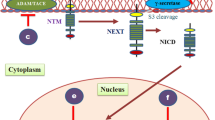
Abstract
Esophageal squamous cell carcinoma (ESCC) is the most common type of esophageal cancer (EC) in Asia. It is a malignant digestive tract tumor with abundant gene mutations. Due to the lack of specific diagnostic markers and early cancer screening markers, most patients are diagnosed at an advanced stage. Genetic and epigenetic changes are closely related to the occurrence and development of ESCC. Here, We review the activation of proto-oncogenes into oncogenes through gene mutation and gene amplification in ESCC from a genetic and epigenetic genome perspective, We also discuss the specific regulatory mechanisms through which these oncogenes mainly affect the biological function and occurrence and development of ESCC through specific regulatory mechanisms. In addition, we summarize the clinical application value of these oncogenes is summarized, and it provides a feasible direction for clinical use as potential therapeutic and diagnostic markers.




Similar content being viewed by others
Abbreviations
- ESCC:
-
Esophageal squamous cell carcinoma
- EC:
-
Esophageal cancer
- EAC:
-
Esophageal adenocarcinoma
- EMT:
-
Epithelial-mesenchymal transition
- OS:
-
Overall survival
- PIK3CA:
-
P110X of 3-phosphatilinositol kinase L3K
- PKMYT1:
-
Protein kinase, membrane-associated tyrosine/threonine
- MLL2:
-
Mixed-lineage leukemia 2
- FLVCR1:
-
Feline leukemia virus subgroup C receptor 1
- TGIF1:
-
TGFB-induced factor homeobox 1
- TAF1L:
-
TATA-box binding protein associated factor 1
- EP300:
-
E1A Binding Protein P300
- GLI1:
-
Glioma-associated oncogene homolog 1
- YAP1:
-
YES-related protein 1
- SOX2:
-
Sex-determining region Y-box 2
- SOX9:
-
Sex-determining region Y-box 9
- FAM135B:
-
Recombinant proteins of the hominid family with sequence similarity 135, member B
- PCARP:
-
Poplar ataxia with retinitis pigmentosa
- FAM84B:
-
Family with sequence similarity 84 member B
- GASC1:
-
Gene amplified in squamous cell carcinoma 1
- MAGE-A11:
-
Melanoma Antigens Genes (MAGE)family member 11
- ALKBH5:
-
Human ALKB homolog H5
- MTOR:
-
Mechanistic target of rapamycin kinase
- GRN:
-
Granulin precursor
- TFCP2:
-
Transcription factor CP2
- ZEB1:
-
Zinc finger E-box binding homeobox 1
- HAT:
-
Histone acetyl transferase
- HDACs:
-
Histone deacetylases
- APOBEC:
-
Apolipoprotein B mRNA-editing enzyme catalytic polypeptide-like
- CSCs:
-
Cancer stem cells
- CDK1:
-
Cyclin-dependent kinase 1
- TGIF1:
-
TGFB-induced factor homeobox 1
- TRAIL:
-
Tumor Necrosis factor-related apoptosis-inducing ligand
- H3K4:
-
Histone 3 lysine 4
- DSBH:
-
Double-stranded β-helix fold
- DDX3:
-
DEAD- (ASP-Glu-Ala-asp -) Box 3
- METTL3:
-
Methyltransferase-like 3
- HNRNPC:
-
Heterogeneous nuclear ribonucleoprotein binding protein
- HIF-1:
-
Hypoxia-inducible factor 1
- RNF168:
-
RING finger protein 168
- KIF4A:
-
Kinesin superfamily member 4A
- EIF3H:
-
Eukaryotic translation initiation factor 3H
- MMPs:
-
Matrix metalloproteinases
- TRIB2:
-
Tribble2
- GLS2:
-
Glutaminase 2
- CCRT:
-
Concurrent chemoradiotherapy
- NF:
-
Normal fibroblasts
- PARK2:
-
Parkin
- CSE1L:
-
Chromosome segregation 1–like
- ADAR1:
-
Adenosine deaminase RNA specific
- ADAM29:
-
A disintegrin and metalloprotease domain 29
- ErbB:
-
Epidermal growth factor
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660.
Song Y, Li L, Ou Y, Gao Z, Li E, Li X, et al. Identification of genomic alterations in oesophageal squamous cell cancer. Nature. 2014;509(7498):91–5. https://doi.org/10.1038/nature13176.
Tramontano AC, Chen Y, Watson TR, Eckel A, Hur C, Kong CY. Esophageal cancer treatment costs by phase of care and treatment modality, 2000–2013. Cancer Med. 2019;8(11):5158–72. https://doi.org/10.1002/cam4.2451.
Liu X, Zhang M, Ying S, Zhang C, Lin R, Zheng J, et al. Genetic alterations in esophageal tissues from squamous dysplasia to carcinoma. Gastroenterology. 2017;153(1):166–77. https://doi.org/10.1053/j.gastro.2017.03.033.
Lima SC, Hernandez-Vargas H, Simao T, Durand G, Kruel CD, Le Calvez-Kelm F, et al. Identification of a DNA methylome signature of esophageal squamous cell carcinoma and potential epigenetic biomarkers. Epigenetics. 2011;6(10):1217–27. https://doi.org/10.4161/epi.6.10.17199.
O’Reilly S, Forastiere AA. Is surgery necessary with multimodality treatment of oesophageal cancer. Ann Oncol. 1995;6(6):519–21. https://doi.org/10.1093/oxfordjournals.annonc.a059237.
Wu J, Yang J, