Abstract
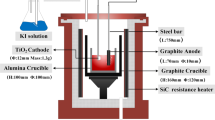
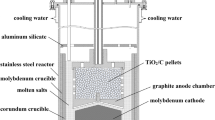
In this paper, a new electrolysis device was presented with corundum crucible as an electrolytic cell in place of a graphite crucible, and in the corundum crucible a sleeve with a cathode pellet with a fluted base placed flat in it was adopted to separate the cathode and anode. The process of electrochemical reduction of solid TiO2 to Ti in situ was studied and characterized by the time–current curves and X-ray diffraction (XRD) patterns of the electrochemical reduction products. The influence of CaCl2 doping in the cathode and the electrolysis device structures on electrochemical reduction mechanisms and the process strengthening was systematically studied. The results show that the oxygen content in the obtained Ti is reduced to 0.51% with a cathode pellet sintering temperature of 1000 °C, sample preparation pressure of 20 MPa and CaCl2 doping amount of 30%. Tiny holes are formed in the cathode pellet by CaCl2 doping in the electrochemical reduction process, which could increase the contact area between the electrolyte and cathode and improve the electrode reaction efficiency. The new electrolysis device could reduce the carbon content in the molten salt, cathode polarization and the electrode reaction overvoltage, inhibit the chances of secondary reactions, increase the contact area between the produced Ca and cathode and strengthen the thermal reduction of TiO2 by Ca.












Similar content being viewed by others
References
Takenaka T, Suzuki T, Ishikawa M, Fukasawa E, Kawakami M. The new concept for electrowinning process of liquid titanium metal in molten salt. Electrochemistry. 1999;67(6):661.
Takenaka T, Matsuo H, Sugawara M, Kawakami M. High temperature electrolysis of Ti and its alloys with a DC-ESR unit. Key Eng Mater. 2010;463(2):85.
Takenaka T, Matsuo H, Kawakami M. Direct production of Ti–Fe alloy in liquid by electrowinning in molten slag. ISIJ Int. 2011;51(11):1762.
Chen GZ, Fray DJ, Farthing TW. Direct electrochemical reduction of titanium dioxide to titanium in molten calcium chloride. Nature. 2000;407(21):361.
Chen GZ, Fray DJ, Farthing TW. Cathodic deoxygenation of the alpha case on titanium and alloys in molten calcium chloride. Metall Mater Trans B. 2001;32(6):1041.
Schwandt C, Fray DJ. Determination of the kinetic pathway in the electrochemical reduction of titanium dioxide in molten calcium chloride. Electrochim Acta. 2005;51(1):66.
Fray DJ. Emerging molten salt technologies for metals production. J Metals. 2001;53(10):27.
Schwandt C, Alexander DTL, Fray DJ. The electro-deoxidation of porous titanium dioxide precursors in molten calcium chloride under cathodic potential control. Electrochim Acta. 2009;54(14):3819.
Alexander DTL, Schwandt C, Fray DJ. Microstructural kinetics of phase transformations during electrochemical reduction of titanium dioxide in molten calcium chloride. Acta Mater. 2006;54(11):2933.
Schwandt C, Doughty GR, Fray DJ. The FFC-Cambridge process for titanium metal winning. Key Eng Mater. 2010;436(436):13.
Alexander DTL, Schwandt C, Fray DJ. The electro-deoxidation of dense titanium dioxide precursors in molten calcium chloride giving a new reaction pathway. Electrochim Acta. 2011;56(9):3286.
Zhao K, Wang YW, Peng JP, Di YZ, Deng XZ, Feng NX. Electrochemical preparation of titanium and titanium–copper alloys with K2Ti6O13 in KF-KCl melts. Rare Met. 2017;36(6):527.
Suzuki RO. Calciothermic reduction of TiO2 and in situ electrolysis of CaO in the molten CaCl2. J Phys Chem Solids. 2005;66(2):461.
Suzuki RO, Inoue S. Calciothermic reduction of titanium oxide and in situ electrolysis in molten CaCl2. Metall Mater Trans B. 2003;34(3):287.
Ono K, Suzuki RO. A new concept for producing Ti sponge: calciothermic reduction. J Metals. 2002;54(2):59.
Zheng H, Ito H, Okabe TH. Production of titanium powder by the calciothermic reduction of titanium concentrates or ore using the preform reduction process. Mater Trans. 2007;48(8):2244.
Okabe TH, Oda T, Mitsuda Y. Titanium powder production by perform reduction process (PRP). J Alloys Compd. 2004;364(1):156.
Kim P, Xie HW, Zhai YC, Zou XY, Lang XC. Direct electrochemical reduction of Dy2O3 in CaCl2 melt. J Appl Electrochem. 2012;42(4):257.
Jin X, Gao P, Wang D, Hu X, Chen GZ. Electrochemical preparation of silicon and its alloys from solid oxides in molten calcium chloride. Angew Chem. 2004;116(6):751.
Yan XY, Fray DJ. Production of niobium powder by direct electrochemical reduction of solid Nb2O5 in a eutectic CaCl2–NaCl melt. Metall Mater Trans B. 2002;33(5):685.
Yan XY, Fray DJ. Using electrodeoxidation to synthesize niobium sponge from solid Nb2O5 in alkali–alkaline–earth metal chloride melts. J Mater Res. 2003;18(2):346.
Xu Q, Deng LQ, Wu Y, Ma T. A study of cathode improvement for electro-deoxidation of Nb2O5 in a eutectic CaCl2–NaCl melt at 1073 K. J Alloys Compd. 2005;396(1–2):288.
Fray DJ, Chen GZ. Reduction of titanium and other metal oxides using electrodeoxidation. Mater Sci Technol. 2004;20(3):295.
Xiao W, Wang DH. Rare metals preparation by electro-reduction of solid compounds in high-temperature molten salts. Rare Met. 2016;35(8):581.
Wang Z, Li J, Hua YX, Xu CY, Zhang Z, Zhang Y. Preparation of Fe–Ti oxide electrode material for production of Ti–Fe alloy by molten salt electrolysis. Chin J Rare Metals. 2016;40(3):252.
Li ZQ, Hu L, Lu J, Liu N, Han M. Research progress in mutual influences between substances during preparation of alloy via molten salt electro-deoxidation. Chin J Rare Metals. 2016;40(11):1169.
Guan CL, Zhang TA, Chen JS. Cathodic processes in electrochemical deoxidation of solid TiO2 to Ti. J Mater Metall. 2008;7(4):267.
Guan CL, Zhang TA, Dou ZH. Investigation of anode and electrode processes of solid state in situ electrochemical reduction from TiO2 to Ti. J Northeast Univ (Nat Sci). 2012;3(9):1307.
Lu ZE. Electrode Process Fundamentals and Applications. Beijing: Higher Education Press; 1992. 47.
Bard AJ, Faulkner LR. Electrochemical Methods Fundamentals and Applications. Beijing: Chemical Industry Press; 2005. 160.
Zha QX. Kinetics Introduction of Electrode Process. Beijing: Science Press; 2002. 91.
Acknowledgements
This study was financially supported by the National Key Basic Research Program of China (No. 2013CB632606-2), the Fundamental Research Funds for the Central Universities (Nos. N110202003 and N130102002) and the National Natural Science Foundation of China (Nos. 51274064 and 51422403).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guan, CL., Zhang, TA., Chen, JS. et al. Process strengthening for electrochemical reduction of solid TiO2 to Ti in situ. Rare Met. 42, 2104–2112 (2023). https://doi.org/10.1007/s12598-018-1158-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-018-1158-z