Abstract
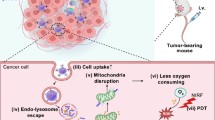
Recently, covalent-organic polymers (COPs), which covalently cross-link different types of organic molecules to form organic network structures, have received significant attention in various fields. However, the design of COPs that allows them to act as therapeutic agents remains to be explored. In the present study, a new class of COPs was fabricated by cross-linking the photosensitizer meso-tetra(p-hydroxyphenyl) porphine (THPP) to a chemotherapeutic pro-drug, cis-platinum (IV); the latter also acts as a reduction-responsive linker. After further conjugation with polyethylene glycol (PEG) in this one-pot reaction, we obtained THPP-Pt-PEG COPs, which can be stored in a lyophilized form and occur as stable nanoparticles in aqueous solution. The THPP-Pt-PEG COPs are effective in killing cancer cells through photodynamic treatment, and exhibited reduction-responsive degradation/drug release behaviors. Upon intravenous injection, the COPs, with a long blood circulation time, showed efficient tumor accumulation. Interestingly, we revealed that after injection of THPP-Pt-PEG COPs, tumors on mice exhibited greatly improved vascular perfusion and largely relieved tumor hypoxia, which favored subsequent photodynamic treatment. Hence, the combined chemo-photodynamic therapy of the COPs offers a remarkably improved therapeutic outcome compared to that with mono-therapies. This work presents a COP-based nanomedicine with high drug loading, lyophilizable formulation, prolonged blood half-life, efficient tumor passive homing, inherent biodegradability, and multiple therapeutic functions to achieve enhanced cancer combination therapy, with promise for clinical translation.

Similar content being viewed by others
References
Delplace, V.; Couvreur, P.; Nicolas, J. Recent trends in the design of anticancer polymer prodrug nanocarriers. Polym. Chem. 2014, 5, 1529–1544.
Sahay, G.; Alakhova, D. Y.; Kabanov, A. V. Endocytosis of nanomedicines. J. Control. Release 2010, 145, 182–195.
Yin, Q.; Shen, J. N.; Zhang, Z. W.; Yu, H. J.; Li, Y. P. Reversal of multidrug resistance by stimuli-responsive drug delivery systems for therapy of tumor. Adv. Drug. Deliv. Rev. 2013, 65, 1699–1715.
Yang, L.; Meng, L.; Zhang, X. B.; Chen, Y.; Zhu, G. Z.; Liu, H. P.; Xiong, X. L.; Sefah, K.; Tan, W. H. Engineering polymeric aptamers for selective cytotoxicity. J. Am. Chem. Soc. 2011, 133, 13380–13386.
Wei, X.; Wang, Y.; Xiong, X.; Guo, X.; Zhang, L.; Zhang, X. B.; Zhou, S. B. Codelivery of a p–p stacked dual anticancer drug combination with nanocarriers for overcoming multidrug resistance and tumor metastasis. Adv. Funct.Mater. 2016, 26, 8266–8280.
Wang, J.; Yang, G.; Guo, X.; Tang, Z. M.; Zhong, Z. D.; Zhou, S. B. Redox-responsive polyanhydride micelles for cancer therapy. Biomaterials 2014, 35, 3080–3090.
Ganta, S.; Devalapally, H.; Shahiwala, A.; Amiji, M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. Control. Release 2008, 126, 187–204.
Li, B.; Xu, H.; Li, Z.; Yao, M. F.; Xie, M.; Shen, H. J.; Shen, S.; Wang, X. S.; Jin, Y. Bypassing multidrug resistance in human breast cancer cells with lipid/polymer particle assemblies. Int. J. Nanomed. 2012, 7, 187–197.
Oh, J. K.; Lee, D. I.; Park, J. M. Biopolymer-based microgels/ nanogels for drug delivery applications. Prog. Polym. Sci. 2009, 34, 1261–1282.
