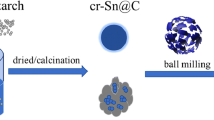
Abstract
Antimony (Sb) is an intriguing anode material for Li-ion batteries (LIBs) owing to its high theoretical capacity of 660 mAh·g−1 and appropriate working potential of ~ 0.8 V (vs. Li+/Li). However, just like all alloying materials, the Sb anode suffers from huge volume expansion (230%) during repeated insertion/extraction of Li+ ions, resulting in structural deterioration and rapid capacity decay. In this work, a novel amorphous Sb/C composite with atomically dispersed Sb particles in carbon matrix is prepared via a straightforward high-energy ball milling approach. The intimate intermixing of amorphous Sb with C provides homogeneous element distribution and isotropic volume expansion during cycling, resulting in persistent structural stability. Meanwhile, the disordered structure of amorphous material shortens the diffusion distance of lithium ions/electrons, promoting fast reaction kinetics and rate capability. Benefiting from the aforementioned effects, the amorphous Sb/C exhibits a high reversible capacity of 537.4 mAh·g−1 at 0.1 A·g−1 and retains 201.0 mAh·g−1 at an ultrahigh current rate of 10.0 A·g−1. Even after 1500 deep cycles at 2.0 A·g−1, the amorphous Sb/C electrode still maintains 86.3% of its initial capacity, which outperforms all existing Sb-based anodes reported so far. Post-mortem analysis further reveals a greatly reduced volume variation of merely 34.6% for the amorphous Sb/C electrode, much lower than that of 223.1% for crystalline Sb materials. This study presents a new approach to stabilizing Sb-based alloy anodes and contributes to the construction of high-performance amorphous anode materials for LIBs, enabling advanced energy storage.
Graphical abstract

摘要
锑 (Sb) 基负极具有660 mAh·g−1的高理论容量和约0.8 V (相对于Li+/Li) 的工作电位, 是一种极具发展潜力的锂离子电池负极材料。然而, Sb负极在嵌脱锂程中会遭受巨大的体积膨胀 (230%), 导致结构稳定性恶化和容量迅速下降, 阻碍了其实际应用。在本工作中, 我们采用简单的高能球磨法首次制备了一种新型无定形Sb/C复合材料。在该复合物中, 非晶态的Sb颗粒均匀分散在碳主体框架中, 这一原子尺度的元素均匀分布使得嵌锂过程产生各向同性的体积膨胀, 从而缓解了结构应力, 并有助于维持长循环过程的结构稳定性。此外, 无定形纳米结构缩短了锂离子/电子的扩散通道, 促进了快速锂化反应动力学和高倍率性能。得益于此, 无定形Sb/C复合材料在0.1 A·g−1电流密度下表现出537.4 mAh·g−1的高可逆容量, 并在10 A·g−1的超高电流下仍能保持201 mAh·g−1的容量。即使在2.0 A·g−1下深度循环1500次后, 该材料仍能保持其初始容量的86.3%, 远超迄今报道的所有Sb基负极。对长循环后电极的形貌深入分析显示, 无定形Sb/C电极的体积膨胀率仅为34.6%, 远低于传统纳米晶态Sb/C负极223.1%的体积膨胀。总的来说, 本工作提出了一种制备超长循环寿命、超高倍率Sb基合金负极的新方法, 并将有助于高性能锂离子电池的开发。







Similar content being viewed by others
References
Choi JW, Aurbach D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat Rev Mater. 2016;1(4):16013. https://doi.org/10.1038/natrevmats.2016.13.
Li M, Lu J, Chen Z, Amine K. 30 Years of lithium-ion batteries. Adv Mater. 2018;2018:e1800561. https://doi.org/10.1002/adma.201800561.
Mao C, Ruther RE, Li J, Du Z, Belharouak I. Identifying the limiting electrode in lithium ion batteries for extreme fast charging. Electrochem Commun. 2018;97:37. https://doi.org/10.1016/j.elecom.2018.10.007.
Nitta N, Wu F, Lee JT, Yushin G. Li-ion battery materials: present and future. Mater Today. 2015;18(5):252. https://doi.org/10.1016/j.mattod.2014.10.040.
