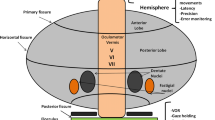
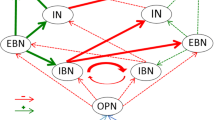
Abstract
Cerebellar ataxias are a wide heterogeneous group of disorders that may present with fine motor deficits as well as gait and balance disturbances that have a significant influence on everyday activities. To review the ocular movements in cerebellar ataxias in order to improve the clinical knowledge of cerebellar ataxias and related subtypes. English papers published from January 1990 to May 2022 were selected by searching PubMed services. The main search keywords were ocular motor, oculomotor, eye movement, eye motility, and ocular motility, along with each ataxia subtype. The eligible papers were analyzed for clinical presentation, involved mutations, the underlying pathology, and ocular movement alterations. Forty-three subtypes of spinocerebellar ataxias and a number of autosomal dominant and autosomal recessive ataxias were discussed in terms of pathology, clinical manifestations, involved mutations, and with a focus on the ocular abnormalities. A flowchart has been made using ocular movement manifestations to differentiate different ataxia subtypes. And underlying pathology of each subtype is reviewed in form of illustrated models to reach a better understanding of each disorder.



Similar content being viewed by others
References
Witek N, Hawkins J, Hall D. Genetic ataxias: update on classification and diagnostic approaches. Curr Neurol Neurosci Rep [Internet]. 2021;21(3):13. https://doi.org/10.1007/s11910-021-01092-4.
Joo B-E, Lee C-N, Park K-W. Prevalence rate and functional status of cerebellar ataxia in Korea. The Cerebellum. 2012;11(3):733–8.
Musselman KE, Stoyanov CT, Marasigan R, Jenkins ME, Konczak J, Morton SM, et al. Prevalence of ataxia in children: a systematic review. Neurology. 2014;82(1):80–9.
Oh AJ, Chen T, Shariati MA, Jehangir N, Hwang TN, Liao YJ. A simple saccadic reading test to assess ocular motor function in cerebellar ataxia. PLoS One. 2018;13(11):e0203924.
Lopez A, Ferrero F, Postolache O. An affordable method for evaluation of ataxic disorders based on electrooculography. Sensors (Basel). 2019;19(17):3756.
Jayadev S, Bird TD. Hereditary ataxias: overview. Genet Med. 2013;15(9):673–83.
Manto M, Gandini J, Feil K, Strupp M. Cerebellar ataxias: an update. Curr Opin Neurol [Internet]. 2020;33(1):150–60. https://doi.org/10.1097/WCO.0000000000000774.
Kassavetis P, Kaski D, Anderson T, Hallett M. Eye movement disorders in movement disorders. Mov Disord Clin Pract [Internet]. 2022;9(3):284–95. https://doi.org/10.1002/mdc3.13413.
Alexandre MF, Rivaud-Pechoux S, Challe G, Durr A, Gaymard B. Functional consequences of oculomotor disorders in hereditary cerebellar ataxias. Cerebellum. 2013;12(3):396–405.
Corral-Juan M, Casquero P, Giraldo-Restrepo N, Laurie S, Martinez-Piñeiro A, Mateo-Montero RC, et al. New spinocerebellar ataxia subtype caused by SAMD9L mutation triggering mitochondrial dysregulation (SCA49). Brain Commun. 2022;4(2):fcac030.
Du Y-C, Ma Y, Shao Y-R, Gan S-R, Dong Y, Wu Z-Y. Factors associated with intergenerational instability of ATXN3 CAG repeat and genetic anticipation in Chinese patients with spinocerebellar ataxia type 3. The Cerebellum. 2020;19(6):902–6.
Klockgether T, Mariotti C, Paulson HL. Spinocerebellar ataxia. Nat Rev Dis Prim. 2019;5(1):24.
Whaley NR, Fujioka S, Wszolek ZK. Autosomal dominant cerebellar ataxia type I: a review of the phenotypic and genotypic characteristics. Orphanet J Rare Dis. 2011;6(1):33.
Harding AE. The clinical features and classification of the late onset autosomal dominant cerebellar ataxias. Brain. 1982;105(1):1–28.
Fujioka S, Sundal C, Wszolek ZK. Autosomal dominant cerebellar ataxia type III: a review of the phenotypic and genotypic characteristics. Orphanet J Rare Dis. 2013;8(1):14.
Orr HT, Chung M, Banfi S, Kwiatkowski TJ, Servadio A, Beaudet AL, et al. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat Genet. 1993;4(3):221–6.
Paulson HL. The spinocerebellar ataxias. J Neuroophthalmol. 2009;29(3):227–37.
Adachi M, Kawanami T, Ohshima H, Hosoya T. Characteristic signal changes in the pontine base on T2- and multishot diffusion-weighted images in spinocerebellar ataxia type 1. Neuroradiology. 2006;48(1):8–13.
Ishida C, Komai K, Yonezawa K, Sakajiri K-I, Nitta E, Kawashima A, et al. An autopsy case of an aged patient with spinocerebellar ataxia type 2. Neuropathology. 2011;31(5):510–8.
Vale J, Bugalho P, Silveira I, Sequeiros J, Guimarães J, Coutinho P. Autosomal dominant cerebellar ataxia: frequency analysis and clinical characterization of 45 families from Portugal. Eur J Neurol. 2010;17(1):124–8.
Rossi M, Perez-Lloret S, Doldan L, Cerquetti D, Balej J, Millar Vernetti P, et al. Autosomal dominant cerebellar ataxias: a systematic review of clinical features. Eur J Neurol. 2014;21(4):607–15.