Cao, Z. Q.; Yu, Q. M.; Xue, H.; Cheng, G.; Jiang, S. Y. Nanoparticles for drug delivery prepared from amphiphilic plga zwitterionic block copolymers with sharp contrast in polarity between two blocks. Angew. Chem., Int. Ed. 2010, 49, 3771–3776.
Poland, C. A.; Duffin, R.; Kinloch, I.; Maynard, A.; Wallace, W. A. H.; Seaton, A.; Stone, V.; Brown, S.; MacNee, W.; Donaldson, K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 2008, 3, 423–428.
Griset, A. P.; Walpole, J.; Liu, R.; Gaffey, A.; Colson, Y. L.; Grinstaff, M. W. Expansile nanoparticles: Synthesis, characterization, and in vivo efficacy of an acid-responsive polymeric drug delivery system. J. Am. Chem. Soc. 2009, 131, 2469–2471.
Hakkarainen, M.; Höglund, A.; Odelius, K.; Albertsson, A.-C. Tuning the release rate of acidic degradation products through macromolecular design of caprolactone-based copolymers. J. Am. Soc. Chem. 2007, 129, 6308–6312.
Joshi, H. M.; Bhumkar, D. R.; Joshi, K.; Pokharkar, V.; Sastry, M. Gold nanopartncles as carriers for efficient transmucosal insulin delivery. Langmuir 2006, 22, 300–305.
Dovydenko, I.; Tarassov, I.; Venyaminova, A.; Entelis, N. Method of carrier-free delivery of therapeutic RNA importable into human mitochondria: Lipophilic conjugates with cleavable bonds. Biomaterials 2016, 76, 408–417.
Hou, W. X.; Zhao, X.; Qian, X. Q.; Pan, F.; Zhang, C. L.; Yang, Y. M.; de la Fuente, J. M.; Cui, D. X. Ph-sensitive self-assembling nanoparticles for tumor near-infrared fluorescence imaging and chemo-photodynamic combination therapy. Nanoscale 2016, 8, 104–116.
Khan, W.; Farah, S.; Nyska, A.; Domb, A. J. Carrier free rapamycin loaded drug eluting stent: In vitro and in vivo evaluation. J. Control. Release 2013, 168, 70–76.
Chen, F.; Zhao, Y. Y.; Pan, Y. M.; Xue, X. D.; Zhang, X.; Kumar, A.; Liang, X. J. Synergistically enhanced therapeutic effect of a carrier-free hcpt/dox nanodrug on breast cancer cells through improved cellular drug accumulation. Mol. Pharm. 2015, 12, 2237–2244.
Zhang, R. Y.; Xing, R. R.; Jiao, T. F.; Ma, K.; Chen, C. J.; Ma, G. H.; Yan, X. H. Carrier-free, chemophotodynamic dual nanodrugs via self-assembly for synergistic antitumor therapy. ACS Appl. Mater. Interfaces 2016, 8, 13262–13269.
Li, W.; Yang, Y. L.; Wang, C.; Liu, Z.; Zhang, X. J.; An, F. F.; Diao, X. J.; Hao, X. J.; Zhang, X. H. Carrier-free, functionalized drug nanoparticles for targeted drug delivery. Chem. Commun. 2012, 48, 8120–8122.
Zhou, M. J.; Zhang, X. J.; Yang, Y. L.; Liu, Z.; Tian, B. S.; Jie, J. S.; Zhang, X. H. Carrier-free functionalized multidrug nanorods for synergistic cancer therapy. Biomaterials 2013, 34, 8960–8967.
Diao, X. J.; Li, W.; Yu, J.; Wang, X. J.; Zhang, X. J.; Yang, Y. L.; An, F. F.; Liu, Z.; Zhang, X. H. Carrier-free, water dispersible and highly luminescent dye nanoparticles for targeted cell imaging. Nanoscale 2012, 4, 5373–5377.
