Abstract
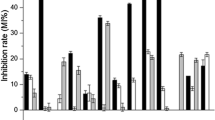
Twenty-eight strains of lactic acid bacteria (LAB) were characterized for the ability to express enzymes of interest (including protease, xylanase, α-amylase, laccase, and glucose oxidase) as well as the ability to produce exopolysaccharide (EPS). The screening of enzyme capability for all LAB strains proceeded in a progressive 3-stage manner that helps to profile the efficiency of LAB strains in expressing chosen enzymes (Stage 1), highlights the strains with affinity for flour as the substrate (Stage 2), and discerns strains that can adapt well in a simulated starter environment (Stage 3). The theoretical ability of LAB to express these enzymes was also assessed using Basic Local Alignment Search Tool (BLAST) analysis to identify the underlying genes in the whole genome sequence. By consolidating both experimental data and information obtained from BLAST, three LAB strains were deemed optimal in expressing enzymes, namely, Lb. delbrueckii subsp. bulgaricus (RBL 52), Lb. rhamnosus (RBL 102), and Lb. plantarum (ATCC 10241). Meanwhile, EPS-producing capabilities were observed for 10 out of 28 LAB strains, among which, Lactococcus lactis subsp. diacetylactis (RBL 37) had the highest total EPS yield (274.15 mg polysaccharide/L culture) and produced 46.2% polysaccharide with a molecular mass of more than 100 kDa.











Similar content being viewed by others
Data Availability
Data were provided in the supplementary information.
References
Khodaei N, Nguyen MM, Mdimagh A, Bayen S, Karboune S (2021) Compositional diversity and antioxidant properties of essential oils: predictive models. LWT 138:110684
Dong Y, Ismail Fliss I, Karboune S (2024) Investigation of in situ and ex situ mode of LAB incorporation and the effect on dough viscoelasticity, bread texture, and overall physical quality postbaking. ACS Food Science & Technology 4(2):316–332
Dong Y, Karboune S (2021) A review of bread qualities and current strategies for bread bioprotection: flavor, sensory, rheological, and textural attributes. Compr Rev Food Sci Food Saf 20:1937–1981
Axel C, Zannini E, Arendt EK (2017) Mold spoilage of bread and its biopreservation: a review of current strategies for bread shelf-life extension. Crit Rev Food Sci Nutr 57(16):3528–3542
Florou-Paneri P, Christaki E, Bonos E (2013) Lactic acid bacteria as source of functional ingredients. Lactic acid bacteria-R & D for food, health and livestock purposes. IntechOpen
Jideani VA, Vogt K (2016) Antimicrobial packaging for extending the shelf life of bread—a review. Crit Rev Food Sci Nutr 56(8):1313–1324
Pozo-Bayón MA, Guichard E, Cayot N (2006) Flavor control in baked cereal products. Food Rev Intl 22(4):335–379
Hui YH, Corke H, Leyn De, Nip WK, Cross NA (2008) Bakery products: science and technology. John Wiley & Sons, Hoboken
Angmo K, Kumari A, Bhalla TC (2016) Probiotic characterization of lactic acid bacteria isolated from fermented foods and beverage of Ladakh. LWT-food Science and Technology 66:428–435
Tieking M, Gänzle MG (2005) Exopolysaccharides from cereal-associated lactobacilli. Trends Food Sci Technol 16(1–3):79–84
Mohania D, Nagpal R, Kumar M, Bhardwaj A, Yadav M, Jain S et al (2008) Molecular approaches for identification and characterization of lactic acid bacteria. J Dig Dis 9(4):190–198
Savijoki K, Ingmer H, Varmanen P (2006) Proteolytic systems of lactic acid bacteria. Appl Microbiol Biotechnol 71(4):394–406
Gobbetti M, De Angelis M, Di Cagno R, Rizzell CG (2008) Sourdough/lactic acid bacteria. Academic Press, In Gluten-free cereal products and beverages, pp 267–288
Naik AS, Waghmare R (2020) Application of glycosyl hydrolases in food industry. Industrial Applications of Glycoside Hydrolases. Springer, Singapore, pp 217–228
Chen Y, Eder S, Schubert S, Gorgerat S, Boschet E, Baltensperger L, Windhab EJ (2021) Influence of amylase addition on bread quality and bread staling. ACS Food Science & Technology 1(6):1143–1150
Mohamed ER, Halaby MS, Nadir AS, El-Masry HG (2019) The effect of alpha-amylase and ascorbic acid as improvers on pan bread quality. Middle East J Appl Sci 9:906–913
Liu M, Bayjanov JR, Renckens B, Nauta A, Siezen RJ (2010) The proteolytic system of lactic acid bacteria revisited: a genomic comparison. BMC Genomics 11(1):1–15
García-Cano I, Rocha-Mendoza D, Ortega-Anaya J, Wang K, Kosmerl E, Jiménez-Flores R (2019) Lactic acid bacteria isolated from dairy products as potential producers of lipolytic, proteolytic and antibacterial proteins. Appl Microbiol Biotechnol 103(13):5243–5257
Griffiths MW, Tellez AM (2013) Lactobacillus helveticus: the proteolytic system. Front Microbiol 4:40677
Azizi S, Azizi MH, Moogouei R, Rajaei P (2020) The effect of quinoa flour and enzymes on the quality of gluten-free bread. Food Sci Nutr 8(5):2373–2382
Kawamura-Konishi Y, Shoda K, Koga H, Honda Y (2013) Improvement in gluten-free rice bread quality by protease treatment. J Cereal Sci 58(1):45–50
Dai Y, Tyl C (2021) A review on mechanistic aspects of individual versus combined uses of enzymes as clean label-friendly dough conditioners in breads. J Food Sci 86(5):1583–1598
Sharma N, Sharma N (2017) Microbial xylanases and their industrial applications as well as future perspectives: a review. Global J Biol Agric Health Sci 6:5–12
Selinheimo E, Autio K, Kruus K, Buchert J (2007) Elucidating the mechanism of laccase and tyrosinase in wheat bread making. J Agric Food Chem 55(15):6357–6365
Niño-Medina G, Gutiérrez-Soto G, Urías-Orona V, Hernández-Luna CE (2017) Effect of laccase from Trametes maxima CU1 on physicochemical quality of bread. Cogent Food & Agriculture 3(1):1328762
Eugenia Steffolani M, Ribotta PD, Pérez GT, León AE (2012) Combinations of glucose oxidase, α-amylase and xylanase affect dough properties and bread quality. Int J Food Sci Technol 47(3):525–534
Primo-Martín C, Wang M, Lichtendonk WJ, Plijter JJ, Hamer RJ (2005) An explanation for the combined effect of xylanase–glucose oxidase in dough systems. J Sci Food Agric 85(7):1186–1196
Yang M, Yue Y, Liu L, Tong L, Wang L, Ashraf J, Zhou S (2021) Investigation of combined effects of xylanase and glucose oxidase in whole wheat buns making based on reconstituted model dough system. LWT 135:110261
Primo-Martin C, Valera R, Martinez-Anaya MA (2003) Effect of pentosanase and oxidases on the characteristics of doughs and the glutenin macropolymer (GMP). J Agric Food Chem 51(16):4673–4679
Di Cagno R, De Angelis M, Limitone A, Minervini F, Carnevali P, Corsetti A et al (2006) Glucan and fructan production by sourdough Weissella cibaria and Lactobacillus plantarum. J Agric Food Chem 54(26):9873–9881
Korcz E, Varga L (2021) Exopolysaccharides from lactic acid bacteria: techno-functional application in the food industry. Trends Food Sci Technol 110:375–384
Tamani RJ, Goh KKT, Brennan CS (2013) Physico-chemical properties of sourdough bread production using selected lactobacilli starter cultures. J Food Qual 36(4):245–252
