329–2337. https://doi.org/10.1007/s42729-020-00300-3
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: Measurement and characterization by UV-VIS. In: Current protocols in food analytical chemistry. John Wiley & Sons, Madison
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as weel as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313. https://doi.org/10.1016/S0176-1617(11)81192-2
Campos PS, Thi ATP (1997) Effect of abscisic acid pretreatment on membrane leakage and lipid composition of Vigna unguiculata leaf discs subjected to osmotic stress. Plant Sci 130:11–18. https://doi.org/10.1016/S0168-9452(97)00199-4
Hermann ER, Câmara GMS (1999) Um método simples para estimar a área foliar de cana-de-açúcar. Stab 17:32–34
Elliott CL, Snyder GH (1991) Autoclave-induced digestion for the colometric determination of silicon in rice straw. J Agric Food Chem 39:1118–1119. https://doi.org/10.1021/jf00006a024
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1996) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Camargo MS, Korndörfer GH, Wyler P (2014) Silicate fertilization of sugarcane cultivated in tropical soils. Field Crops Res 167:64–75. https://doi.org/10.1016/j.fcr.2014.07.009
Anderson DL, Bowen JE (1992) Sugarcane nutrition. Associação Brasileira de Potassa e do Fósforo (Potafós), Piracicaba
Berthelsen S, Noble A, Kingston G, Hurney A, Rudd A, Garside A (2003) Improving yield and CCS in sugarcane through the application of silicon based amendments. Sugar Research and Development Corporation, CSIRO Land and Water. Available in https://elibrary.sugarresearch.com.au/handle/11079/12957. Acessed 10 Jan 2022
McCray JM, Ji S (2012) Calibration of sugarcane response to calcium silicate on Florida Histosols. J Plant Nutr 35:1192–1209. https://doi.org/10.1080/01904167.2012.676131
Camargo MS, Korndörfer GH, Foltran DE, Henrique CM, Rossetto R (2010) Silicon uptake, yield and Diatraea saccharalis incidence in sugarcane cultivars. Bragantia 69:937–944. https://doi.org/10.1590/S0006-87052010000400020
Camargo MS, Keeping MG (2021) Silicon in sugarcane: availability in soil, fertilization, and uptake. SILICON. https://doi.org/10.1007/s12633-020-00935-y
Silva MA, Jifon JF, Silva JAG, Sharma V (2007) Use of physiological parameters as fast tools to screen for drought tolerance in sugarcane. Braz J Plant Physiol 19:193–201. https://doi.org/10.1590/S1677-04202007000300003
Endres L, Silva JV, Ferreira VM, Barbosa GVS (2010) Photosynthesis and water relations in Brazilian sugarcane. Open Agric J 11:31–37. https://doi.org/10.2174/1874331501004010031
Silva MA, Jifon JF, Santos CM, Jadoski CJ, Silva JAG (2013) Photosynthetic capacity and water use efficiency in sugarcane genotypes subject to water deficit during early growth phase. Braz Arch Biol Technol 56:735–748. https://doi.org/10.1590/S1516-89132013000500004
Zhao D, Glaz B, Comstock JC (2013) Sugarcane leaf photosynthesis and growth characters during development of waterdeficit stress. Crop Sci 53:1066–1075. https://doi.org/10.2135/cropsci2012.09.0554
Hattori T, Sonobe K, Araki H, Inanaga S, AnP MS (2008) Silicon application by sorghum through the alleviation of stress-induced increase in hydraulic resistance. J Plant Nutr 31:1482–1495
De Tombeur F, Vander Linden C, Cornélis JT, Godin B, Philippe Compère P, Delvaux B (2020) Soil and climate affect foliar silicification patterns and silica-cellulose balance in sugarcane (Saccharum officinarum). Plant Soil 452:529–546. https://doi.org/10.1007/s11104-020-04588-z
Inman-Bamber N, Lakshmanan P, Park S (2012) Sugarcane for waterlimited environments: Theoretical assessment of suitable traits. Field Crops Res 134:95–104. https://doi.org/10.1016/j.fcr.2012.05.004
Marchiori PER, Machado ES, Sales CRG, Espinoza-Núñez E, Magalhães Filho JR, Souza GM, Pires RCM, Ribeiro RV (2017) Physiological plasticity is important for maintaining sugarcane growth under water deficit. Front Plant Sci. https://doi.org/10.3389/fpls.2017.02148
Sales CRG, Ribeiro RV, Machado DFS, Machado RS, Dovis VL, Lagora AMMA (2012) Trocas gasosas e balanço de carboidratos em plantas de cana-de-açúcar sob condições de estresses radiculares. Bragantia 73:319–327. https://doi.org/10.1590/S0006-87052012000300001
Acknowledgements
The authors would like to thank Sao Paulo State Research Foundation (FAPESP) for financial support (2018/05843-0; 2022/03805-9) of the first author, and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-Brazil) for the fellowships to the fifth (Grant 316424/2021-8), and seventh author (Grant 305952/2018-8).
Funding
The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
Mônica Sartori Camargo planned, designed, and carried out the experiments, analyzed and interpreted the data, and was responsible for the manuscript preparation and for the project FAPESP 2018/05843–0. Gustavo Jonas Baltieri carried out the experiment, including measurements of the water potential in soil, irrigation calculations, and manual irrigation, and took the biometric measurements (fellowship 20/00038–1). Hariane Luiz Santos, and Melina Rodrigues Alves Carnietto took all physiological measurements. André Rodrigues dos Reis conducted the sugar analysis and interpreted the data. Mônica Sartori Camargo, Ana Claudia Pacheco, and Marcelo de Almeida Silva interpreted the physiological data and contributed to writing the latest version of the manuscript.
Corresponding author
Ethics declarations
Research Involving Human Participants and or Animals
The authors declare this study does not contain studies with human participants or animals.
Consent to Participate
All authors give their consent for participate of this paper.
Consent for Publication
All authors give their consent for submission and publishing the this paper.
Competing Interests
The authors declare no competing interests.
Conflict of Interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Camargo, M.S., Baltieri, G.J., Santos, H.L. et al. Silicon Fertilization Enhances Photosynthetic Activity and Sugar Metabolism in Sugarcane Cultivars under Water Deficit at the Ripening Phase. Silicon 15, 3021–3033 (2023). https://doi.org/10.1007/s12633-022-02236-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-022-02236-y