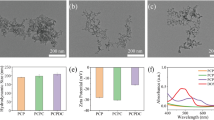
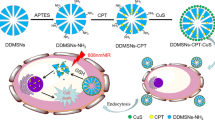
Abstract
Construction of multifunctional stimuli-responsive nanotherapeutics enabling improved intratumoral penetration of therapeutics and reversal of multiple-drug resistance (MDR) is potent to achieve effective cancer treatment. Herein, we report a general method to synthesize pH-dissociable calcium carbonate (CaCO3) hollow nanoparticles with amorphous CaCO3 as the template, gallic acid (GA) as the organic ligand, and ferrous ions as the metallic center via a one-pot coordination reaction. The obtained GA–Fe@CaCO3 exhibits high loading efficiencies to both oxidized cisplatin prodrug and doxorubicin, yielding drug loaded GA–Fe@CaCO3 nanotherapeutics featured in pH-responsive size shrinkage, drug release, and Fenton catalytic activity. Compared to nonresponsive GA–Fe@silica nanoparticles prepared with silica nanoparticles as the template, such GA–Fe@CaCO3 confers significantly improved intratumoral penetration capacity. Moreover, both types of drug-loaded GA–Fe@CaCO3 nanotherapeutics exhibit synergistic therapeutic efficacies to corresponding MDR cancer cells because of the GA–Fe mediated intracellular oxidative stress amplification that could reduce the efflux of engulfed drugs by impairing the mitochondrial-mediated production of adenosine triphosphate (ATP). As a result, it is found that the doxorubicin loaded GA–Fe@CaCO3 exhibits superior therapeutic effect towards doxorubicin-resistant 4T1 breast tumors via combined chemodynamic and chemo-therapies. This work highlights the preparation of pH-dissociable CaCO3-based nanotherapeutics to enable effective tumor penetration for enhanced treatment of drug-resistant tumors.

Similar content being viewed by others
References
Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157.
Bor, G.; Mat Azmi, I. D.; Yaghmur, A. Nanomedicines for cancer therapy: Current status, challenges and future prospects. Ther. Deliv. 2019, 10, 113–132.
Zhang, R. X.; Wong, H. L.; Xue, H. Y.; Eoh, J. Y.; Wu, X. Y. Nanomedicine of synergistic drug combinations for cancer therapy-Strategies and perspectives. J. Control. Release 2016, 240, 489–503.
Theocharis, A. D.; Skandalis, S. S.; Gialeli, C.; Karamanos, N. K. Extracellular matrix structure. Adv. Drug Deliver. Rev. 2016, 97, 4–27.
Heldin, C. H.; Rubin, K.; Pietras, K.; Östman, A. High interstitial fluid pressure—an obstacle in cancer therapy. Nat. Rev. Cancer 2004, 4, 806–813.
Böckelmann, L. C.; Schumacher, U. Targeting tumor interstitial fluid pressure: Will it yield novel successful therapies for solid tumors? Expert Opin. Ther. Targets 2019, 23, 1005–1014.
Chen, Q.; Wang, C.; Zhang, X. D.; Chen, G. J.; Hu, Q. Y.; Li, H. J.; Wang, J. Q.; Wen, D.; Zhang, Y. Q.; Lu, Y. F. et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat. Nanotechnol. 2019, 14, 89–97.
Minchinton, A. I.; Tannock, I. F. Drug penetration in solid tumours. Nat. Rev. Cancer 2006, 6, 583–592.
Zhou, Q.; Shao, S. Q.; Wang, J. Q.; Xu, C. H.; Xiang, J. J.; Piao, Y.; Zhou, Z. X.; Yu, Q. S.; Tang, J. B.; Liu, X. R. et al. Enzyme-activatable polymer-drug conjugate augments tumour penetration and treatment efficacy. Nat. Nanotechnol. 2019, 14, 799–809.
Sun, Y. Tumor microenvironment and cancer therapy resistance. Cancer Lett. 2016, 380, 205–215.
Wang, H. M.; Feng, Z. Q. Q.; Wu, D. D.; Fritzsching, K. J.; Rigney, M.; Zhou, J.; Jiang, Y. J.; Schmidt-Rohr, K.; Xu, B. Enzyme-regulated supramolecular assemblies of cholesterol conjugates against drugresistant ovarian cancer cells. J. Am. Chem. Soc. 2016, 138, 10758–10761.
Wang, S.; Huang, P.; Chen, X. Y. Stimuli-responsive programmed specific targeting in nanomedicine. ACS Nano 2016, 10, 2991–2994.