Symons LJ, Brennan CS (2004) The influence of (1→ 3)(1→ 4)-β-D-Glucan-rich fractions from barley on the physicochemical properties and in vitro reducing sugar release of white wheat breads. J Food Sci 69(6):463–467
Petkova M, Stefanova P, Gotcheva V, Kuzmanova I, Angelov A (2020) Microbiological and physiochemical characterization of traditional bulgarian sourdoughs and screening of lactic acid bacteria for amylolytic activity. J Chem Technol Metallurgy 55(5)
Seemann T (2014) Prokka: rapid prokaryotic genome annotation. Bioinformatics 30(14):2068–2069
Coêlho DF, Saturnino TP, Fernandes FF, Mazzola PG, Silveira E, Tambourgi EB (2016) Azocasein substrate for determination of proteolytic activity: reexamining a traditional method using bromelain samples. Biomed Res Int. https://doi.org/10.1155/2016/8409183
Karboune S, L’Hocine L, Anthoni J, Geraert PA, Kermasha S (2009) Properties of selected hemicellulases of a multi-enzymatic system from Penicillium funiculosum. Biosci Biotechnol Biochem 73(6):1286–1292
Li M, Karboune S (2021) Laccase-catalyzed conjugation of potato protein (PPT) with selected pectic polysaccharides (PPS): conjugation efficiency and emulsification properties. Food Chem 342:128212
Romo-Rodríguez P, Acevedo-Aguilar FJ, Lopez-Torres A, Wrobel K, Wrobel K, Gutiérrez-Corona JF (2015) Cr (VI) reduction by gluconolactone and hydrogen peroxide, the reaction products of fungal glucose oxidase: cooperative interaction with organic acids in the biotransformation of Cr (VI). Chemosphere 134:563–570
Li J, Karboune S (2019) Characterization of the composition and the techno-functional properties of mannoproteins from Saccharomyces cerevisiae yeast cell walls. Food Chem 297:124867
Sundarram A, Murthy TPK (2014) α-amylase production and applications: a review. Journal of Applied & Environmental Microbiology 2(4):166–175
Toe CJ, Foo HL, Loh TC, Mohamad R, Abdul Rahim R, Idrus Z (2019) Extracellular proteolytic activity and amino acid production by lactic acid bacteria isolated from Malaysian foods. Int J Mol Sci 20(7):1777
Papamanoli E, Tzanetakis N, Litopoulou-Tzanetaki E, Kotzekidou P (2003) Characterization of lactic acid bacteria isolated from a Greek dry-fermented sausage in respect of their technological and probiotic properties. Meat Sci 65(2):859–867
Essid I, Medini M, Hassouna M (2009) Technological and safety properties of Lactobacillus plantarum strains isolated from a Tunisian traditional salted meat. Meat Sci 81(1):203–208
Llorente-Bousquets A, Pérez-Munguía S, Farres A (2008) Novel extracellular proteolytic activity in Pediococcus acidilactici ATCC 8042. Can J Microbiol 54(8):694–699
Gänzle MG, Vermeulen N, Vogel RF (2007) Carbohydrate, peptide and lipid metabolism of lactic acid bacteria in sourdough. Food Microbiol 24(2):128–138
Upadhyay VK, McSweeney PLH, Magboul AAA, Fox PF (2004) Proteolysis in cheese during ripening. Cheese: chemistry, physics and microbiology 1(3):391–434
Cizeikiene D, Jagelaviciute J, Stankevicius M, Maruska A (2020) Thermophilic lactic acid bacteria affect the characteristics of sourdough and whole-grain wheat bread. Food Biosci 38:100791
Gänzle MG (2014) Enzymatic and bacterial conversions during sourdough fermentation. Food Microbiol 37:2–10
Matthews A, Grimaldi A, Walker M, Bartowsky E, Grbin P, Jiranek V (2004) Lactic acid bacteria as a potential source of enzymes for use in vinification. Appl Environ Microbiol 70(10):5715–5731
Harris AD, Ramalingam C (2010) Xylanases and its application in food industry: a review. J Exp Sci 1(7):1–11