Moscovich M, Okun MS, Favilla C, Figueroa KP, Pulst SM, Perlman S, et al. Clinical evaluation of eye movements in spinocerebellar ataxias: a prospective multicenter study. J neuro-ophthalmology Off J North Am Neuro-Ophthalmology Soc. 2015;35(1):16–21.
Nishiguchi KM, Aoki M, Nakazawa T, Abe T. Macular degeneration as a common cause of visual loss in spinocerebellar ataxia type 1 (SCA1) patients. Ophthalmic Genet. 2019;40(1):49–53.
Burk K, Abele M, Fetter M, Dichgans J, Skalej M, Laccone F, et al. Autosomal dominant cerebellar ataxia type I clinical features and MRI in families with SCA1, SCA2 and SCA3. Brain. 1996;119(5):1497–505.
Rodriguez-Labrada R, Velazquez-Perez L, Auburger G, Ziemann U, Canales-Ochoa N, Medrano-Montero J, et al. Spinocerebellar ataxia type 2: measures of saccade changes improve power for clinical trials. Mov Disord. 2016;31(4):570–8.
Kim JS, Kim JS, Youn J, Seo D-W, Jeong Y, Kang J-H, et al. Ocular motor characteristics of different subtypes of spinocerebellar ataxia: distinguishing features. Mov Disord. 2013;28(9):1271–7.
Burk K, Fetter M, Abele M, Laccone F, Brice A, Dichgans J, et al. Autosomal dominant cerebellar ataxia type I: oculomotor abnormalities in families with SCA1, SCA2, and SCA3. J Neurol. 1999;246(9):789–97.
Harmuth T, Weber JJ, Zimmer AJ, Sowa AS, Schmidt J, Fitzgerald JC, et al. Mitochondrial dysfunction in spinocerebellar ataxia type 3 is linked to VDAC1 deubiquitination. Int J Mol Sci. 2022;23(11):5933.
Koeppen AH. The neuropathology of spinocerebellar ataxia type 3/Machado-Joseph disease. Adv Exp Med Biol. 2018;1049:233–41.
Clausi S, De Luca M, Chiricozzi FR, Tedesco AM, Casali C, Molinari M, et al. Oculomotor deficits affect neuropsychological performance in oculomotor apraxia type 2. Cortex. 2013;49(3):691–701.
Hamilton SR. Neuro-ophthalmology of movement disorders. Curr Opin Ophthalmol. 2000;11(6):403–7.
Kang SL, Shaikh AG, Ghasia FF. Vergence and strabismus in neurodegenerative disorders. Front Neurol. 2018;9:299.
Butteriss D, Chinnery P, Birchall D. Radiological characterization of spinocerebellar ataxia type 6. Br J Radiol. 2005;78(932):694–6.
Franklin GL, Meira AT, Camargo CHF, Nascimento FA, Teive HAG. Upward gaze palsy: a valuable sign to distinguish spinocerebellar ataxias. The Cerebellum. 2020;19(5):685–90.
Flanigan K, Gardner K, Alderson K, Galster B, Otterud B, Leppert MF, et al. Autosomal dominant spinocerebellar ataxia with sensory axonal neuropathy (SCA4): clinical description and genetic localization to chromosome 16q22.1. Am J Hum Genet. 1996;59(2):392–9.
Hellenbroich Y, Bernard V, Zühlke C. Spinocerebellar ataxia type 4 and 16q22.1-linked Japanese ataxia are not allelic. J Neurol. 2008;255(4):612–3.
Hellenbroich Y, Gierga K, Reusche E, Schwinger E, Deller T, de Vos RAI, et al. Spinocerebellar ataxia type 4 (SCA4): Initial pathoanatomical study reveals widespread cerebellar and brainstem degeneration. J Neural Transm. 2006;113(7):829–43.
Maschke M, Oehlert G, Xie T-D, Perlman S, Subramony SH, Kumar N, et al. Clinical feature profile of spinocerebellar ataxia type 1-8 predicts genetically defined subtypes. Mov Disord. 2005;20:1405–12.
Accogli A, St-Onge J, Addour-Boudrahem N, Lafond-Lapalme J, Laporte AD, Rouleau GA, et al. Heterozygous missense pathogenic variants within the second spectrin repeat of SPTBN2 lead to infantile-onset cerebellar ataxia. J Child Neurol. 2020;35(2):106–10.
Dick KA, Ikeda Y, Day JW, Ranum LPW. Spinocerebellar ataxia type 5. Handb Clin Neurol. 2012;103:451–9.
Burk K, Zuhlke C, Konig IR, Ziegler A, Schwinger E, Globas C, et al. Spinocerebellar ataxia type 5: clinical and molecular genetic features of a German kindred. Neurology. 2004;62(2):327–9.
Rosini F, Pretegiani E, Battisti C, Dotti MT, Federico A, Rufa A. Eye movement changes in autosomal dominant spinocerebellar ataxias. Neurol Sci. 2020;41:1719–34.
Nicita F, Nardella M, Bellacchio E, Alfieri P, Terrone G, Piccini G, et al. Heterozygous missense variants of SPTBN2 are a frequent cause of congenital cerebellar ataxia. Clin Genet. 2019;96(2):169–75.
Spagnoli C, Frattini D, Gozzi F, Rizzi S, Salerno GG, Cimino L, et al. Infantile-onset spinocerebellar ataxia type 5 (SCA5) with optic atrophy and peripheral neuropathy. The Cerebellum. 2021;20(3):481–3.
Rentiya Z, Hutnik R, Mekkam YQ, Bae J. The pathophysiology and clinical manifestations of spinocerebellar ataxia type 6. Cerebellum. 2020;19:459–64.