Hu, M. X.; Huang, P.; Wang, Y.; Su, Y.; Zhou, L. Z.; Zhu, X. Y.; Yan, D. Y. Synergistic combination chemotherapy of camptothecin and floxuridine through self-assembly of amphiphilic drug-drug conjugate. Bioconjugate Chem. 2015, 26, 2497–2506.
Huang, P.; Wang, D. L.; Su, Y.; Huang, W.; Zhou, Y. F.; Cui, D. X.; Zhu, X. Y.; Yan, D. Y. Combination of small molecule prodrug and nanodrug delivery: Amphiphilic drug-drug conjugate for cancer therapy. J. Am. Chem. Soc. 2014, 136, 11748–11756.
Ding, S. Y.; Wang, W. Covalent organic frameworks (COFs): From design to applications. Chem. Soc. Rev. 2012, 42, 548–568.
Feng, X.; Ding, X. S.; Jiang, D. L. Covalent organic frameworks. Chem. Soc. Rev. 2012, 41, 6010–6022.
Day, N. U.; Wamser, C. C.; Walter, M. G. Porphyrin polymers and organic frameworks. Polymer Int. 2015, 64, 833–857.
Dolmans, D. E. J. G. J.; Fukumura, D.; Jain, R. K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387.
Paszko, E.; Ehrhardt, C.; Senge, M. O.; Kelleher, D. P.; Reynolds, J. V. Nanodrug applications in photodynamic therapy. Photodiagn. Photodyn. Ther. 2011, 8, 14–29.
Konan, Y. N.; Berton, M.; Gurny, R.; Allémann, E. Enhanced photodynamic activity of meso-tetra(4-hydroxyphenyl)porphyrin by incorporation into sub-200 nm nanoparticles. Eur. J. Pharm. Sci. 2003, 18, 241–249.
Liu, J. J.; Yang, Y.; Zhu, W. W.; Yi, X.; Dong, Z. L.; Xu, X. N.; Chen, M. W.; Yang, K.; Lu, G.; Jiang, L. X. et al. Nanoscale metal-organic frameworks for combined photodynamic & radiation therapy in cancer treatment. Biomaterials 2016, 97, 1–9.
Yang, Y.; Liu, J. J.; Liang, C.; Feng, L. Z.; Fu, T. T.; Dong, Z. L.; Chao, Y.; Li, Y. G.; Lu, G.; Chen, M. W. et al. Nanoscale metal–organic particles with rapid clearance for magnetic resonance imaging-guided photothermal therapy. ACS Nano 2016, 10, 2774–2781.
Liu, J. J.; Wang, C.; Wang, X. J.; Wang, X.; Cheng, L.; Li, Y. G.; Liu, Z. Mesoporous silica coated single-walled carbon nanotubes as a multifunctional light-responsive platform for cancer combination therapy. Adv. Funct. Mater. 2015, 25, 384–392.
Fang, Q. R.; Wang, J. H.; Gu, S.; Kaspar, R. B.; Zhuang, Z. B.; Zheng, J.; Guo, H. X.; Qiu, S. L.; Yan, Y. S. 3D porous crystalline polyimide covalent organic frameworks for drug delivery. J. Am. Chem. Soc. 2015, 137, 8352–8355.
Bai, L. Y.; Phua, S. Z. F.; Lim, W. Q.; Jana, A.; Luo, Z.; Tham, H. J. P.; Zhao, L. Z.; Gao, Q.; Zhao, Y. L. Nanoscale covalent organic frameworks as smart carriers for drug delivery. Chem. Commun. 2016, 52, 4128–4131.
Vyas, V. S.; Vishwakarma, M.; Moudrakovski, I.; Haase, F.; Savasci, G.; Ochsenfeld, C.; Spatz, J. P.; Lotsch, B. V. Exploiting noncovalent interactions in an imine-based covalent organic framework for quercetin delivery. Adv. Mater. 2016, 28, 8749–8754.