Goswani GK, Rawat S (2015) Microbial xylanase and their applications. Int J Curr Res Acad Rev 3(6):436–450
Camacho NA, Aguilar G (2003) Production, purification, and characterization of a low-molecular-mass xylanase from Aspergillus sp. and its application in baking. Appl biochem biotech 104(3):159–171
Pontonio E, Mahony J, Di Cagno R, Motherway MOC, Lugli GA, O’Callaghan A et al (2016) Cloning, expression and characterization of a β-D-xylosidase from Lactobacillus rossiae DSM 15814 T. Microb Cell Fact 15(1):1–12
Michlmayr H, Hell J, Lorenz C, Böhmdorfer S, Rosenau T, Kneifel W (2013) Arabinoxylan oligosaccharide hydrolysis by family 43 and 51 glycosidases from Lactobacillus brevis DSM 20054. Appl Environ Microbiol 79(21):6747–6754
Lasrado LD, Gudipati M (2013) Purification and characterization of β-D-xylosidase from Lactobacillus brevis grown on xylo-oligosaccharides. Carbohyd Polym 92(2):1978–1983
Madhukumar MS, Muralikrishna G (2012) Fermentation of xylo-oligosaccharides obtained from wheat bran and Bengal gram husk by lactic acid bacteria and bifidobacteria. J Food Sci Technol 49(6):745–752
Patel MJ, Ng JHY, Hawkins WE, Pitts KF, Chakrabarti-Bell S (2012) Effects of fungal α-amylase on chemically leavened wheat flour doughs. J Cereal Sci 56(3):644–651
Díaz-Ruiz G, Guyot J, Ruiz-Teran F, Morlon-Guyot J, Wacher C (2003) Microbial and physiological characterization of weakly amylolytic but fast-growing lactic acid bacteria: a functional role in supporting microbial diversity in pozol, a Mexican fermented maize beverage. Appl Environ Microbiol 69(8):4367–4374
Songré-Ouattara LT, Mouquet-Rivier C, Icard-Vernière C, Humblot C, Diawara B, Guyot JP (2008) Enzyme activities of lactic acid bacteria from a pearl millet fermented gruel (ben-saalga) of functional interest in nutrition. Int J Food Microbiol 128(2):395–400
Sann AI, Morlon-Guyot J, Guyot JP (2002) New efficient amylase-producing strains of Lactobacillus plantarum and L fermentum isolated from different Nigerian traditional fermented foods. Int J Food Microbiol 72(1–2):53–62
Reddy G, Altaf MD, Naveena BJ, Venkateshwar M, Kumar EV (2008) Amylolytic bacterial lactic acid fermentation—a review. Biotechnol Adv 26(1):22–34
Petrova P, Petrov K (2012) Direct starch conversion into L-(+)-lactic acid by a novel amylolytic strain of Lactobacillus paracasei B41. Starch-stärke 64(1):10–17
Petrova P, Petrov K (2020) Lactic acid fermentation of cereals and pseudocereals: ancient nutritional biotechnologies with modern applications. Nutrients 12(4):1118
Demirkol DO, Dornbusch K, Feller KH, Timur S (2011) Microfluidic devices and true-color sensor as platform for glucose oxidase and laccase assays. Eng Life Sci 11(2):182–188
Selinheimo E, Kruus K, Buchert J, Hopia A, Autio K (2006) Effects of laccase, xylanase and their combination on the rheological properties of wheat doughs. J Cereal Sci 43(2):152–159
Minussi RC, Pastore GM, Durán N (2002) Potential applications of laccase in the food industry. Trends Food Sci Technol 13(6–7):205–216
Adelakun OE, Kudanga T, Parker A, Green IR, le Roes-Hill ML, Burton SG (2012) Laccase-catalyzed dimerization of ferulic acid amplifies antioxidant activity. J Mol Catal B Enzym 74:29–35
Aljawish A, Chevalot I, Jasniewski J, Paris C, Scher J, Muniglia L (2014) Laccase-catalysed oxidation of ferulic acid and ethyl ferulate in aqueous medium: a green procedure for the synthesis of new compounds. Food Chem 145:1046–1054