Sinke RJ, Ippel EF, Diepstraten CM, Beemer FA, Wokke JH, van Hilten BJ, et al. Clinical and molecular correlations in spinocerebellar ataxia type 6: a study of 24 Dutch families. Arch Neurol. 2001;58:1839–44.
Christova P, Anderson JH, Gomez CM. Impaired eye movements in presymptomatic spinocerebellar ataxia type 6. Arch Neurol. 2008;65(4):530–6.
Gomez CM, Thompson RM, Gammack JT, Perlman SL, Dobyns WB, Truwit CL, et al. Spinocerebellar ataxia type 6: Gaze-evoked and vertical nystagmus, Purkinje cell degeneration, and variable age of onset. Ann Neurol. 1997;42(6):933–50.
Martin J-J. Spinocerebellar ataxia type 7. Handb Clin Neurol. 2012;103:475–91.
Seidel K, Siswanto S, Brunt ERP, den Dunnen W, Korf H-W, Rüb U. Brain pathology of spinocerebellar ataxias. Acta Neuropathol. 2012;124(1):1–21.
Isashiki Y, Kii Y, Ohba N, Nakagawa M. Retinopathy associated with Machado--Joseph disease (spinocerebellar ataxia 3) with CAG trinucleotide repeat expansion. Am J Ophthalmol. 2001;131(6):808–10.
Stephen CD, Schmahmann JD. Eye movement abnormalities are ubiquitous in the spinocerebellar ataxias. Cerebellum. 2019;18(6):1130–6.
Oh AK, Jacobson KM, Jen JC, Baloh RW. Slowing of voluntary and involuntary saccades: an early sign in spinocerebellar ataxia type 7. Ann Neurol. 2001;49(6):801–4.
Koob MD, Moseley ML, Schut LJ, Benzow KA, Bird TD, Day JW, et al. An untranslated CTG expansion causes a novel form of spinocerebellar ataxia (SCA8). Nat Genet. 1999;21(4):379–84.
Zeman A. Spinocerebellar ataxia type 8 in Scotland: genetic and clinical features in seven unrelated cases and a review of published reports. J Neurol Neurosurg Psychiatry. 2004;75(3):459–65.
Kim JS, Son TO, Youn J, Ki C-S, Cho JW. Non-ataxic phenotypes of SCA8 mimicking amyotrophic lateral sclerosis and Parkinson disease. J Clin Neurol. 2013;9(4):274.
Zhou Y, Yuan Y, Liu Z, Zeng S, Chen Z, Shen L, et al. Genetic and clinical analyses of spinocerebellar ataxia type 8 in mainland China. J Neurol. 2019;266:2979–86.
Hernandez-Castillo CR, Diaz R, Vaca-Palomares I, Torres DL, Chirino A, Campos-Romo A, et al. Extensive cerebellar and thalamic degeneration in spinocerebellar ataxia type 10. Parkinsonism Relat Disord. 2019;66:182–8.
Teive HAG, Munhoz RP, Arruda WO, Lopes-Cendes I, Raskin S, Werneck LC, et al. Spinocerebellar ataxias: genotype-phenotype correlations in 104 Brazilian families. Clinics (Sao Paulo). 2012;67(5):443–9.
Ashizawa T. Spinocerebellar ataxia type 10. Handb Clin Neurol. 2012;103:507–19.
Sullivan R, Yau WY, O’Connor E, Houlden H. Spinocerebellar ataxia: an update. J Neurol. 2019;266(2):533–44.
Johnson J, Wood N, Giunti P, Houlden H. Clinical and genetic analysis of spinocerebellar ataxia type 11. The Cerebellum. 2008;7(2):159–64.
Cohen RL, Margolis RL. Spinocerebellar ataxia type 12: clues to pathogenesis. Curr Opin Neurol. 2016;29(6):735–42.
Ganaraja VH, Holla VV, Stezin A, Kamble N, Yadav R, Purushottam M, et al. Clinical, radiological, and genetic profile of spinocerebellar ataxia 12: a hospital-based cohort analysis. Tremor Other Hyperkinet Mov. 2022;12(1):1–11.
Stevanin G, Dürr A. Spinocerebellar ataxia 13 and 25. Handb Clin Neurol. 2012;103:549–53.
Herman-Bert A, Stevanin G, Netter J-C, Rascol O, Brassat D, Calvas P, et al. Mapping of spinocerebellar ataxia 13 to chromosome 19q13.3-q13.4 in a family with autosomal dominant cerebellar ataxia and mental retardation. Am J Hum Genet [Internet]. 2000;67(1):229–35. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0002929707624495. Accessed 20 Aug 2022
Kim M, Oh SH, Cho JW, Lee J-H. Spinocerebellar ataxia 13 presenting with pure cerebellar syndrome in a Korean family. J Mov Disord [Internet]. 2020;13(3):244–6. Available from: http://e-jmd.org/journal/view.php?doi=10.14802/jmd.20064. Accessed 20 Aug 2022
Chen D-H, Raskind WH, Bird TD. Spinocerebellar ataxia type 14. Handb Clin Neurol. 2012;103:555–9.
Gardner RJM, Knight MA, Hara K, Tsuji S, Forrest SM, Storey E. Spinocerebellar ataxia type 15. Cerebellum. 2005;4(1):47–50.
Tipton PW, Guthrie K, Strongosky A, Reimer R, Wszolek ZK. Spinocerebellar ataxia 15: a phenotypic review and expansion. Neurol Neurochir Pol [Internet]. 2017;51(1):86–91. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0028384316302031. Accessed 20 Aug 2022
Wang L, Hao Y, Yu P, Cao Z, Zhang J, Zhang X, et al. Identification of a splicing mutation in ITPR1 via WES in a Chinese early-onset spinocerebellar ataxia family. The Cerebellum [Internet]. 2018;17(3):294–9. https://doi.org/10.1007/s12311-017-0896-z.