Li, F. Y.; Lu, J. X.; Kong, X. Q.; Hyeon, T.; Ling, D. S. Dynamic nanoparticle assemblies for biomedical applications. Adv. Mater. 2017, 29, 1605897.
Sun, Q. X.; Ojha, T.; Kiessling, F.; Lammers, T.; Shi, Y. Enhancing tumor penetration of nanomedicines. Biomacromolecules 2017, 18, 1449–1459.
Li, H. J.; Du, J. Z.; Du, X. J.; Xu, C. F.; Sun, C. Y.; Wang, H. X.; Cao, Z. T.; Yang, X. Z.; Zhu, Y. H.; Nie, S. M. et al. Stimuli-responsive clustered nanoparticles for improved tumor penetration and therapeutic efficacy. Proc. Natl. Acad. Sci. USA 2016, 113, 4164–4169.
Markman, J. L.; Rekechenetskiy, A.; Holler, E.; Ljubimova, J. Y. Nanomedicine therapeutic approaches to overcome cancer drug resistance. Adv. Drug Deliver. Rev. 2013, 65, 1866–1879.
Mou, Q. B.; Ma, Y.; Ding, F.; Gao, X. H.; Yan, D. Y.; Zhu, X. Y.; Zhang, C. Two-in-one chemogene assembled from drug-integrated antisense oligonucleotides to reverse chemoresistance. J. Am. Chem. Soc. 2019, 141, 6955–6966.
Szakács, G.; Paterson, J. K.; Ludwig, J. A.; Booth-Genthe, C.; Gottesman, M. M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234.
Gottesman, M. M.; Fojo, T.; Bates, S. E. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58.
Wang, H.; Gao, Z.; Liu, X. Y.; Agarwal, P.; Zhao, S. T.; Conroy, D. W.; Ji, G.; Yu, J. H.; Jaroniec, C. P.; Liu, Z. G. et al. Targeted production of reactive oxygen species in mitochondria to overcome cancer drug resistance. Nat. Commun. 2018, 9, 562.
Zhang, C.; Bu, W. B.; Ni, D. L.; Zhang, S. J.; Li, Q.; Yao, Z. W.; Zhang, J. W.; Yao, H. L.; Wang, Z.; Shi, J. L. Synthesis of iron nanometallic glasses and their application in cancer therapy by a localized Fenton reaction. Angew. Chem., Int. Ed. 2016, 55, 2101–2106.
Tang, Z. M.; Liu, Y. Y.; He, M. Y.; Bu, W. B. Chemodynamic therapy: Tumour microenvironment-mediated Fenton and Fenton-like reactions. Angew. Chem., Int. Ed. 2019, 58, 946–956.
Liu, Y.; Zhen, W. Y.; Wang, Y. H.; Liu, J. H.; Jin, L. H.; Zhang, T. Q.; Zhang, S. T.; Zhao, Y.; Song, S. Y.; Li, C. Y. et al. One-dimensional Fe2P acts as a fenton agent in response to nir ii light and ultrasound for deep tumor synergetic theranostics. Angew. Chem., Int. Ed. 2019, 58, 2407–2412.
Xue, C. C.; Li, M. H.; Zhao, Y.; Zhou, J.; Hu, Y.; Cai, K. Y.; Zhao, Y. L.; Yu, S. H.; Luo, Z. Tumor microenvironment-activatable Fedoxorubicin preloaded amorphous CaCO3 nanoformulation triggers ferroptosis in target tumor cells. Sci. Adv. 2020, 6, eaax1346.
Brookes, P. S.; Yoon, Y.; Robotham, J. L.; Anders, M. W.; Sheu, S. S. Calcium, ATP, and ROS: A mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 2004, 287, C817–C833.
Dong, Z. L.; Feng, L. Z.; Zhu, W. W.; Sun, X. Q.; Gao, M.; Zhao, H.; Chao, Y.; Liu, Z. CaCO3 nanoparticles as an ultra-sensitive tumor-pH-responsive nanoplatform enabling real-time drug release monitoring and cancer combination therapy. Biomaterials 2016, 110, 60–70.
Zhao, Y.; Luo, Z.; Li, M. H.; Qu, Q. Y.; Ma, X.; Yu, S. H.; Zhao, Y. L. A preloaded amorphous calcium carbonate/doxorubicin@silica nanoreactor for pH-responsive delivery of an anticancer drug. Angew. Chem., Int. Ed. 2015, 54, 919–922
Dong, Z. L.; Feng, L. Z.; Chao, Y.; Hao, Y.; Chen, M. C.; Gong, F.; Han, X.; Zhang, R.; Cheng, L.; Liu, Z. Amplification of tumor oxidative stresses with liposomal fenton catalyst and glutathione inhibitor for enhanced cancer chemotherapy and radiotherapy. Nano Lett. 2018, 19, 805–815.