Bankar SB, Bule MV, Singhal RS, Ananthanarayan L (2009) Glucose oxidase—an overview. Biotechnol Adv 27(4):489–501
Callejón S, Sendra R, Ferrer S, Pardo I (2014) Identification of a novel enzymatic activity from lactic acid bacteria able to degrade biogenic amines in wine. Appl Microbiol Biotechnol 98(1):185–198
Callejón S, Sendra R, Ferrer S, Pardo I (2017) Recombinant laccase from Pediococcus acidilactici CECT 5930 with ability to degrade tyramine. PLoS ONE 12(10):e0186019
Guarcello R, De Angelis M, Settanni L, Formiglio S, Gaglio R, Minervini F et al (2016) Selection of amine-oxidizing dairy lactic acid bacteria and identification of the enzyme and gene involved in the decrease of biogenic amines. Appl Environ Microbiol 82(23):6870–6880
Olmeda I, Casino P, Collins RE, Sendra R, Callejón S, Huesa J, Pardo I (2021) Structural analysis and biochemical properties of laccase enzymes from two Pediococcus species. Microb Biotechnol 14(3):1026–1043
McPhillips K, Waters DM, Parlet C, Walsh DJ, Arendt EK, Murray PG (2014) Purification and characterisation of a β-1, 4-xylanase from Remersonia thermophila CBS 54.069 and its application in bread making. Appl biochem biotech 172(4):1747–1762
Waters DM, Ryan LA, Murray PG, Arendt EK, Tuohy MG (2011) Characterisation of a Talaromyces emersonii thermostable enzyme cocktail with applications in wheat dough rheology. Enzyme Microb Technol 49(2):229–236
Al Loman A, Ju LK (2016) Towards complete hydrolysis of soy flour carbohydrates by enzyme mixtures for protein enrichment: a modeling approach. Enzyme Microb Technol 86:25–33
Coda R, Di Cagno R, Gobbetti M, Rizzello CG (2014) Sourdough lactic acid bacteria: exploration of non-wheat cereal-based fermentation. Food Microbiol 37:51–58
Coda R, Di Cagno R, Rizzello CG, Nionelli L, Edema MO, Gobbetti M (2011) Utilization of African grains for sourdough bread making. J Food Sci 76(6):329–335
Gobbetti M, De Angelis M, Corsetti A, Di Cagno R (2005) Biochemistry and physiology of sourdough lactic acid bacteria. Trends Food Sci Technol 16(1–3):57–69
Gobbetti M, Rizzello CG, Di Cagno R, De Angelis M (2014) How the sourdough may affect the functional features of leavened baked goods. Food Microbiol 37:30–40
Thiel C, Gänzle M, Vogel RF (2002) Contribution of sourdough lactobacilli, yeast, and cereal enzymes to the generation of amino acids in dough relevant for bread flavor. Cereal Chem 79(1):45–51
Zotta T, Ricciardi A, Parente E (2007) Enzymatic activities of lactic acid bacteria isolated from Cornetto di Matera sourdoughs. Int J Food Microbiol 115(2):165–172
Moslehishad M, Mirdamadi S, Ehsani MR, Ezzatpanah H, Moosavi-Movahedi AA (2013) The proteolytic activity of selected lactic acid bacteria in fermenting cow’s and camel’s milk and the resultant sensory characteristics of the products. Int J Dairy Technol 66(2):279–285
Gandhi A, Shah NP (2014) Cell growth and proteolytic activity of Lactobacillus acidophilus, Lactobacillus helveticus, Lactobacillus delbrueckii ssp. bulgaricus, and Streptococcus thermophilus in milk as affected by supplementation with peptide fractions. Int J Food Sci Nutr 65(8):937–941
Manini F, Casiraghi MC, Poutanen K, Brasca M, Erba D, Plumed-Ferrer C (2016) Characterization of lactic acid bacteria isolated from wheat bran sourdough. LWT-food Science and Technology 66:275–283
Rollan G, De Angelis M, Gobbetti M, De Valdez GF (2005) Proteolytic activity and reduction of gliadin-like fractions by sourdough lactobacilli. J Appl Microbiol 99(6):1495–1502
