Abstract
Silicon, a quasi-essential element that is often associated with rigidity and hardiness is an alleviator of biotic and abiotic stresses. Lodging affects the ability of plants especially cereal plants such as rice, Oryza sativa to grow upright, thus reducing yield and increasing harvesting costs. Bending or lodging resistance could be increased by strengthening the rice culm. Here, we tested stage and method of silicon application, each with three levels of treatments to find the right combination to enhance yield and lodging resistance in MR219, a popular rice variety in Malaysia. Application of silicon at the onset of reproductive stage via topdressing improved agronomic and physiological parameters. Plants applied with Si at the reproductive stage by means of topdressing showed a significantly higher leaf area. Number of tillers, weight per panicle and weight of 100 grains were significantly higher when silicon was applied as topdressing at the reproductive stage. Number of spikelets per panicle was significantly higher when silicon was supplied as topdressing or soil incorporation. Percentage of filled spikelets was significantly higher when silicon supplementation was done as topdressing at either reproductive or maturity stages. Mode of application which favoured the increase in soluble lignin and neutral detergent fiber was topdressing and soil incorporation. Meanwhile, cellulose and acid detergent lignin was highest when silicon was applied at the reproductive and maturity stages. Application of silicon at the reproductive stage by means of topdressing was effective in improving growth, yield and stem strength of the rice plant since the method has higher retention time and has residual effect. Foliar application was deemed unsuitable as it can only be applied in minimal amount while showing a temporary effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nutrient management is one of the important aspects in agriculture and crop production. It involves proper application of fertilizers or nutrients at the right time, rate and method. This practice could potentially save management cost, reduce yield loss and curb incidence of toxicity. Silicon (Si) which is gaining prominence is thought to improve growth, development, mechanical strength, and disease resistance in plants especially Si-accumulators. Rice, a Si-accumulator, often enjoys the benefit of Si fertilization in China since they are the major consumers of this cheap carbohydrate (Ning et al. 2014).
Silicon is commonly found ubiquitously in soils but not as an element itself but as silicates or silica and often makes up the soil solution. Silicon present in the range of 0.1 to 10% (Epstein 1999) depending on its mode of accumulation is the only element that is not toxic to plants if taken in excess. This range is considered higher than those of essential macronutrients but Si has yet to attain the status of essential nutrient mainly because it is not involved in metabolism and moreover, the plant is able to complete its life cycle in nutrient solution devoid of this element (Arnon and Stout 1939). However, recent classification by Epstein and Bloom (2003), has termed Si as quasi-essential since Si deficient plants could show symptoms of abnormalities in terms of growth and development. Though it is the second most abundant on earth, Si cannot be taken up readily by plants’ transport system if it is not in monosilicilic form.
Method of fertilizer application including soil incorporation, topdressing and foliar application is crucial as it affects nutrient accessibility. The former two are the preferred method for solid fertilizer and could be supplied at higher rates. In addition, these methods are favourable for nutrients that are taken up by means of vascular transportation. On the other hand, foliar spray is relatively easy to apply at manageable rates while not affecting the soil pH since Si can act as liming agent when applied at high rates in the form of solid fertilizer (Tubana et al. 2012; Haynes et al. 2013). However, in regards to Si, plant response to foliar application is arguable as uptake of Si is via roots; hence foliar application had been deemed ineffective.
Growth of rice plant is divided into three distinct stages; vegetative, reproductive and maturity (Yoshida 1981). It is absolutely necessary to determine the exact stage of application to increase productivity and efficiency of nutrient uptake as wrong timing would be an absolute waste of resources and prove costly. Amount of nutrient uptake is dependent upon the uptake pattern of the crop and may differ according to the growth stage. Moreover, application at the appropriate stage would reduce nutrient loss. Nutrient requirement vary according to crop, nutrient mobility, soil type, and soil fertility.
Silicon is often applied in the form of slag, potassium silicate, sodium silicate, silica, wollastonite, and silica gel. Application of Si at different growth stage could yield an array of results, depending on the form of Si used. Moreover, the ease of application could also make the application of Si more viable and economical. Thus, the main objective of this experiment was to determine the appropriate time and method of Si application in increasing yield, growth and lodging resistance of MR219.
Methods and materials
Crop establishment
A total of four seeds of MR219, a Malaysian rice variety, with emerging radicles were directly sown on moist soil surface with no standing water in polyethylene containers with a diameter of 31.5 cm filled with approximately 15 kg of puddled silty clay soil. The pH, CEC and Si content of the soil were 5.2, 15.88 meq/100 g soil and 11.41 mg/kg−1, respectively. The experiment was carried out in a screenhouse at Universiti Putra Malaysia, Selangor, Malaysia located at an altitude of 30 m above sea level, altitude of 30° 21 N and longitude of 101° 70 E (Dorairaj and Ismail 2017). The humidity, minimum and maximum temperatures were 88%, 22 °C and 34.5 °C, respectively.