Chu Y, Guo L, Xi B, Feng Z, Wu F, Lin Y, Liu J, Sun D, Feng J, Qian Y, Xiong S. Embedding MnO@Mn3O4 nanoparticles in an N-doped-carbon framework derived from Mn-organic clusters for efficient lithium storage. Adv Mater. 2018;30(6):1704244. https://doi.org/10.1002/adma.201704244.
Chu Y, Xi B, Xiong S. One-step construction of MoO2 uniform nanoparticles on graphene with enhanced lithium storage. Chin Chem Lett. 2021;32(6):1983. https://doi.org/10.1016/j.cclet.2020.10.024.
Zhang Q, Xi B, Xiong S, Qian Y. Carbon coated SiO nanoparticles embedded in hierarchical porous N-doped carbon nanosheets for enhanced lithium storage. Inorg Chem Front. 2021;8(18):4282. https://doi.org/10.1039/d1qi00778e.
Chu Y, Xiong S. Mixed transition-metal oxides@carbon core-shell nanostructures derived from heterometallic clusters for enhanced lithium storage. Chin Chem Lett. 2022;33(1):486. https://doi.org/10.1016/j.cclet.2021.06.074.
Xin F, Whittingham MS. Challenges and development of tin-based anode with high volumetric capacity for Li-ion batteries. Electrochem Energy Rev. 2020;3(4):643. https://doi.org/10.1007/s41918-020-00082-3.
Nam KH, Jeong S, Yu BC, Choi JH, Jeon KJ, Park CM. Li-compound anodes: a classification for high-performance Li-Ion battery anodes. ACS Nano. 2022;16(9):13704. https://doi.org/10.1021/acsnano.2c05172.
Cheng H, Shapter JG, Li Y, Gao G. Recent progress of advanced anode materials of lithium-ion batteries. J Energy Chem. 2021;57:451. https://doi.org/10.1016/j.jechem.2020.08.056.
Qian J, Qiao D, Ai X, Cao Y, Yang H. Reversible 3-Li storage reactions of amorphous phosphorus as high capacity and cycling-stable anodes for Li-ion batteries. Chem Commun. 2012;48(71):8931. https://doi.org/10.1039/c2cc34388f.
He J, Wei Y, Zhai T, Li H. Antimony-based materials as promising anodes for rechargeable lithium-ion and sodium-ion batteries. Mater Chem Front. 2018;2(3):437. https://doi.org/10.1039/c7qm00480j.
Qian J, Chen Y, Wu L, Cao Y, Ai X, Yang H. High capacity Na-storage and superior cyclability of nanocomposite Sb/C anode for Na-ion batteries. Chem Commun. 2012;48(56):7070. https://doi.org/10.1039/c2cc32730a.
Liang SZ, Wang XY, Xia YG, Xia SL, Metwalli E, Qiu B, Ji Q, Yin SS, Xie S, Fang K, Zheng LY, Wang MM, Zuo XX, Li RJ, Liu ZP, Zhu J, Müller-Buschbaum P, Cheng YJ. Scalable synthesis of hierarchical antimony/carbon micro-/nanohybrid lithium/sodium-ion battery anodes based on dimethacrylate monomer. Acta Metall Sin. 2018;31(9):910. https://doi.org/10.1007/s40195-018-0733-5.
Liu XH, Huang JY. In situ TEM electrochemistry of anode materials in lithium ion batteries. Energy Environ Sci. 2011;4(10):3844. https://doi.org/10.1039/C1EE01918J.
Yuk JM, Seo HK, Choi JW, Lee JY. Anisotropic lithiation onset in silicon nanoparticle anode revealed by in situ graphene liquid cell electron microscopy. ACS Nano. 2014;8(7):7478. https://doi.org/10.1021/nn502779n.
Liu J, Yu L, Wu C, Wen Y, Yin K, Chiang FK, Hu R, Liu J, Sun L, Gu L, Maier J, Yu Y, Zhu M. New nanoconfined galvanic replacement synthesis of hollow Sb@C yolk-shell spheres constituting a stable anode for high-rate Li/Na-ion batteries. Nano Lett. 2017;17(3):2034. https://doi.org/10.1021/acs.nanolett.7b00083.