Gerez C, Rollan GC, De Valdez GF (2006) Gluten breakdown by lactobacilli and pediococci strains isolated from sourdough. Lett Appl Microbiol 42(5):459–464
Petrova P, Petrov K, Stoyancheva G (2013) Starch-modifying enzymes of lactic acid bacteria–structures, properties, and applications. Starch-stärke 65(1–2):34–47
Waglay A, Karboune S (2016) Enzymatic generation of peptides from potato proteins by selected proteases and characterization of their structural properties. Biotechnol Prog 32:420–429
Kieliszek M, Pobiega K, Piwowarek K, Kot AM (2021) Characteristics of the proteolytic enzymes produced by lactic acid bacteria. Mol 26(7):1858
Pfeiler EA, Klaenhammer TR (2007) The genomics of lactic acid bacteria. Trends Microbiol 15(12):546–553
Akbulut S, Baltaci MO, Adiguzel G, Adiguzel A (2021) Identification and biotechnological characterization of lactic acid bacteria isolated from white cheese samples. J Pure Appl Microbiol 16(4):2912–2922
Adiguzel G, Fai O, Sisecioglu M, Sari B, Baltaci O, Akbulut S, Adiguzel A (2019) A novel endo-β-1, 4-xylanase from Pediococcus acidilactici GC25; purification, characterization and application in clarification of fruit juices. Int J Biol Macromol 129:571–578
Sivashankari S, Shanmughavel P (2006) Functional annotation of hypothetical proteins–a review. Bioinformation 1(8):335
Petrov K, Urshev Z, Petrova P (2008) L (+)-lactic acid production from starch by a novel amylolytic Lactococcus lactis subsp. lactis B84. Food Microbiology 25(4):550–557
Kim JH, Sunako M, Ono H, Murooka Y, Fukusaki E, Yamashita M (2008) Characterization of gene encoding amylopullulanase from plant-originated lactic acid bacterium, Lactobacillus plantarum L137. J Biosci Bioeng 106(5):449–459
Xu Y, Ding J, Gong S, Li M, Yang T, Zhang J (2020) Physicochemical properties of potato starch fermented by amylolytic Lactobacillus plantarum. Int J Biol Macromol 158:656–661
Blandino A, Al-Aseeri M, Pandiella S, Cantero D, Webb C (2003) Cereal-based fermented foods and beverages. Food Res Int 36(6):527–543
Wasko A, Polak-Berecka M, Targonski Z (2010) A new protein of α-amylase activity from Lactococcus lactis. J Microbiol Biotechnol 20(9):1307–1313
Muyanja CMBK, Narvhus JA, Treimo J, Langsrud T (2003) Isolation, characterisation and identification of lactic acid bacteria from bushera: a Ugandan traditional fermented beverage. Int J Food Microbiol 80(3):201–210
Turpin W, Humblot C, Guyot JP (2011) Genetic screening of functional properties of lactic acid bacteria in a fermented pearl millet slurry and in the metagenome of fermented starchy foods. Appl Environ Microbiol 77(24):8722–8734
Ghanimah M, El-Ghaish S, Salah S, Swelam S (2023) Optimal conditions for the production of exopolysaccharide by Pediococcus acidilactici and impact of the bacterium, its EPS and dextran on the quality of Kariesh cheese. Int J Dairy Technol 76(3):512–520
Lebeer S, Verhoeven TLA, Francius G, Schoofs G, Lambrichts I, Dufrêne Y, De Keersmaecker SCJ (2009) Identification of a gene cluster for the biosynthesis of a long, galactose-rich exopolysaccharide in Lactobacillus rhamnosus GG and functional analysis of the priming glycosyltransferase. Applied and Environ- mental Microbiology 75(11):3554–3563
Zannini E, Waters DM, Coffey A, Arendt EK (2016) Production, properties, and industrial food application of lactic acid bacteria-derived exopolysaccharides. Appl Microbiol Biotechnol 100(3):1121–1135
Tallon R, Bressollier P, Urdaci MC (2003) Isolation and characterization of two exopolysaccharides produced by Lactobacillus plantarum EP56. Res Microbiol 154(10):705–712