Treatment and experimental design
The experiment was conducted in a randomized complete block design (RCBD) with three replicates and four plants per replicate. A total of three methods (topdressing, soil incorporation, foliar spray) and time of application (vegetative, reproductive, maturity) were tested in this two-factorial experiment in addition to control/untreated. Silicon fertilizer (SiO2 = 66%) was applied at the rate of 4 g per pot for both topdressing and soil incorporation whereas 400 ppm of Si was used in foliar spray at 21, 56 and 82 days after sowing (DAS) corresponding to vegetative, reproductive and maturity stages, respectively (Dorairaj et al. 2017). For foliar spray, 1% Tween 20 (Sigma-Aldrich) was added as a surfactant.
Flag leaf area, chlorophyll content and photosynthetic rate
Flag leaf area, chlorophyll content and photosynthetic rate were measured two weeks before harvest from fifteen leaves per replicate. A Leaf Area Measurement System was used to measure the leaf area whereas closed infra-red gas analyzer LI-6400 Portable Photosynthesis System (LI-COR, Lincoln, Nebraska, USA) was used to determine the photosynthetic rate. Chlorophyll content of the flag leaf samples were measured using Scanning Spectrophotometer (Shimadzu). Flag leaf samples measuring 3 cm2 were soaked in 20 ml of 80% acetone in the dark for ten days to ensure the release of total chlorophyll from the tissue. Then, 3.5 ml of supernatant was sampled to measure the absorbance of chlorophyll a and b at 664 nm and 647 nm, respectively. The content of chlorophyll a and b were calculated according to the following formulae adopted from Coombs et al. (1985):
Photosynthesis measurements were taken under a light intensity of 800 µm m−2 S−1 PAR, temperature of 30 ± 2 °C, external carbon dioxide concentration of 390 µm mol−1, relative air humidity of 70% at 0900–1100 h (Dorairaj and Ismail 2017).
Yield and yield components
Yield components such as number of spikelets per panicle, percentage of filled spikelets, weight of 100 grains and weight per panicle were measured at harvest (110–115 DAS) on ten tillers per replicate. Number of tillers per pot and percentage of effective tillers per pot were measured one week before harvest.
Bending resistance
Bending resistance was assessed using a modified model of Kaack and Schwarz (2001) at harvest (110–115 DAS). Rice stems were cut at 25 cm from base of the plant and placed on the platform of the apparatus (Instron Ltd, Texture Analyzer). It was equipped with a 5 kg load cell and a knife set with blade serving as a plunger to force the stem to bend with a velocity of 5.0 mm/s to a 45° angle (Dorairaj and Ismail 2017).
X-Ray fluorescence analysis
Dried aboveground sample that was ground to powder form was first extracted with methanol and dried at 65 °C for 72 h. Next, the sample was compacted to form a thin layer befitting the sample cups. A total of eight samples per replicate were analyzed. Silicon content was determined using Energy Dispersive X-ray Fluorescence Spectrometer (EDX 720, Shimadzu, Japan).
Thioglycolic acid lignin quantification
Thioglycolic acid lignin was determined according to the modified method of Brinkmann et al. (2002). Briefly, dried ground aboveground plant sample was washed with distilled water followed by 80% methanol extraction to isolate structural biomass for lignin analysis. Aliquots of 2 mg of the plant sample were weighed into microcentrifuge tubes and mixed with 1.5 ml of 2 N hydrochloric acid (HCl) and 0.3 ml thiogylcolic acid. After incubation at 95 °C for 4 h, the samples were rapidly cooled on ice and centrifuged for 10 min at 16,000 g. The supernatant was discarded while the pellets were washed with distilled water. Next, the pellets were incubated with 1 ml of 0.5 N NaOH for 18 h on a shaker at room temperature while the suspension was centrifuged for 10 min at 16,000g. The supernatant was transferred into a new microcentrifuge tube whereas the pellet was re-suspended in NaOH, vigorously mixed and centrifuged. The resulting supernatant was combined with the first alkaline supernatant and mixed with 0.3 ml concentrated HCl. Finally, the samples were incubated for 4 h at 4 °C to precipitate the lignothioglycolate derivates after which the supernatant was discarded, and the pellet solubilized in 1 ml of 0.5 N NaOH. Absorbance was measured at 280 nm using Multiskan Microplate Spectrophotometer Thermo Scientific USA (Dorairaj and Ismail 2017). Calibration curves were generated by subjecting increasing amounts of 0.5–2.5 mg of commercial lignin (alkaline spruce lignin, Aldrich) to the same procedure.