Li X-Y, Qu J-K, Yin H-Y. Electrolytic alloy-type anodes for metal-ion batteries. Rare Met. 2021;40(2):329. https://doi.org/10.1007/s12598-020-01537-8.
He M, Kravchyk K, Walter M, Kovalenko MV. Monodisperse antimony nanocrystals for high-rate Li-ion and Na-ion battery anodes: nano versus bulk. Nano Lett. 2014;14(3):1255. https://doi.org/10.1021/nl404165c.
Yang Q, Zhou J, Zhang G, Guo C, Li M, Zhu Y, Qian Y. Sb nanoparticles uniformly dispersed in 1-D N-doped porous carbon as anodes for Li-ion and Na-ion batteries. J Mater Chem A. 2017;5(24):12144. https://doi.org/10.1039/c7tă0f.
Hou H, Jing M, Yang Y, Zhu Y, Fang L, Song W, Pan C, Yang X, Ji X. Sodium/Lithium storage behavior of antimony hollow nanospheres for rechargeable batteries. ACS Appl Mater Inter. 2014;6(18):16189. https://doi.org/10.1021/am504310k.
Gao Y, Tian W, Huo C, Zhang K, Guo S, Zhang S, Song X, Jiang L, Huo K, Zeng H. Tailoring natural layered β-phase antimony into few layer antimonene for Li storage with high rate capabilities. J Mater Chem A. 2019;7(7):3238. https://doi.org/10.1039/c8ta11218e.
Yuan Y, Yao W, Yurkiv V, Liu T, Song B, Mashayek F, Shahbazian-Yassar R, Lu J. Beyond volume variation: anisotropic and protrusive lithiation in bismuth nanowire. ACS Nano. 2020;14(11):15669. https://doi.org/10.1021/acsnano.0c06597.
Yi Z, Han Q, Ju S, Wu Y, Cheng Y, Wang L. Fabrication of one-dimensional Sb@TiO2 composites as anode materials for Lithium-Ion batteries. J Electrochem Soc. 2016;163(13):A2641. https://doi.org/10.1149/2.0881613jes.
Li N, Liao S, Sun Y, Song HW, Wang CX. Uniformly dispersed self-assembled growth of Sb2O3/Sb@graphene nanocomposites on a 3D carbon sheet network for high Na-storage capacity and excellent stability. J Mater Chem A. 2015;3(11):5820. https://doi.org/10.1039/c4ta06825d.
Yi Z, Han Q, Zan P, Wu Y, Cheng Y, Wang L. Sb nanoparticles encapsulated into porous carbon matrixes for high-performance lithium-ion battery anodes. J Power Sour. 2016;331:16. https://doi.org/10.1016/j.jpowsour.2016.09.027.
Lv H, Qiu S, Lu G, Fu Y, Li X, Hu C, Liu J. Nanostructured antimony/carbon composite fibers as anode material for lithium-ion battery. Electrochim Acta. 2015;151:214. https://doi.org/10.1016/j.electacta.2014.11.013.
Xu X, Si L, Zhou XS, Tu FZ, Zhu XS, Bao JC. Chemical bonding between antimony and ionic liquid-derived nitrogen-doped carbon for sodium-ion battery anode. J Power Sour. 2017;349:37. https://doi.org/10.1016/j.jpowsour.2017.03.026.
Barnes P, Zuo Y, Dixon K, Hou D, Lee S, Ma Z, Connell JG, Zhou H, Deng C, Smith K, Gabriel E, Liu Y, Maryon OO, Davis PH, Zhu H, Du Y, Qi J, Zhu Z, Chen C, Zhu Z, Zhou Y, Simmonds PJ, Briggs AE, Schwartz D, Ong SP, Xiong H. Electrochemically induced amorphous-to-rock-salt phase transformation in niobium oxide electrode for Li-ion batteries. Nat Mater. 2022;21(7):795. https://doi.org/10.1038/s41563-022-01242-0.
Pan W, Cai X, Yang C, Zhou L. Amorphous Si/TiC/graphite composite fabricated by high-energy ball-milling as an anode for lithium-ion batteries. J Electron Mater. 2021;50(5):2584. https://doi.org/10.1007/s11664-021-08767-1.
Li Y, Lai XQ, Qu JP, Lai QZ, Yi TF. Research progress in regulation strategies of high-performance antimony-based anode materials for sodium ion batteries. Acta Phys-Chim Sin. 2022;38(11):2204049. https://doi.org/10.3866/Pku.Whxb202204049.
Wei Y, Liu X, Yao R, Qian J, Yin Y, Li D, Chen Y. Embedding the high entropy alloy nanoparticles into carbon matrix toward high performance Li-ion batteries. J Alloys Compd. 2023;938:168610. https://doi.org/10.1016/j.jallcom.2022.168610.
Huang M, Xi B, Mi L, Zhang Z, Chen W, Feng J, Xiong S. Rationally designed three-layered TiO2 @amorphous MoS3 @carbon hierarchical microspheres for efficient potassium storage. Small. 2022;2022:e2107819. https://doi.org/10.1002/smll.202107819.
Zhao L, Dvorak DJ, Obrovac MN. Layered amorphous silicon as negative electrodes in lithium-ion batteries. J Power Sour. 2016;332:290. https://doi.org/10.1016/j.jpowsour.2016.09.124.
Han Y, Lin N, Xu T, Li T, Tian J, Zhu Y, Qian Y. An amorphous Si material with a sponge-like structure as an anode for Li-ion and Na-ion batteries. Nanoscale. 2018;10(7):3153. https://doi.org/10.1039/c7nr08886h.
Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys Rev Lett. 1996;77(18):3865. https://doi.org/10.1103/PhysRevLett.77.3865.
Vanderbilt D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys Rev B. 1990;41(11):7892. https://doi.org/10.1103/PhysRevB.41.7892.
Hoover WG. Canonical dynamics: equilibrium phase-space distributions. Phys Rev A. 1985;31(3):1695. https://doi.org/10.1103/PhysRevA.31.1695.
Govind N, Petersen M, Fitzgerald G, King-Smith D, Andzelm J. A generalized synchronous transit method for transition state location. Comput Mater Sci. 2003;28(2):250. https://doi.org/10.1016/s0927-0256(03)00111-3.
Halgren TA, Lipscomb WN. The synchronous-transit method for determining reaction pathways and locating molecular transition states. Chem Phys Lett. 1977;49(2):225. https://doi.org/10.1016/0009-2614(77)80574-5.
Henkelman G, Jonsson H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J Chem Phys. 2000;113(22):9978. https://doi.org/10.1063/1.1323224.
Henkelman G, Uberuaga BP, Jonsson H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J Chem Phys. 2000;113(22):9901. https://doi.org/10.1063/1.1329672.
Smidstrup S, Pedersen A, Stokbro K, Jonsson H. Improved initial guess for minimum energy path calculations. J Chem Phys. 2014;140(21): 214106. https://doi.org/10.1063/1.4878664.
Qian J, Xiong Y, Cao Y, Ai X, Yang H. Synergistic Na-storage reactions in Sn4P3 as a high capacity and cycle-stable anode of Na-ion batteries. Nano Lett. 2014;14(4):1865. https://doi.org/10.1021/nl404637q.
Nzabahimana J, Liu Z, Guo S, Wang L, Hu X. Top-down synthesis of silicon/carbon composite anode materials for lithium-ion batteries: mechanical milling and etching. Chemsuschem. 2020;13(8):1923. https://doi.org/10.1002/cssc.201903155.
Qian J, Wu X, Cao Y, Ai X, Yang H. High capacity and rate capability of amorphous phosphorus for sodium ion batteries. Angew Chem Int Ed. 2013;52(17):4633. https://doi.org/10.1002/anie.201209689.
Ramireddy T, Rahman MM, Xing T, Chen Y, Glushenkov AM. Stable anode performance of an Sb–carbon nanocomposite in lithium-ion batteries and the effect of ball milling mode in the course of its preparation. J Mater Chem A. 2014;2(12):4282. https://doi.org/10.1039/c3ta14643j.
Zhu YJ, Han XG, Xu YH, Liu YH, Zheng SY, Xu K, Hu LB, Wang CS. Electrospun Sb/C fibers for a stable and fast sodium-ion battery anode. ACS Nano. 2013;7(7):6378. https://doi.org/10.1021/nn4025674.
Vorokh AS. Scherrer formula: estimation of error in determining small nanoparticle size. Nanosyst-Phys Chem. 2018;9(3):364. https://doi.org/10.17586/2220-8054-2018-9-3-364-369.
Shkodich NF, Vadchenko SG, Nepapushev AA, Kovalev DY, Kovalev ID, Ruvimov S, Rogachev AS, Mukasyan AS. Crystallization of amorphous Cu50Ti50 alloy prepared by high-energy ball milling. J Alloys Compd. 2018;741:575. https://doi.org/10.1016/j.jallcom.2018.01.062.
Hou HS, Jing MJ, Yang YC, Zhang Y, Song WX, Yang XM, Chen J, Chen QY, Ji XB. Antimony nanoparticles anchored on interconnected carbon nanofibers networks as advanced anode material for sodium-ion batteries. J Power Sour. 2015;284:227. https://doi.org/10.1016/j.jpowsour.2015.03.043.
Yang Y, Yang X, Zhang Y, Hou H, Jing M, Zhu Y, Fang L, Chen Q, Ji X. Cathodically induced antimony for rechargeable Li-ion and Na-ion batteries: the influences of hexagonal and amorphous phase. J Power Sour. 2015;282:358. https://doi.org/10.1016/j.jpowsour.2015.02.071.
Yang T, Zhong J, Liu J, Yuan Y, Yang D, Mao Q, Li X, Guo Z. A general strategy for antimony-based alloy nanocomposite embedded in swiss-cheese-like nitrogen-doped porous carbon for energy storage. Adv Funct Mater. 2021. https://doi.org/10.1002/adfm.202009433.
Yang J, Li JB, Wang TY, Notten PHL, Ma H, Liu ZG, Wang CY, Wang GX. Novel hybrid of amorphous Sb/N-doped layered carbon for high-performance sodium-ion batteries. Chem Eng J. 2021;407:127169. https://doi.org/10.1016/j.cej.2020.127169.
Zhang W, Du X, Tan Y, Hu J, Li Z, Tang B. Amorphous cobalt boron alloy@graphene oxide nanocomposites for pseudocapacitor applications. J Mater Sci Technol. 2017;33(5):438. https://doi.org/10.1016/j.jmst.2016.06.012.
Li HB, Yu MH, Wang FX, Liu P, Liang Y, Xiao J, Wang CX, Tong YX, Yang GW. Amorphous nickel hydroxide nanospheres with ultrahigh capacitance and energy density as electrochemical pseudocapacitor materials. Nat Commun. 2013;4:1894. https://doi.org/10.1038/ncomms2932.
Yang L, Yang B, Chen X, Wang H, Dang J, Liu X. Bimetallic alloy SbSn nanodots filled in electrospun N-doped carbon fibers for high performance Na-ion battery anode. Electrochim Acta. 2021;389:138246. https://doi.org/10.1016/j.electacta.2021.138246.
Zheng J, Yang Y, Fan X, Ji G, Ji X, Wang H, Hou S, Zachariah MR, Wang C. Extremely stable antimony–carbon composite anodes for potassium-ion batteries. Energy Environ Sci. 2019;12(2):615. https://doi.org/10.1039/c8ee02836b.
Hassoun J, Derrien G, Panero S, Scrosati B. The role of the morphology in the response of Sb–C nanocomposite electrodes in lithium cells. J Power Sour. 2008;183(1):339. https://doi.org/10.1016/j.jpowsour.2008.05.017.
Ku JH, Ryu JH, Kim SH, Han OH, Oh SM. reversible lithium storage with high mobility at structural defects in amorphous molybdenum dioxide electrode. Adv Funct Mater. 2012;22(17):3658. https://doi.org/10.1002/adfm.201102669.