Wang, S. Z.; Gao, R. M.; Zhou, F. M.; Selke, M. Nanomaterials and singlet oxygen photosensitizers: Potential applications in photodynamic therapy. J. Mater. Chem. 2004, 14, 487–493.
Rieter, W. J.; Pott, K. M.; Taylor, K. M. L.; Lin, W. B. Nanoscale coordination polymers for platinum-based anticancer drug delivery. J. Am. Chem. Soc. 2008, 130, 11584–11585.
Zhu, Z.; Tang, Z. W.; Phillips, J. A.; Yang, R. H.; Wang, H.; Tan, W. H. Regulation of singlet oxygen generation using single-walled carbon nanotubes. J. Am. Chem. Soc. 2008, 130, 10856–10857.
Yang, J.; Liu, W. W.; Sui, M. H.; Tang, J. B.; Shen, Y. Q. Platinum (IV)-coordinate polymers as intracellular reductionresponsive backbone-type conjugates for cancer drug delivery. Biomaterials 2011, 32, 9136–9143.
Feng, L. Z.; Gao, M.; Tao, D. L.; Chen, Q.; Wang, H. R.; Dong, Z. L.; Chen, M. W.; Liu, Z. Cisplatin-prodrugconstructed liposomes as a versatile theranostic nanoplatform for bimodal imaging guided combination cancer therapy. Adv. Funct.Mater. 2016, 26, 2207–2217.
Wilson, W. R.; Hay, M. P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 2011, 11, 393–410.
Brown, J. M.; Wilson, W. R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 2004, 4, 437–447.
Bertout, J. A.; Patel, S. A.; Simon, M. C. The impact of O2 availability on human cancer. Nat. Rev. Cancer 2008, 8, 967–975.
Harris, A. L. Hypoxia-a key regulatory factor in tumour growth. Nat. Rev. Cancer 2002, 2, 38–47.
Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995, 1, 27–30.
Zhong, X. S.; Liu, L. Z.; Skinner, H. D.; Cao, Z. X.; Ding, M.; Jiang, B. H. Mechanism of vascular endothelial growth factor expression mediated by cisplatin in human ovarian cancer cells. Biochem. Biophys. Res. Commun. 2007, 358, 92–98.
Olson, T. A.; Mohanraj, D.; Carson, L. F.; Ramakrishnan, S. Vascular permeability factor gene expression in normal and neoplastic human ovaries. Cancer Res. 1994, 54, 276–280.
Gong, H.; Chao, Y.; Xiang, J.; Han, X.; Song, G. S.; Feng, L. Z.; Liu, J. J.; Yang, G. B.; Chen, Q.; Liu, Z. Hyaluronidase to enhance nanoparticle-based photodynamic tumor therapy. Nano Lett. 2016, 16, 2512–2521.
Li, Q.; Tian, Y. T.; Li, D. D.; Sun, J. F.; Shi, D. L.; Fang, L.; Gao, Y.; Liu, H. Y. The effect of lipocisplatin on cisplatin efficacy and nephrotoxicity in malignant breast cancer treatment. Biomaterials 2014, 35, 6462–6472.
Acknowledgements
This article was partially supported by the National Basic Research Programs of China (No. 2016YFA0201200), the National Natural Science Foundation of China (No. 51525203), Collaborative Innovation Center of Suzhou Nano Science and Technology, and a Project Funded by the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
12274_2017_1858_MOESM1_ESM.pdf
Nanoscale covalent organic polymers as a biodegradable nanomedicine for chemotherapy-enhanced photodynamic therapy of cancer
Rights and permissions
About this article
Cite this article
Wang, H., Zhu, W., Feng, L. et al. Nanoscale covalent organic polymers as a biodegradable nanomedicine for chemotherapy-enhanced photodynamic therapy of cancer. Nano Res. 11, 3244–3257 (2018). https://doi.org/10.1007/s12274-017-1858-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-017-1858-y