Miyoshi Y, Yamada T, Tanimura M, Taniwaki T, Arakawa K, Ohyagi Y, et al. A novel autosomal dominant spinocerebellar ataxia (SCA16) linked to chromosome 8q22.1-24.1. Neurology [Internet]. 2001;57(1):96–100. https://doi.org/10.1212/WNL.57.1.96.
Toyoshima Y, Takahashi H. Spinocerebellar ataxia type 17 (SCA17). Adv Exp Med Biol. 2018;1049:219–31.
Ivanova E, Nuzhnyi E, Abramycheva N, Klyushnikov S, Fedotova E, Illarioshkin S. Mutation analysis of the TATA box-binding protein (TBP) gene in Russian patients with spinocerebellar ataxia and Huntington disease-like phenotype. Clin Neurol Neurosurg [Internet]. 2022;222:107473. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0303846722003547. Accessed 20 Aug 2022
Rossi M, Hamed M, Rodríguez-Antigüedad J, Cornejo-Olivas M, Breza M, Lohmann K, et al. Genotype–phenotype correlations for ATX-TBP (SCA17): mdsgene systematic review. Mov Disord. 2023;38(3):368–77. https://doi.org/10.1002/mds.29278.
Hubner J, Sprenger A, Klein C, Hagenah J, Rambold H, Zuhlke C, et al. Eye movement abnormalities in spinocerebellar ataxia type 17 (SCA17). Neurology. 2007;69(11):1160–8.
Mariotti C, Alpini D, Fancellu R, Soliveri P, Grisoli M, Ravaglia S, et al. Spinocerebellar ataxia type 17 (SCA17): oculomotor phenotype and clinical characterization of 15 Italian patients. J Neurol [Internet]. 2007;254:1538–46. https://doi.org/10.1007/s00415-007-0579-7.
Lin P, Zhang D, Xu G, Yan C. Identification of IFRD1 variant in a Han Chinese family with autosomal dominant hereditary spastic paraplegia associated with peripheral neuropathy and ataxia. J Hum Genet. 2018;63(4):521–4.
Schelhaas HJ, van de Warrenburg BPC. Clinical, psychological, and genetic characteristics of spinocerebellar ataxia type 19 (SCA19). Cerebellum. 2005;4(1):51–4.
Chung M-Y, Lu Y-C, Cheng N-C, Soong B-W. A novel autosomal dominant spinocerebellar ataxia (SCA22) linked to chromosome 1p21-q23. Brain. 2003;126(Pt 6):1293–9.
Storey E, Gardner RJM. Spinocerebellar ataxia type 20. Handb Clin Neurol. 2012;103:567–73.
Tilikete C, Desestret V. Hypertrophic olivary degeneration and palatal or oculopalatal tremor. Front Neurol. 2017;8:302.
Knight MA, Gardner RJM, Bahlo M, Matsuura T, Dixon JA, Forrest SM, et al. Dominantly inherited ataxia and dysphonia with dentate calcification: spinocerebellar ataxia type 20. Brain. 2004;127(Pt 5):1172–81.
Storey E, Knight MA, Forrest SM, Gardner RJM. Spinocerebellar ataxia type 20. Cerebellum. 2005;4(1):55–7.
Delplanque J, Devos D, Vuillaume I, De Becdelievre A, Vangelder E, Maurage CA, et al. Slowly progressive spinocerebellar ataxia with extrapyramidal signs and mild cognitive impairment (SCA21). Cerebellum. 2008;7(2):179–83.
Traschütz A, van Gaalen J, Oosterloo M, Vreeburg M, Kamsteeg E-J, Deininger N, et al. The movement disorder spectrum of SCA21 (ATX-TMEM240): 3 novel families and systematic review of the literature. Parkinsonism Relat Disord. 2019;62:215–20.
Yahikozawa H, Miyatake S, Sakai T, Uehara T, Yamada M, Hanyu N, et al. A Japanese family of spinocerebellar ataxia type 21: clinical and neuropathological studies. Cerebellum. 2018;17(5):525–30.
Verbeek DS. Spinocerebellar ataxia type 23: a genetic update. Cerebellum. 2009;8(2):104–7.
Liu Y-T, Tang B-S, Wang J-L, Guan W-J, Shen L, Shi Y-T, et al. Spinocerebellar ataxia type 23 is an uncommon SCA subtype in the Chinese Han population. Neurosci Lett. 2012;528(1):51–4.
Jezierska J, Stevanin G, Watanabe H, Fokkens MR, Zagnoli F, Kok J, et al. Identification and characterization of novel PDYN mutations in dominant cerebellar ataxia cases. J Neurol. 2013;260(7):1807–12.
Stevanin G, Bouslam N, Thobois S, Azzedine H, Ravaux L, Boland A, et al. Spinocerebellar ataxia with sensory neuropathy (SCA25) maps to chromosome 2p. Ann Neurol. 2004;55(1):97–104.
Yu G-Y, Howell MJ, Roller MJ, Xie T-D, Gomez CM. Spinocerebellar ataxia type 26 maps to chromosome 19p13.3 adjacent to SCA6. Ann Neurol. 2005;57(3):349–54.
Groth CL, Berman BD. Spinocerebellar ataxia 27: a review and characterization of an evolving phenotype. Tremor Other Hyperkinet Mov (N Y). 2018;8:534.
Brusse E, de Koning I, Maat-Kievit A, Oostra BA, Heutink P, van Swieten JC. Spinocerebellar ataxia associated with a mutation in the fibroblast growth factor 14 gene (SCA27): a new phenotype. Mov Disord. 2006;21(3):396–401.