Neutral detergent fiber
One gram of dried straw sample were placed in a 600 ml Berzelius beaker. Samples were assessed in triplicates. Next, 100 ml of neutral detergent solution was mixed with 2 ml of decalin and 0.5 g of sodium sulphide (Van Soest et al. 1991). Beaker was then placed on a hot plate for about an hour till it comes to boiling after which it was filtered through a sintered crucible. This crucible was placed on a Buchner flask that was fitted with a vacuum pump to facilitate the filtration process. The residue was washed with acetone and then distilled water. Sintered crucible with the residue was placed in an oven for 24 h at 105 °C. Next, it was placed on a dessicator before measuring the weight. Neutral detergent fiber (NDF) was calculated according to Van Soest et al. (1991) method as shown below:
Acid detergent fiber
One gram of dried straw sample were placed in a 600 ml Berzelius beaker which was mixed with 100 ml of acid detergent solution. Samples were assessed in triplicates. Beaker was then placed on a hot plate for about an hour till it comes to boiling after which it was filtered through a sintered crucible (Van Soest et al. 1991). This crucible was placed on a Buchner flask that was fitted with a vacuum pump. This is to facilitate the filtration process. The residue was washed with acetone and then distilled water. Sintered crucible with the residue was placed in an oven for 12 h at 105 °C. Next, it was placed on a dessicator before measuring the weight. Acid detergent fiber (ADF) was calculated according to Van Soest et al. (1991) method as shown below:
Acid detergent lignin
Samples from ADF analysis were used to determine the lignin content. 10 ml of cool 72% sulphuric acid (H2SO4) was added to the dried sintered crucible with residue. The draining acid was refilled keeping the mixture for 3 h in 72% H2SO4. This acid was then filtered off under vacuum pump. The residue was washed with distilled water until it was acid-free. The sample in crucible was dried at 105 °C for 2 h, cooled in a dessicator and weighed (W2). The sample was then placed in a furnace for about 2 h at 550 °C, cooled and weighed (W3) to determine ash content of residue (Dyckmans et al. 2002).
Statistical analyses
All data were expressed as means and analyzed using the ANOVA procedure in the SAS Statistical software package (version 9.2 for windows). Differences among treatments were determined using the least significant difference (LSD) test at the 0.05 probability level.
Results
Flag leaf area, chlorophyll content and photosynthetic rate
Total chlorophyll content was not statistically significant in terms of method and time of application (Table 1). No interaction was observed. Contents of chlorophyll a were significantly different in terms of method though no interaction was present (Table 1). Topdressed plants had the highest chlorophyll a content followed by soil incorporated, though they were insignificant. However, the former method of application was significantly higher than foliar applied plants. In terms of chlorophyll b content, no significant difference was detected.
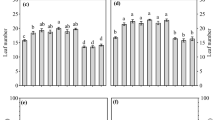
Flag leaf area was significantly different in terms of method and stage of application and showed presence of interaction between the two factors (Table 1). Plants applied with Si at the reproductive stage showed a significantly higher leaf area than those applied at maturity and vegetative. As for method of application, leaf area of topdressed plants were significantly higher than soil incorporated and foliar applied plants. The highest flag leaf area was obtained when Si was applied as topdressing at reproductive stage, followed by soil incorporation and foliar spray (Fig. 1). Similar result was obtained when Si was applied at maturity stage. However, for vegetative stage, highest value was obtained when Si was applied as soil incorporation followed by topdressing and foliar spray.
Effect of combination of silicon treatments on flag leaf area. Veg = vegetative, Rep = reproductive, Mat = maturity, Top = topdressing on soil surface, Soil = soil incorporation, Foliar = foliar spray. Vertical bars represent standard deviation. Means with same letter are not significantly different at p ≤ 0.05 according to Fisher’s least significant difference (LSD)
Photosynthetic rate was significantly different in terms of method of application but not time of application (Table 1). However, statistical analyses revealed presence of interaction between the main effects. As for method of application, foliar applied plants had the highest photosynthetic rate followed by topdressing and soil incorporation at 9.06 and 8.31 µmol CO2/m2/s, respectively. These methods were significantly different from one another. Photosynthetic rate was highest when Si was applied at vegetative stage as foliar spray while soil incorporated plants show highest rate when Si was applied at maturity stage (Fig. 2). Meanwhile, the photosynthetic rate of topdressed plants was highest when Si was applied at reproductive stage followed by maturity and vegetative stages.
Effect of combination of silicon treatments on photosynthetic rate. Rep = reproductive, Mat = maturity, Top = topdressing on soil surface, Soil = soil incorporation, Foliar = foliar spray. Vertical bars represent standard deviation. Means with same letter are not significantly different at p ≤ 0.05 according to Fisher’s least significant difference (LSD)
Yield and yield components
Total number of tillers was significantly different in terms of time and method of application (Table 2). Tiller number of soil incorporated and topdressed plants were significantly higher than foliar applied plants. Meanwhile those applied at reproductive stage had the highest tiller number followed by vegetative and maturity. However, tiller number of plants treated with Si at reproductive stage was not different from vegetative. The highest number of tillers per pot was obtained when Si was applied at reproductive stage as soil incorporation whereas foliar sprayed plants at both reproductive and maturity stages yielded the lowest number (Fig. 3). Topdressed plants gave highest number of tillers when applied at maturity stage followed closely by reproductive stage. As for percentage of effective tillers, no significant difference was observed.
Effect of combination of silicon treatments on total number of tillers. Veg = vegetative, Rep = reproductive, Mat = maturity, Top = topdressing on soil surface, Soil = soil incorporation, Foliar = foliar spray. Vertical bars represent standard deviation. Means with same letter are not significantly different at p ≤ 0.05 according to Fisher’s least significant difference (LSD)
Number of spikelets per panicle was significantly different in terms of method of Si application (Table 2). Si applied as soil incorporation had the highest number of spikelets per panicle followed by topdressing and foliar. However soil incorporated and topdressed plants were not statistically different. Likewise, stage of application showed no significance. Percentages of filled spikelets were significantly different in terms of method and stage of application but these factors were insignificant in terms of interaction (Table 2). Si applied at reproductive and maturity stages had significantly higher percentage of filled spikelets than vegetative stage. Meanwhile, topdressed plants was significantly higher than both soil incorporated and foliar applied plants.
Weight of 100 grains was significantly different whereby interaction between time and method were observed (Table 2). Application of Si at reproductive stage resulted in highest weight followed by maturity; both of which were not statistically different whereas vegetative stage yielded the lowest value at 2.69 g. The weight of 100 grains was highest when Si was applied as topdressing at reproductive stage (3.37 g) and lowest when it was applied at vegetative stage as topdressing and foliar spray at maturity stage (2.50 g) (Fig. 4). For vegetative stage, foliar sprayed plants gave highest value followed by soil incorporated and topdressed plants. As for maturity stage, the weight was highest when Si was applied as soil incorporation followed by topdressing and foliar spray.
Effect of combination of silicon treatments on weight of 100 grains. Veg = vegetative, Rep = reproductive, Mat = maturity, Top = topdressing on soil surface, Soil = soil incorporation, Foliar = foliar spray. Vertical bars represent standard deviation. Means with same letter are not significantly different at p ≤ 0.05 according to Fisher’s least significant difference (LSD)
Likewise, weight of panicle was statistically different in terms of method and time; interaction was present (Table 2). Plants treated with Si at reproductive stage had the heaviest panicle which was statistically different from those treated at maturity and vegetative. As for method of application, topdressing and soil incorporation were statistically not different. However these two methods were significantly different from foliar applied plants. At reproductive stage, the panicle was heaviest when Si was applied as topdressing followed by soil incorporation and foliar spray (Fig. 5). Lowest weight was obtained at vegetative stage when Si was applied as foliar spray. At this stage, heaviest panicle was obtained when Si was applied as topdressing followed very closely by soil incorporation. Similar trend was observed for maturity stage. Soil incorporation and foliar spray yielded highest panicle when Si was applied at reproductive and maturity stages, respectively.
Effect of combination of silicon treatments on weight per panicle. Veg = vegetative, Rep = reproductive, Mat = maturity, Top = topdressing on soil surface, Soil = soil incorporation, Foliar = foliar spray. Vertical bars represent standard deviation. Means with same letter are not significantly different at p ≤ 0.05 according to Fisher’s least significant difference (LSD)
Bending resistance
Bending resistance was significantly different in terms of method of application with topdressing having the highest resistance followed by soil incorporation and foliar (Table 3). The former two were not significantly different from each other. Both time of application and interaction were found to be insignificant.
Silicon content
Statistical analysis revealed there were differences in terms of method and time of application but no interaction was present (Table 3). Plants treated with Si at the reproductive stage showed the highest Si content which was significantly different from those treated at maturity and vegetative. The best method of application was found to be topdressing though it was not statistically different from soil incorporation. However, topdressed plants showed significantly higher Si content as compared to foliar applied plants.
Thioglycolic acid lignin
Significant difference in thioglycolic acid lignin was observed in terms of method and time of Si application as main effects (Table 4). No interaction was present. Multiple mean comparisons showed the highest content was of those treated at reproductive stage followed by maturity stage; both of which were statistically not different. Application at the first stage of growth herein vegetative had the lowest lignin content which was significantly different from the other two stages tested. As for method of application, lignin content of topdressed and soil incorporated were statistically same. Foliar applied Si showed lowest lignin content at 103.35 mg/g.
Neutral detergent fiber
Neutral detergent fiber (NDF) was significantly different in terms of method and stage of application in addition to presence of interaction between the two factors (Table 4). NDF contents of topdressed and soil incorporated plants were significantly higher than foliar applied plants. As for stage of application, plants treated with Si at reproductive and maturity stage were insignificant but the NDF was higher than plants treated with Si at vegetative. At all stages, neutral detergent fiber was highest when Si was applied as topdressing followed by soil incorporation and foliar spray. Meanwhile, both topdressed and soil incorporated plants showed highest fiber content when Si was applied at reproductive stage followed by maturity and vegetative stage. Foliar applied plants showed highest content when applied at maturity stage followed by reproductive and vegetative.
Cellulose
Cellulose content was significantly different in terms of the two methods tested, stage and method of application (Table 4). Plants treated with Si at reproductive and maturity stages were significantly higher than those applied at vegetative stage though no statistical differences were observed between the former two levels. On the other hand, topdressed and soil incorporated plants had significantly higher cellulose content in comparison to foliar applied plants.
Acid detergent lignin (ADL)
As in the case of cellulose, lignin content was significantly different in terms of both factors tested (Table 4). ADL content was highest in plants treated with Si at reproductive stage followed by maturity and vegetative stages. The former two were not significantly different, however it was statistically different from vegetative. On the other hand, foliar applied plants showed highest ADL content followed by topdressed plants which were insignificant. Application of Si as foliar showed lowest value though not statistically different from topdressed plants.
Discussion
The three growth stages of rice; vegetative, reproductive and maturity are distinctive and manipulation of physiological activities via exogenous application of nutrients should be carried out at proper timing to maximize the output. Besides, the mechanism of transport is nutrient specific, hence the uptake of nutrients by plants is dependant upon both the source and method of nutrient application. The degree of Si accumulation is reliant upon the uptake methods of active, passive or rejective which are responsible for high, medium, and low Si accumulation in the plants, respectively (Takahashi et al. 1990). Among monocots, plants in the families of Poaceae and Cyperaceae are high Si-accumulators. Rice which is a Si-accumulator takes up Si at a faster rate than water as it follows the active mode (Takahashi et al. 1990). Meanwhile, in passive mode, uptake of Si and water are at equal rate as portrayed by cucumber. In contrast, tomato which follows the rejective mode tends to remove Si, that is shown by the increasing concentration of Si in the uptake solution (Mitani and Ma 2005).
Plants benefit from Si fertilization as numerous studies had accounted for its role in tolerance of biotic and abiotic stresses. The cumulative effect of these stress mitigation is improved growth and development. Rice productivity had shown improvement when grown in Si supplemented soil as in this study. It has been reported that productivity is compromised in tropical region as the soil is five to ten times deficient in Si compared to temperate regions (Datnoff and Rodrigues 2005).
In this study, tiller number per pot and tillers per hill were higher in Si-treated plants particularly when this non-essential element is incorporated at reproductive stage by means of topdressing or soil incorporation. Number of spikelets per panicle were higher when Si was applied as topdressing and soil incorporation whereby it increased by 19% and 22%, respectively, compared to foliar applied plants. Compared to this parameter, percentage of filled spikelets could potentially reveal more on the effects of Si supplementation as it is an indicator of sterility or fertility. Plants treated at stages of reproductive and maturity showed lowest seed sterility with 3% and 1.8% increase in grain filling as compared to those treated at vegetative as the percentage of filled spikelets was highest. In terms of method, topdressed plants showed an increase of about 3.6% in grain filling compared to the other two methods. Meanwhile, the weight of 100 grains of plants treated at reproductive stage by topdressing was 13% and 22% higher than soil incorporation and foliar spray, respectively. These former two treatment combination showed about 25% increase compared to all foliar combinations.
The results of yield components in this study were in agreement to a study on rice in Iran which revealed that 400 kg/ ha of calcium silicate showed the highest increase in dry matter and grain yield (Kumleh and Kavossi 2003). Nevertheless, the role and effects of Si in rice is best studied using low silicon mutant (lsi1). Tamai and Ma (2008) revealed that there were no significant differences in height and dry weight in mutant and wild type rice. However, grain yield and percentage of filled spikelets were way higher in the wild rice. Grain yield reduced in the range of 79–98% in lsi1 which was due to a lower percentage of filled spikelets. This drastic reduction reaffirms that high Si accumulation is a necessity to increase rice yield. Excessive transpiration is thought to be the main cause of sterility in lsi1 since it does not possess the Si-cuticle double layer deposited beneath the epidermal cells which the wild type plant possess. This layer is responsible for reducing water loss through cuticles (Ma and Takashashi 2002; Trenholm et al. 2004). On top of that, this barrier also contributed to mitigation of salt toxicity by means of salt dilution (Romero-Aranda et al. 2006).
Meanwhile, Si fertilizers increased Si content in stem and leaf samples in addition to 1000 grain weight, tiller number, dry weight and yield (Gholami and Falah 2013). This corresponded to results obtained by Chaoming et al. (1999) who found that Si increased filled spikelets percentage, thousand seed weight, total number of spikelets per panicle and grain yield besides reducing lodging.
All plants use chlorophyll to utilize carbon dioxide and water to form sugar compounds in the process of photosynthesis. These photosynthates are then translocated to various parts of the plants known as sinks. Rice yield commensurate with the accumulation of sugar compound as Xie et al. (2011) found that 90% of grain yield originates from the photosynthetic carbon assimilation of leaves after heading, especially from flag leaf. Thus, leaf area, its standing position and chlorophyll content are key factors in this food making process particularly in efficient capture of solar energy.
In the current study, application of Si did not influence chlorophyll content as no significant differences were observed in all three components of it. On the other hand, flag leaf area was significantly different in terms of both factors with presence of interaction. Once again plants treated at reproductive stage by topdressing produced highest leaf area. This was in contrast to photosynthetic rate whereby foliar applied plants showed the highest rate followed topdressing and soil incorporation. Plants treated by foliar spray at vegetative and reproductive stage performed best and there was about 9% increase in photosynthetic rate in these combinations compared to maturity. Having high chlorophyll content does not warrant for increased photosynthetic rate at all times. The process of fixing carbon dioxide to synthesize sugar molecule is complex, as it involves the acquisition of raw materials such as water and light besides the green pigment found in chloroplast (Lawlor 2001). The limiting factor could be temperature, carbon dioxide concentration, light intensity and water; not necessarily chlorophyll content. In addition, the concentration of rubisco, the enzyme that catalyzes the conversion of carbon dioxide found in the atmosphere into glucose could also limit the photosynthesis rate (Chapin and Eviner 2007).
Freckling reduces the active leaf area for photosynthesis whereas Si-treated leaves are often more erect avoiding mutual shading thus improving light interception and promoting photosynthesis. Lau et al. (1978) hypothesized that Si deposited on leaf epidermal cells could serve as ‘windows’ allowing more light to pass through the photosynthetic mesophyll tissue, thus increasing the photosynthetic rate and number of tillers. Moreover, studies by IRRI indicate that Si-deficient plants always have a reduced number of panicles per square meter and percentage of filled grains (Meena et al. 2014).
A lodged culm could hinder light capture thus reducing photosynthesis efficiency which would lead to reduced dry matter (Hitaka 1969). Severe lodging will affect grain filling as it may inhibit transport of minerals, nutrients and assimilates (Kashiwagi et al. 2005). A lodged plant is often structurally very weak thus providing favourable environment for disease development. It is not an exaggeration to say this phenomenon affects the quality and quality of rice (Kono 1995).
Plant cell wall which is composed of hemicelluloses, cellulose and lignin are intertwined and is very much involved in mechanical strength of the plant structure (Jones et al. 2001; Tanaka et al. 2003). Findings from the current study showed Si was significantly correlated to higher lodging resistance and lignin deposition (Table 5). Application of Si elevated lignin content in plants which in turn increased the lodging resistance. Straw strength and cell wall lignification can affect the ability of the plant to resist any external force such as wind. Similarly, cellulose which was also significantly correlated to Si content (Table 5) is important for culm strengthening. Moreover, a lodging tolerant wheat cultivar exhibited a higher deposition of Si in the culm epidermis than a lodging susceptible cultivar (Ma and Yamaji 2006).
Conversely, in wheat (Li 1998) and rice (Taylor et al. 1999; Yang et al. 2001), the soluble carbohydrate content of the basal internodes of the stem was positively correlated to lodging resistance. Comparatively, Jones et al. (2001) reported that both lignin and cellulose contents are associated to stem rigidity whereas Huang (1988) revealed that strong stems possessed higher lignin content in the basal internodes compared to weak stems. Likewise, according to Zhu et al. (2004) and Jones et al. (2001), lignin content play a more pivotal role in increasing the mechanical support and tensile strength of stem in comparison to cellulose content which was in contrast to Wang et al. (2006).
We measured both ADL and TGA lignin in addition to cellulose and NDF content. The Van Soest method is commonly employed for forage analysis and involves harsh chemicals and large amount of sample. In addition, it is very time consuming as number of samples that can be processed in a day is very small and involves use of expensive glasswares. In contrast, TGA lignin can be processed in large batches at any given time and relatively quick though not easy. As such it is suggested that for researches on stem strength involving lodging, lignin is quantified as TGA.
Optimum results were obtained when Si was applied as topdressing followed by soil incorporation. Plants treated with Si as foliar application did not show any signs of growth improvement. Soil and topdressing are the preferred method for nutrient application as they are easily transported via roots, can be applied in higher rate and have higher retention time. Topdressing is preferred for after planting application as soil incorporation may disturb soil structure and root anchorage. Foliar application needs several applications to meet the nutrient demand. Though this method allows plants to utilize nutrients more rapidly than the other two methods, it is affected by climate change whereby the nutrients run off in rain water and leaves could suffer from toxicity and scorching if applied above permitted amount. Besides, the plant should have sufficient leaf area for the optimum absorption of nutrients (Fageria et al. 2009).