Arnold S, Gentile A, Li YJ, Wang QS, Marchionna S, Ruffo R, Presser V. Design of high-performance antimony/MXene hybrid electrodes for sodium-ion batteries. J Mater Chem A. 2022;10(19):10569. https://doi.org/10.1039/d2ta00542e.
Chen H, Yang Y, Boyle DT, Jeong YK, Xu R, de Vasconcelos LS, Huang Z, Wang H, Wang H, Huang W, Li H, Wang J, Gu H, Matsumoto R, Motohashi K, Nakayama Y, Zhao K, Cui Y. Free-standing ultrathin lithium metal–graphene oxide host foils with controllable thickness for lithium batteries. Nat Energy. 2021;6(8):790. https://doi.org/10.1038/s41560-021-00833-6.
Liu MC, Yang ZZ, Shen YF, Guo SH, Zhang JY, Ai XP, Yang HX, Qian JF. Chemically presodiated Sb with a fluoride-rich interphase as a cycle-stable anode for high-energy sodium ion batteries. J Mater Chem A. 2021;9(9):5639. https://doi.org/10.1039/d0ta10880d.
Jiao S, Ren X, Cao R, Engelhard MH, Liu Y, Hu D, Mei D, Zheng J, Zhao W, Li Q, Liu N, Adams BD, Ma C, Liu J, Zhang JG, Xu W. Stable cycling of high-voltage lithium metal batteries in ether electrolytes. Nat Energy. 2018;3(9):739. https://doi.org/10.1038/s41560-018-0199-8.
Zhou CC, Su Z, Gao XL, Cao R, Yang SC, Liu XH. Ultra-high-energy lithium-ion batteries enabled by aligned structured thick electrode design. Rare Met. 2022;41(1):14. https://doi.org/10.1007/s12598-021-01785-2.
Ye L, Zhang C, Zhou Y, Ülgüt B, Zhao Y, Qian J. Guided lithium nucleation and growth on lithiophilic tin-decorated copper substrate. J Energy Chem. 2022;74:412. https://doi.org/10.1016/j.jechem.2022.07.027.
Wu C, Zhou Y, Zhu XL, Zhan MZ, Yang HX, Qian JF. Research progress on high concentration electrolytes for li metal batteries. Acta Phys-Chim Sin. 2021;37(2):2008044. https://doi.org/10.3866/Pku.Whxb202008044.
Chen B, Chen L, Zu L, Feng Y, Su Q, Zhang C, Yang J. Zero-strain high-capacity silicon/carbon anode enabled by a MOF-derived space-confined single-atom catalytic strategy for lithium-ion batteries. Adv Mater. 2022;34(21):e2200894. https://doi.org/10.1002/adma.202200894.
Zheng MH, Liu Y, Ru Q, Zhang J, Pan ZK, Gao YQ, Ling FCC, Wei L. Novel antimony phosphate loaded on grid-like N, S-doped carbon for facilitating sodium-ion storage. Chem Eng J. 2021;415:128942. https://doi.org/10.1016/j.cej.2021.128942.
Zhou L, Jiao P, Fang L, Liu L, Hao Z, Wang H, Kang YM, Zhang K, Chen J. Two-phase transition induced amorphous metal phosphides enabling rapid, reversible alkali-metal ion storage. ACS Nano. 2021;15(8):13486. https://doi.org/10.1021/acsnano.1c04041.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 22279093 and 22075216), the Natural Science Foundation of Hubei Province, China (No. 2022CFB096), and the Fundamental Research Funds for Central University (Nos. 2042022gf0005 and 2042021kf0194). The authors would like to thank Prof. Burak Ülgüt at Bilkent University for his assistance with EIS analysis and Dr. Shaobo Mo from the Core Facility of Wuhan University for the support of TEM tests.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Ze-Zhou Yang and Cheng-Yi Zhang have contributed equally to this work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, ZZ., Zhang, CY., Ou, YQ. et al. Amorphous Sb/C composite with isotropic expansion property as an ultra-stable and high-rate anode for lithium-ion batteries. Rare Met. 43, 2039–2052 (2024). https://doi.org/10.1007/s12598-023-02548-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-023-02548-x