Strupp M, Maul S, Konte B, Hartmann AM, Giegling I, Wollenteit S, et al. A variation in FGF14 is associated with downbeat nystagmus in a genome-wide association study. Cerebellum. 2020;19(3):348–57.
Mariotti C, Brusco A, Di Bella D, Cagnoli C, Seri M, Gellera C, et al. Spinocerebellar ataxia type 28: a novel autosomal dominant cerebellar ataxia characterized by slow progression and ophthalmoparesis. Cerebellum. 2008;7(2):184–8.
Politi LS, Bianchi Marzoli S, Godi C, Panzeri M, Ciasca P, Brugnara G, et al. MRI evidence of cerebellar and extraocular muscle atrophy differently contributing to eye movement abnormalities in SCA2 and SCA28 diseases. Invest Ophthalmol Vis Sci. 2016;57(6):2714–20.
Cagnoli C, Mariotti C, Taroni F, Seri M, Brussino A, Michielotto C, et al. SCA28, a novel form of autosomal dominant cerebellar ataxia on chromosome 18p11.22–q11.2. Brain [Internet]. 2006;129(1):235–42. Available from: http://academic.oup.com/brain/article/129/1/235/311787/SCA28-a-novel-form-of-autosomal-dominant. Accessed 20 Aug 2022
Zambonin JL, Bellomo A, Ben-Pazi H, Everman DB, Frazer LM, Geraghty MT, et al. Spinocerebellar ataxia type 29 due to mutations in ITPR1: a case series and review of this emerging congenital ataxia. Orphanet J Rare Dis. 2017;12(1):121.
Huang L, Chardon JW, Carter MT, Friend KL, Dudding TE, Schwartzentruber J, et al. Missense mutations in ITPR1 cause autosomal dominant congenital nonprogressive spinocerebellar ataxia. Orphanet J Rare Dis. 2012;7:67.
Storey E, Bahlo M, Fahey M, Sisson O, Lueck CJ, Gardner RJM. A new dominantly inherited pure cerebellar ataxia, SCA 30. J Neurol Neurosurg Psychiatry. 2009;80(4):408–11.
Nakamura K, Yoshida K, Matsushima A, Shimizu Y, Sato S, Yahikozawa H, et al. Natural history of spinocerebellar ataxia type 31: a 4-year prospective study. Cerebellum. 2017;16(2):518–24.
Adachi T, Kitayama M, Nakano T, Adachi Y, Kato S, Nakashima K. Autopsy case of spinocerebellar ataxia type 31 with severe dementia at the terminal stage. Neuropathology. 2015;35(3):273–9.
Beaudin M, Sellami L, Martel C, Touzel-Deschênes L, Houle G, Martineau L, et al. Characterization of the phenotype with cognitive impairment and protein mislocalization in SCA34. Neurol Genet. 2020;6(2):e403.
Ozaki K, Ansai A, Nobuhara K, Araki T, Kubodera T, Ishii T, et al. Prevalence and clinicoradiological features of spinocerebellar ataxia type 34 in a Japanese ataxia cohort. Parkinsonism Relat Disord. 2019;65:238–42.
Ozaki K, Irioka T, Uchihara T, Yamada A, Nakamura A, Majima T, et al. Neuropathology of SCA34 showing widespread oligodendroglial pathology with vacuolar white matter degeneration: a case study. Acta Neuropathol Commun [Internet]. 2021;9(1):172. https://doi.org/10.1186/s40478-021-01272-w.
Ozaki K, Doi H, Mitsui J, Sato N, Iikuni Y, Majima T, et al. A novel mutation in ELOVL4 leading to spinocerebellar ataxia (SCA) with the hot cross bun sign but lacking erythrokeratodermia: a broadened spectrum of SCA34. JAMA Neurol. 2015;72(7):797–805.
Lin C-C, Gan S-R, Gupta D, Alaedini A, Green PH, Kuo S-H. Hispanic spinocerebellar ataxia type 35 (SCA35) with a novel frameshift mutation. Cerebellum. 2019;18(2):291–4.
Guo Y-C, Lin J-J, Liao Y-C, Tsai P-C, Lee Y-C, Soong B-W. Spinocerebellar ataxia 35: novel mutations in TGM6 with clinical and genetic characterization. Neurology. 2014;83(17):1554–61.
Wang Q, Zhang C, Liu S, Liu T, Ni R, Liu X, et al. Long-read sequencing identified intronic (GGCCTG)n expansion in NOP56 in one SCA36 family and literature review. Clin Neurol Neurosurg [Internet]. 2022;223:107503. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0303846722003845. Accessed 20 Aug 2022
Aguiar P, Pardo J, Arias M, Quintáns B, Fernández-Prieto M, Martínez-Regueiro R, et al. PET and MRI detection of early and progressive neurodegeneration in spinocerebellar ataxia type 36. Mov Disord [Internet]. 2017;32(2):264–73. https://doi.org/10.1002/mds.26854.
Lopez S, He F. Spinocerebellar ataxia 36: from mutations toward therapies. Front Genet [Internet]. 2022;4:13. https://doi.org/10.3389/fgene.2022.837690/full.
Xie Y, Chen Z, Long Z, Chen R-T, Jiang Y-Z, Liu M-J, et al. Identification of the largest SCA36 pedigree in Asia: with multimodel neuroimaging evaluation for the first time. The Cerebellum [Internet]. 2022;21(3):358–67. https://doi.org/10.1007/s12311-021-01304-0.
Garcia-Murias M, Quintans B, Arias M, Seixas AI, Cacheiro P, Tarrio R, et al. “Costa da Morte” ataxia is spinocerebellar ataxia 36: clinical and genetic characterization. Brain. 2012;135(5):1423–35. https://doi.org/10.1093/brain/aws069.