Thus, foliar spray may be more suitable for application of micronutrients whereas soil and topdressing can be done for both macro and micronutrients. Nonetheless, foliar fertilization is a cost effective method of supplementing the plants’ nutrients for a more efficient fertilization (Girma et al. 2007) and could be formulated in combination of fungicides and insecticides to aid the supply of nutrients to crops in the form of sprays (Fageria et al. 2009). On the other hand, yield seems to be maximized when Si was supplied at the reproductive stage which is also referred to as pre-heading (Yoshida 1981). Clearly it is because this growth phase influences and determines the most important yield components, namely, panicle number, spikelet number and panicle fertility (Ma et al. 1989).
Conclusion
Application of silicon at the reproductive stage by means of topdressing was effective in improving growth, yield and stem strength of the rice plant. Foliar application is unsuitable as it can only be applied in minimal amount and has a temporary effect. Topdressing is preferred as it has higher retention time and has residual effect.
Author contribution statement
DD and MRI designed the study, discussed the results, read and approved the manuscript. DD performed the research and wrote the manuscript. MRI secured funding and reviewed the manuscript. URS and TKB provided critical reviews and approved the manuscript.
References
Arnon DI, Stout PR (1939) The essentiality of certain elements in minute quantity for plants with special reference to copper. Plant Physiol 14:371–375
Brinkmann K, Blaschke L, Polle A (2002) Comparison of different methods for lignin determination as a basis for calibration of near-infrared reflectance spectroscopy and implications of lignoproteins. J Chem Ecol 28:2483–2501
Chang SJ, Tzeng DDS, Li CC (2002) Effect of silicon nutrient on bacterial blight resistance of rice (Oryza sativa L.). In: Matoh T (ed) Second silicon in agriculture conference. Press-Net, Kyoto, pp 31–33
Chaoming Z, Jianfei L, Liping C (1999) Yield effects on the application of silicon fertilizer early hybrid rice. J Artic 2:79–80
Chapin FS, Eviner VT (2007) Biogeochemistry of terrestrial net primary production. Treatise Geochem 8:1–35
Coombs J, Hall OO, Long SP, Scurlock JMO (1985) Techniques in bioproductivity and photosynthesis. Pergamon Press, Oxford
Datnof LE, Rodrigues FA (2005) The role of silicon in suppressing rice diseases. APSnet Features. https://doi.org/10.1094/APSnetFeature-2005-0205
Dorairaj D, Ismail MR (2017) Distribution of silicified microstructures, regulation of cinnamyl alcohol dehydrogenase and lodging resistance in silicon and paclobutrazol mediated oryza sativa. Front Physiol 8:491
Dorairaj D, Ismail MR, Sinniah UR, Tan KB (2017) Influence of silicon on growth, yield and lodging resistance of MR219, a lowland rice of Malaysia. J Plant Nutr 40(8):1111–1124
Dyckmans J, Flessa H, Brinkmann K, Mai C, Polle A (2002) Carbon and nitrogen dynamics in acid detergent fibre lignins of beech (Fagus sylvatica L.) during the growth phase. Plant Cell Environ. 25:469–478
Epstein E (1999) Silicon. Annu Rev Plant Physiol Plant Mol Biol 50:641–664
Epstein E, Bloom AJ (2003) Mineral nutrition of plants: principles and perspectives. John Wiley and Sons, New York
Fageria NK, Barbosa FMP, Moreira A, Guimaraes CM (2009) Foliar fertilization of crop plants. J Plant Nutr 32(6):1044–1064
Gholami Y, Falah A (2013) Effects of two different sources of silicon on dry matter production, yield and yield components of rice, Tarom Hashemi variety and 843 Lines. Int J Agric Crop Sci 5:227–231
Girma K, Martin KL, Freeman KW, Mosali J, Teal RK, Raun WR, Moges SM, Arnall DB (2007) Determination of optimum rate and growth for foliar applied phosphorus in corn. Commun Soil Sci Plant Anal 38:1137–1154
Haynes RJ, Belyaeva ON, Kingston G (2013) Evaluation of industrial wastes as sources of fertilizer silicon using chemical extractions and plant uptake. J Plant Nutr Soil Sci 176:238–248
Hitaka H (1969) Studies on the lodging of rice plants. Jpn Agric Res Q 4(3):1–6
Huang YL (1988) Morphological factors and control techniques of lodging in wheat. Jiangsu Agric Sci 10:5–8
Jones L, Ennos AR, Turner SR (2001) Cloning and characterization of irregular xylem4 (irx4): a severely lignin-deficient mutant of Arabidopsis. Plant J 26:205–216
Kaack K, Schwarz KU (2001) Morphological and mechanical properties of Miscanthus in relation to harvesting, lodging, and growth conditions. Ind Crops Prod 14:145–154
Kashiwagi T, Sasaki H, Ishimaru K (2005) Factors responsible for decreasing sturdiness of the lower part in lodging of rice (Oryza sativa L.). Plant Prod Sci 2:166–172