Ruas-Madiedo P, De Los Reyes-Gavilán CG (2005) Invited review: methods for the screening, isolation, and characterization of exopolysaccharides produced by lactic acid bacteria. J Dairy Sci 88(3):843–856
Badel S, Bernardi T, Michaud P (2011) New perspectives for Lactobacilli exopolysaccharides. Biotechnol Adv 29(1):54–66
Imran MYM, Reehana N, Jayaraj KA, Ahamed AAP, Dhanasekaran D, Thajuddin N et al (2016) Statistical optimization of exopolysaccharide production by Lactobacillus plantarum NTMI05 and NTMI20. Int J Biol Macromol 93:731–745
Malick A, Khodaei N, Benkerroum N, Karboune S (2017) Production of exopolysaccharides by selected Bacillus strains: optimization of media composition to maximize the yield and structural characterization. Int J Biol Macromol 102:539–549
De Vuyst L, Vancanneyt M (2007) Biodiversity and identification of sourdough lactic acid bacteria. Food Microbiol 24(2):120–127
De Vuyst L, Neysens P (2005) The sourdough microflora: biodiversity and metabolic interactions. Trends Food Sci Technol 16(1–3):43–56
Liu A, Jia Y, Zhao L, Gao Y, Liu G, Chen Y et al (2018) Diversity of isolated lactic acid bacteria in Ya’an sourdoughs and evaluation of their exopolysaccharide production characteristics. LWT 95:17–22
Gänzle M, Ripari V (2016) Composition and function of sourdough microbiota: from ecological theory to bread quality. Int J Food Microbiol 239:19–25
Pessione E (2012) Lactic acid bacteria contribution to gut microbiota complexity: lights and shadows. Front Cell Infect Microbiol 2:86
Buron-Moles G, Chailyan A, Dolejs I, Forster J, Mikš MH (2019) Uncovering carbohydrate metabolism through a genotype-phenotype association study of 56 lactic acid bacteria genomes. Appl Microbiol Biotechnol 103(7):3135–3152
du Toit M, Engelbrecht L, Lerm E, Krieger-Weber S (2011) Lactobacillus: the next generation of malolactic fermentation starter cultures—an overview. Food Bioprocess Technol 4(6):876–906
Oshiro M, Zendo T, Nakayama J (2021) Diversity and dynamics of sourdough lactic acid bacteriota created by a slow food fermentation system. J Biosci Bioeng 131(4):333–340
Arena MP, Russo P, Spano G, Capozzi V (2020) From microbial ecology to innovative applications in food quality improvements: the case of sourdough as a model matrix. J 3(1):9–19
Corsetti A, Settanni L (2007) Lactobacilli in sourdough fermentation. Food Res Int 40(5):539–558
Qiao N, Wittouck S, Mattarelli P, Zheng J, Lebeer S, Felis GE, Gänzle MG (2022) After the storm—perspectives on the taxonomy of Lactobacillaceae. JDS Communications 3(3):222–227
Oberg TS, McMahon DJ, Culumber MD, McAuliffe O, Oberg CJ (2022) Invited review: review of taxonomic changes in dairy-related lactobacilli. J Dairy Sci 105(4):2750–2770
Tamrat S, Borrell JS, Biswas MK, Gashu D, Wondimu T, Vásquez-Londoño CA et al (2020) Micronutrient composition and microbial community analysis across diverse landraces of the Ethiopian orphan crop enset. Food Res Int 137:109636
Al Daccache M, Koubaa M, Maroun RG, Salameh D, Louka N, Vorobiev E (2020) Impact of the physicochemical composition and microbial diversity in apple juice fermentation process: a review. Molecules 25(16):3698
Johanson A, Goel A, Olsson L, Franzén CJ (2020) Respiratory physiology of Lactococcus lactis in chemostat cultures and its effect on cellular robustness in frozen and freeze-dried starter cultures. Appl Environ Microbiol 86(6):e02785-e2819