Arias M, García-Murias M, Sobrido MJ. Spinocerebellar ataxia 36 (SCA36): «Costa da Morte ataxia». Neurologia. 2017;32(6):386–93.
Loureiro JR, Oliveira CL, Mota C, Castro AF, Costa C, Loureiro JL, et al. Mutational mechanism for DAB1 (ATTTC) n insertion in SCA37: ATTTT repeat lengthening and nucleotide substitution. Hum Mutat [Internet]. 2019;40(4):404–12. https://doi.org/10.1002/humu.23704.
Rosenbohm A, Pott H, Thomsen M, Rafehi H, Kaya S, Szymczak S, et al. Familial cerebellar ataxia and amyotrophic lateral sclerosis/frontotemporal dementia with DAB1 and C9ORF72 repeat expansions: an 18-year study. Mov Disord. 2022;37(12):2427–39. https://doi.org/10.1002/mds.29221.
Corral-Juan M, Serrano-Munuera C, Rábano A, Cota-González D, Segarra-Roca A, Ispierto L, et al. Clinical, genetic and neuropathological characterization of spinocerebellar ataxia type 37. Brain. 2018;141(7):1981–97.
Serrano-Munuera C, Corral-Juan M, Stevanin G, San Nicolás H, Roig C, Corral J, et al. New subtype of spinocerebellar ataxia with altered vertical eye movements mapping to chromosome 1p32. JAMA Neurol. 2013;70(6):764–71.
Seixas AI, Loureiro JR, Costa C, Ordóñez-Ugalde A, Marcelino H, Oliveira CL, et al. A pentanucleotide ATTTC repeat insertion in the non-coding region of DAB1, mapping to SCA37, causes spinocerebellar ataxia. Am J Hum Genet. 2017;101(1):87–103.
Di Gregorio E, Borroni B, Giorgio E, Lacerenza D, Ferrero M, Lo Buono N, et al. ELOVL5 mutations cause spinocerebellar ataxia 38. Am J Hum Genet. 2014;95(2):209–17.
Borroni B, Di Gregorio E, Orsi L, Vaula G, Costanzi C, Tempia F, et al. Clinical and neuroradiological features of spinocerebellar ataxia 38 (SCA38). Parkinsonism Relat Disord. 2016;28:80–6.
Gazulla J, Orduna-Hospital E, Benavente I, Rodríguez-Valle A, Osorio-Caicedo P, Alvarez-de Andrés S, et al. Contributions to the study of spinocerebellar ataxia type 38 (SCA38). J Neurol. 2020;267:2288–95.
Johnson JO, Stevanin G, van de Leemput J, Hernandez DG, Arepalli S, Forlani S, et al. A 7.5-Mb duplication at chromosome 11q21-11q22.3 is associated with a novel spastic ataxia syndrome. Mov Disord [Internet]. 2015;30(2):262–6. https://doi.org/10.1002/mds.26059.
Tsoi H, Yu ACS, Chen ZS, Ng NKN, Chan AYY, Yuen LYP, et al. A novel missense mutation in CCDC88C activates the JNK pathway and causes a dominant form of spinocerebellar ataxia. J Med Genet. 2014;51(9):590–5.
Ngo K, Aker M, Petty LE, Chen J, Cavalcanti F, Nelson AB, et al. Expanding the global prevalence of spinocerebellar ataxia type 42. Neurol Genet. 2018;4(3):e232.
Kimura M, Yabe I, Hama Y, Eguchi K, Ura S, Tsuzaka K, et al. SCA42 mutation analysis in a case series of Japanese patients with spinocerebellar ataxia. J Hum Genet. 2017;62(9):857–9.
Depondt C, Donatello S, Rai M, Wang FC, Manto M, Simonis N, et al. MME mutation in dominant spinocerebellar ataxia with neuropathy (SCA43). Neurol Genet. 2016;2(5):e94.
Watson LM, Bamber E, Schnekenberg RP, Williams J, Bettencourt C, Lickiss J, et al. Dominant mutations in GRM1 cause spinocerebellar ataxia type 44. Am J Hum Genet. 2017;101(3):451–8.
Parodi L, Coarelli G, Stevanin G, Brice A, Durr A. Hereditary ataxias and paraparesias: clinical and genetic update. Curr Opin Neurol. 2018;31(4):462–71.
Ganguly J, Mukherjee S, Basu P, Mondal B, Chatterjee K, Roy A, et al. Think of SCA45 in late-onset familial ataxias: the first report from the indian subcontinent with a novel variant. Mov Disord Clin Pract [Internet]. 2022 Nov;9(8):1140–3. https://doi.org/10.1002/mdc3.13580.
Tonholo Silva TY, Rosa ABR, Quaio CR, Verbeek D, Pedroso JL, Barsottini O. Does SCA45 cause very late-onset pure cerebellar ataxia? Neurol Genet [Internet]. 2021 Jun;7(3):e581. https://doi.org/10.1212/NXG.0000000000000581.
Lai K-L, Liao Y-C, Tsai P-C, Hsiao C-T, Soong B-W, Lee Y-C. Investigating PUM1 mutations in a Taiwanese cohort with cerebellar ataxia. Parkinsonism Relat Disord. 2019;66:220–3.
Gennarino VA, Palmer EE, McDonell LM, Wang L, Adamski CJ, Koire A, et al. A mild PUM1 mutation is associated with adult-onset ataxia, whereas haploinsufficiency causes developmental delay and seizures. Cell. 2018;172(5):924–936.e11.
De Michele G, Lieto M, Galatolo D, Salvatore E, Cocozza S, Barghigiani M, et al. Spinocerebellar ataxia 48 presenting with ataxia associated with cognitive, psychiatric, and extrapyramidal features: a report of two Italian families. Parkinsonism Relat Disord. 2019;65:91–6.
Palvadeau R, Kaya-Güleç ZE, Şimşir G, Vural A, Öztop-Çakmak Ö, Genç G, et al. Cerebellar cognitive-affective syndrome preceding ataxia associated with complex extrapyramidal features in a Turkish SCA48 family. Neurogenetics. 2020;21(1):51–8.
Lieto M, Riso V, Galatolo D, De Michele G, Rossi S, Barghigiani M, et al. The complex phenotype of spinocerebellar ataxia type 48 in eight unrelated Italian families. Eur J Neurol. 2020;27(3):498–505.
Cocozza S, Pontillo G, De Michele G, Perillo T, Guerriero E, Ugga L, et al. The “crab sign”: an imaging feature of spinocerebellar ataxia type 48. Neuroradiology. 2020;62:1095–103.
Silver MR, Sethi KD, Mehta SH, Nichols FT, Morgan JC. Case report of optic atrophy in dentatorubropallidoluysian atrophy (DRPLA). BMC Neurol. 2015;15:260.
Muñoz E, Milà M, Sánchez A, Latorre P, Ariza A, Codina M, et al. Dentatorubropallidoluysian atrophy in a spanish family: a clinical, radiological, pathological, and genetic study. J Neurol Neurosurg Psychiatry. 1999;67(6):811–4.
Rocha Cabrero F, De Jesus O. Dentatorubral pallidoluysian atrophy. Treasure Island (FL); 2022.
Vinton A, Fahey MC, O’Brien TJ, Shaw J, Storey E, Gardner RJM, et al. Dentatorubral-pallidoluysian atrophy in three generations, with clinical courses from nearly asymptomatic elderly to severe juvenile, in an Australian family of Macedonian descent. Am J Med Genet A. 2005;136(2):201–4.
Gordon N. Episodic ataxia and channelopathies. Brain Dev. 1998;20(1):9–13.
Graves TD, Griggs RC, Bundy BN, Jen JC, Baloh RW, Hanna MG, et al. Episodic ataxia type 1: natural history and effect on quality of life. The Cerebellum. 2022:1–9.
Choi J-H, Oh EH, Choi SY, Kim HJ, Lee SK, Choi JY, et al. Vestibular impairments in episodic ataxia type 2. J Neurol. 2022;269(5):2687–95.
Anheim M, Tranchant C, Koenig M. The autosomal recessive cerebellar ataxias. N Engl J Med. 2012;366(7):636–46.
Gama MTD, Braga-Neto P, Rangel DM, Godeiro C, Alencar R, Embiruçu EK, et al. Autosomal recessive cerebellar ataxias in South America: a multicenter study of 1338 patients. Mov Disord [Internet]. 2022 May 4;37(8):1773–4. https://doi.org/10.1002/mds.29046.
Al-Din AS, Al-Kurdi A, Al-Salem MK, Al-Nassar KE, Al-Zuhair A, Rudwan MA, et al. Autosomal recessive ataxia, slow eye movements, dementia and extrapyramidal disturbances. J Neurol Sci. 1990;96(2–3):191–205.
Vankan P. Prevalence gradients of Friedreich’s ataxia and R1b haplotype in Europe co-localize, suggesting a common Palaeolithic origin in the Franco-Cantabrian ice age refuge. J Neurochem. 2013;126:11–20.
Campuzano V, Montermini L, Moltò MD, Pianese L, Cossée M, Cavalcanti F, et al. Friedreich’s ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science (80- ). 1996 Mar;271(5254):1423–7.
Harding AE. Friedreich’s ataxia: a clinical and genetic study of 90 families with an analysis of early diagnostic criteria and intrafamilial clustering of clinical features. Brain. 1981;104(3):589–620.
Lecocq C, Charles P, Azulay J-P, Meissner W, Rai M, N’Guyen K, et al. Delayed-onset Friedreich’s ataxia revisited. Mov Disord. 2016;31(1):62–9.
Santos R, Lefevre S, Sliwa D, Seguin A, Camadro J-M, Lesuisse E. Friedreich ataxia: molecular mechanisms, redox considerations, and therapeutic opportunities. Antioxid Redox Signal. 2010;13(5):651–90.
Parkinson MH, Boesch S, Nachbauer W, Mariotti C, Giunti P. Clinical features of Friedreich’s ataxia: classical and atypical phenotypes. J Neurochem. 2013;126(Suppl):103–17.
Spieker S, Schulz JB, Petersen D, Fetter M, Klockgether T, Dichgans J. Fixation instability and oculomotor abnormalities in Friedreich’s ataxia. J Neurol. 1995;242(8):517–21.
Bogdanova-Mihaylova P, Plapp HM, Chen H, Early A, Cassidy L, Walsh RA, et al. Longitudinal assessment using optical coherence tomography in patients with Friedreich’s ataxia. Tomography. 2021;7(4):915–31.
Fahey MC, Cremer PD, Aw ST, Millist L, Todd MJ, White OB, et al. Vestibular, saccadic and fixation abnormalities in genetically confirmed Friedreich ataxia. Brain. 2008;131(Pt 4):1035–45.
Furman JM, Perlman S, Baloh RW. Eye movements in Friedreich’s ataxia. Arch Neurol. 1983;40(6):343–6.
Bhidayasiri R, Perlman SL, Pulst S-M, Geschwind DH. Late-onset Friedreich ataxia. Arch Neurol. 2005;62:1865.
Perlman S, Becker-Catania S, Gatti RA. Ataxia-telangiectasia: diagnosis and treatment. Semin Pediatr Neurol. 2003;10(3):173–82.