Kono M (1995) Physiological aspects of lodging. In: Matsuo T, Kumazawa K, Ishii R, Ishihara K, Hirata H (eds) Science of the rice plant, vol 2, Physiology 2, Food and Agricultural Policy Research Centre, Tokyo, pp 971–982
Kumleh SA, Kavossi M (2003) Evaluation of interaction of silica and phosphorous on the growth and grain yield of rice (Oryza sativa L.). Iran J Agric Sci 35(3):581–586
Lau E, Goldoftas M, Baldwin VD, Dayanandan P, Srinivasan J, Kaufman PB (1978) Structure and localization of silica in the leaf and internodal epidermal system of the marsh grass, Phragmites australis. Can J Bot 56:1096–1107
Lawlor DW (2001) Photosynthesis. BIOS, UK
Li QQ (1998) Creation, evaluation and utilization of winter wheat germplasm. Shangdon Science and Technology Press, Jinan. pp 203–219
Ma JF, Takashashi E (2002) Soil, fertilizer, and plant silicon research in Japan. Elsevier Science, Amsterdam
Ma JF, Yamaji N (2006) Silicon uptake and accumulation in higher plants. Trends Plant Sci 11:392–397
Ma J, Nishimura K, Takahashi E (1989) Effect of silicon on the growth of rice plant at different growth stages. Soil Sci Plant Nutr 35:347–356
Meena VD, Dotaniya ML, Vassanda C, Rajendiran S, Kundu AS, Rao AS (2014). A case for silicon fertilization to improve crop yields in tropical soils. Proc Natl Acad Sci India B 84:505–518
Mitani N, Ma JF (2005) Uptake system of silicon in different plant species. J Exp Bot 56:1255–1261
Ning DF, Song A, Fan FL, Li ZJ, Liang YC (2014) Effects of slag-based silicon fertilizer on rice growth and brown-spot resistance PLoS One 9(7):e102681
Romero-Aranda MR, Jurado O, Cuartero J (2006) Silicon alleviates the deleterious salt effect on tomato plant growth by improving plant water status. J Plant Physiol 163:847–855
Takahashi E, Ma JF, Miyake Y (1990) The possibility of silicon as an essential element for higher plants. Comments Agric Food Chem 2:99–122
Tamai K, Ma JF (2008) Reexamination of silicon effects on rice growth and production under field conditions using a low silicon mutant. Plant Soil 307:21–27
Tanaka K, Murata K, Yamazaki M, Onosato K, Miyao A, Hirochika H (2003) Three distinct rice cellulose synthase catalytic subunit genes required for cellulose synthesis in the secondary wall. Plant Physiol 133:73–83
Taylor NG, Scheible W-R, Cutler S, Somerville CR, Turner SR (1999) The irregular xylem3 locus of Arabidopsis encodes a cellulose synthase required for secondary cell wall synthesis. Plant Cell 11:769–779
Trenholm LE, Datnoff LE, Nagata RT (2004) Influence of silicon on drought and shade tolerance of St. Augustine grass. Hortic Technol 14(4):487–490
Tubana B, Narayanaswamy C, Lofton J, Kanke Y, Dalen M, Datnoff L (2012) Impact of silicon fertilization to sugarcane grown on alluvial soils in Louisiana. J Am Soc Sugar Cane Tech 32:75
Van Soest PJ, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 74:3583–3597
Wang J, Zhu JM, Lin QQ, Li XJ, Teng NJ, Li ZHSH, Li B, Zhang AM (2006) The effect of the anatomical structure and chemical components of the culm on lodging resistance in wheat. Sci Bull 51(5):1–7
Xie XJ, Shen SHH, Li YX, Zhao XY, Li BB, Xu DF (2011) Effect of photosynthetic characteristic and dry matter accumulation of rice under high temperature at heading stage. Afr J Agric Res 6(7):1931–1940
Yang J, Zhang J, Wang Z, Zhu Q, Liu L (2001) Water-deficit induced senescence and its relationship to the remobilization of pre-stored carbon in wheat during grain filling. Agron J 93:196–20
Yoshida S (1981) Physiological analysis of rice yield. In: Fundamentals of rice crop science. International Rice Research Institute, The Phillipines, pp 231–251
Acknowledgement
The authors thank the Ministry of Education Malaysia for providing the financial support through Translational Research Grant for PadiU Putra: Accelerating rice food security and socioeconomics for rice farming communities. We also thank the Ministry of Education Malaysia for its continuous support by providing us the Long–term Research Grant Scheme (LRGS) for the Development of climate ready rice for sustainable national rice food security. DD would like to thank the same Ministry for MyBrain15 scholarship.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Communicated by H. Peng.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dorairaj, D., Ismail, M.R., Sinniah, U.R. et al. Silicon mediated improvement in agronomic traits, physiological parameters and fiber content in Oryza sativa. Acta Physiol Plant 42, 38 (2020). https://doi.org/10.1007/s11738-020-3024-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-020-3024-5