Sun W, Jiang B, Zhang Y, Guo J, Zhao D, Pu Z, Bao Y (2020) Enabling the biosynthesis of malic acid in Lactococcus lactis by establishing the reductive TCA pathway and promoter engineering. Biochem Eng J 161:107645
Singh D, Johnson TA, Tyagi N, Malhotra R, Behare PV, Kumar S, Tyagi AK (2023) Synergistic effect of LAB strains (Lb. fermentum and Pediococcus acidilactisci) with exogenous fibrolytic enzymes on quality and fermentation characteristics of sugarcane tops silage. Sugar Tech 25(1):141–153
Säde E, Björkroth J (2019) Introduction to the genera Pediococcus, Leuconostoc, Weissella, and Carnobacterium. CRC Press, In Lactic Acid Bacteria, pp 65–85
Hill D, Sugrue I, Tobin C, Hill C, Stanton C, Ross RP (2018) The Lactobacillus casei group: history and health related applications. Front Microbiol 9:2107
Giraffa G, Chanishvili N, Widyastuti Y (2010) Importance of lactobacilli in food and feed biotechnology. Res Microbiol 161(6):480–487
Huo W, Zhang Y, Zhang L, Shen C, Chen L, Liu Q et al (2022) Effect of lactobacilli inoculation on protein and carbohydrate fractions, ensiling characteristics and bacterial community of alfalfa silage. Front Microbiol 13:1070175
Coelho MC, Malcata FX, Silva CC (2022) Lactic acid bacteria in raw-milk cheeses: from starter cultures to probiotic functions. Foods 11(15):2276
Patel M, Surti M, Siddiqui AJ, Alreshidi M, Ashraf SA, Adnan M (2022) Transcriptome-based characterization of interaction between fermenting microorganisms during production of bakery products. In African Fermented Food Products-New Trends. Springer, Cham, pp 143–156
Warburton A, Silcock P, Eyres GT (2022) Impact of sourdough culture on the volatile compounds in wholemeal sourdough bread. Food Res Int 161:111885
Landis EA, Oliverio AM, McKenney EA, Nichols LM, Kfoury N, Biango-Daniels M, Wolfe BE (2021) The diversity and function of sourdough starter microbiomes. Elife 10:e61644
Primo-Martín C, Martínez-Anaya MA (2003) Influence of pentosanase and oxidases on water-extractable pentosans during a straight breadmaking process. J Food Sci 68(1):31–41
Flander L, Rouau X, Morel MH, Autio K, Seppänen-Laakso T, Kruus K, Buchert J (2008) Effects of laccase and xylanase on the chemical and rheological properties of oat and wheat doughs. J Agric Food Chem 56(14):5732–5742
Acknowledgements
The authors are thankful to Dongyun Jung for the help with the genome sequencing and Pratibha Sharma for maintaining the strains.
Funding
This study was financially supported by the “Ministère de l’Agriculture, des Pêcheries, et de l’Alimentation du Québec” (MAPAQ), through the “Consortium de Recherche Innovation Transformation Alimentaire” (RITA) and by local Québec Industries (e.g., Première Moisson, Boulangerie St-Méthode, France Délices, Moulins de Soulanges, Lallemand).
Author information
Authors and Affiliations
Contributions
YD: investigation, methodology, formal analysis, data curation, and writing—the initial draft. JR and IF: methodology, validation, and writing—review and editing. SK: conceptualization, validation, supervision, project administration, and writing—review and editing. All authors reviewed and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dong, Y., Ronholm, J., Fliss, I. et al. Screening of Lactic Acid Bacteria Strains for Potential Sourdough and Bread Applications: Enzyme Expression and Exopolysaccharide Production. Probiotics & Antimicro. Prot. (2024). https://doi.org/10.1007/s12602-024-10270-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s12602-024-10270-y