Chun HH, Gatti RA. Ataxia–telangiectasia, an evolving phenotype. DNA Repair (Amst). 2004;3(8–9):1187–96.
Crawford TO. Ataxia telangiectasia. Semin Pediatr Neurol. 1998;5(4):287–94.
Shaikh AG, Marti S, Tarnutzer AA, Palla A, Crawford TO, Straumann D, et al. Ataxia telangiectasia: a “disease model” to understand the cerebellar control of vestibular reflexes. J Neurophysiol. 2011;105(6):3034–41.
Lewis RF, Lederman HM, Crawford TO. Ocular motor abnormalities in ataxia telangiectasia. Ann Neurol. 1999;46(3):287–95.
Shaikh AG, Marti S, Tarnutzer AA, Palla A, Crawford TO, Straumann D, et al. Gaze fixation deficits and their implication in ataxia-telangiectasia. J Neurol Neurosurg Psychiatry. 2009;80(8):858–64.
Cogan DG. A type of congenital ocular motor apraxia presenting jerky head movements*. Am J Ophthalmol. 1953;36(4):433–41.
Harris CM, Shawkat F, Russell-Eggitt I, Wilson J, Taylor D. Intermittent horizontal saccade failure (‘ocular motor apraxia’) in children. Br J Ophthalmol. 1996;80(2):151–8.
Onodera O. Spinocerebellar ataxia with ocular motor apraxia and DNA repair. Neuropathology. 2006;26(4):361–7.
Federighi P, Ramat S, Rosini F, Pretegiani E, Federico A, Rufa A. Characteristic eye movements in ataxia-telangiectasia-like disorder: an explanatory hypothesis. Front Neurol. 2017;8:596.
Khan AO, Oystreck DT, Koenig M, Salih MA. Ophthalmic features of ataxia telangiectasia-like disorder. J AAPOS Off Publ Am Assoc Pediatr Ophthalmol Strabismus. 2008;12(2):186–9.
Moreira M-C, Barbot C, Tachi N, Kozuka N, Uchida E, Gibson T, et al. The gene mutated in ataxia-ocular apraxia 1 encodes the new HIT/Zn-finger protein aprataxin. Nat Genet. 2001;29(2):189–93.
Sano Y, Date H, Igarashi S, Onodera O, Oyake M, Takahashi T, et al. Aprataxin, the causative protein for EAOH is a nuclear protein with a potential role as a DNA repair protein. Ann Neurol. 2004;55(2):241–9.
Sekijima Y, Ohara S, Nakagawa S, Tabata K, Yoshida K, Ishigame H, et al. Hereditary motor and sensory neuropathy associated with cerebellar atrophy (HMSNCA): clinical and neuropathological features of a Japanese family. J Neurol Sci. 1998;158(1):30–7.
Le Ber I, Moreira M-C, Rivaud-Pechoux S, Chamayou C, Ochsner F, Kuntzer T, et al. Cerebellar ataxia with oculomotor apraxia type 1: clinical and genetic studies. Brain. 2003;126(Pt 12):2761–72.
Wolf NI, Koenig M. Progressive cerebellar atrophy: hereditary ataxias and disorders with spinocerebellar degeneration. Handb Clin Neurol. 2013;113:1869–78.
Anheim M, Monga B, Fleury M, Charles P, Barbot C, Salih M, et al. Ataxia with oculomotor apraxia type 2: clinical, biological and genotype/phenotype correlation study of a cohort of 90 patients. Brain. 2009;132:2688–98.
Le Ber I, Brice A, Dürr A. New autosomal recessive cerebellar ataxias with oculomotor apraxia. Curr Neurol Neurosci Rep. 2005;5(5):411–7.
Palau F, Espinós C. Autosomal recessive cerebellar ataxias. Orphanet J Rare Dis. 2006;1:47.
Patterson MC, Hendriksz CJ, Walterfang M, Sedel F, Vanier MT, Wijburg F. Recommendations for the diagnosis and management of Niemann-Pick disease type C: an update. Mol Genet Metab. 2012;106(3):330–44.
Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, et al. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science (80- ). 1997 Jul;277(5323):228–31.
Devaraj R, Mahale RR, Sindhu DM, Stezin A, Kamble N, Holla VV, et al. Spectrum of movement disorders in Niemann-Pick disease type C. Tremor Other Hyperkinet Mov (N Y). 2022;12:28.
Tang Y, Li H, Liu J-P. Niemann-Pick disease type C: from molecule to clinic. Clin Exp Pharmacol Physiol. 2010;37(1):132–40.
Rottach KG, Von Maydell RD, Das VE, Zivotofsky AZ, Discenna AO, Gordon JL, et al. Evidence for independent feedback control of horizontal and vertical saccades from Niemann-Pick type C disease. Vision Res. 1997;37(24):3627–38.
Solomon D, Winkelman AC, Zee DS, Gray L, Büttner-Ennever J. Niemann-Pick type C disease in two affected sisters: ocular motor recordings and brain-stem neuropathology. Ann N Y Acad Sci. 2005 Apr;1039(1):436–45.
Abel LA, Walterfang M, Fietz M, Bowman EA, Velakoulis D. Saccades in adult Niemann-Pick disease type C reflect frontal, brainstem, and biochemical deficits. Neurology. 2009;72(12):1083–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Compliance Statement
This study has been approved by Shahid Beheshti Medical University research ethics committee, and was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1:
Table 1 A summary of the onset age, mutations, pathology, and ocular movement abnormalities in cerebellar ataxias (DOCX 206 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Salari, M., Etemadifar, M., Rashedi, R. et al. A Review of Ocular Movement Abnormalities in Hereditary Cerebellar Ataxias. Cerebellum 23, 702–721 (2024). https://doi.org/10.1007/s12311-023-01554-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-023-01554-0