Wang, H. R.; Zhu, W. W.; Feng, L. Z.; Chen, Q.; Chao, Y.; Dong, Z. L.; Liu, Z. Nanoscale covalent organic polymers as a biodegradable nanomedicine for chemotherapy-enhanced photodynamic therapy of cancer. Nano Res. 2018, 11, 3244–3257.
Vyas, S.; Zaganjor, E.; Haigis, M. C. Mitochondria and cancer. Cell 2016, 166, 555–566.
Feng, L. Z.; Dong, Z. L.; Tao, D. L.; Zhang, Y. C.; Liu, Z. The acidic tumor microenvironment: A target for smart cancer nano-theranostics. Natl. Sci. Rev. 2017, 5, 269–286.
Wong, C.; Stylianopoulos, T.; Cui, J.; Martin, J.; Chauhan, V. P.; Jiang, W.; Popović, Z.; Jain, R. K.; Bawendi, M. G.; Fukumura, D. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc. Natl. Acad. Sci. USA 2011, 108, 2426–2431.
Dreher, M. R.; Liu, W. G.; Michelich, C. R.; Dewhirst, M. W.; Yuan, F.; Chilkoti, A. Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. J. Natl. Cancer Inst. 2006, 98, 335–344.
Alexis, F.; Pridgen, E.; Molnar, L. K.; Farokhzad, O. C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharmaceutics 2008, 5, 505–515.
Zhang, Y. N.; Poon, W.; Tavares, A. J.; McGilvray, I. D.; Chan, W. C. W. Nanoparticle-liver interactions: Cellular uptake and hepatobiliary elimination. J. Control. Release 2016, 240, 332–348.
Pan, L. M.; He, Q. J.; Liu, J. N.; Chen, Y.; Ma, M.; Zhang, L. L.; Shi, J. L. Nuclear-targeted drug delivery of TAT peptide-conjugated monodisperse mesoporous silica nanoparticles. J. Am. Chem. Soc. 2012, 134, 5722–5725.
Feng, L. Z.; Gao, M.; Tao, D. L.; Chen, Q.; Wang, H. R.; Dong, Z. L.; Chen, M. W.; Liu, Z. Cisplatin-prodrug-constructed liposomes as a versatile theranostic nanoplatform for bimodal imaging guided combination cancer therapy. Adv. Funct. Mater. 2016, 26, 2207–2217.
Dong, Z. L.; Gong, H.; Gao, M.; Zhu, W. W.; Sun, X. Q.; Feng, L. Z.; Fu, T. T.; Li, Y. G.; Liu, Z. Polydopamine nanoparticles as a versatile molecular loading platform to enable imaging-guided cancer combination therapy. Theranostics 2016, 6, 1031–1042.
Chen, Q.; Feng, L. Z.; Liu, J. J.; Zhu, W. W.; Dong, Z. L.; Wu, Y. F.; Liu, Z. Intelligent albumin–MnO2 nanoparticles as pH-/H2O2-responsive dissociable nanocarriers to modulate tumor hypoxia for effective combination therapy. Adv. Mater. 2016, 28, 7129–7136.
Liu, J. J.; Wang, H. R.; Yi, X.; Chao, Y.; Geng, Y. H.; Xu, L. G.; Yang, K.; Liu, Z. pH-sensitive dissociable nanoscale coordination polymers with drug loading for synergistically enhanced chemoradiotherapy. Adv. Funct. Mater. 2017, 27, 1703832.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 51802209), the National Research Programs from Ministry of Science and Technology (MOST) of China (No. 2016YFA0201200), the Natural Science Foundation of Jiangsu Province (No. BK20180848), the China Postdoctoral Science Foundation (No. 2018T110545), the Collaborative Innovation Center of Suzhou Nano Science and Technology, and the 111 Program from the Ministry of Education of China. We also thank the website of BioRender.com for its assistance in the creation of schematic figures.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
12274_2020_2972_MOESM1_ESM.pdf
Metal-polyphenol-network coated CaCO3 as pH-responsive nanocarriers to enable effective intratumoral penetration and reversal of multidrug resistance for augmented cancer treatments
Rights and permissions
About this article
Cite this article
Dong, Z., Hao, Y., Li, Q. et al. Metal-polyphenol-network coated CaCO3 as pH-responsive nanocarriers to enable effective intratumoral penetration and reversal of multidrug resistance for augmented cancer treatments. Nano Res. 13, 3057–3067 (2020). https://doi.org/10.1007/s12274-020-2972-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-020-2972-9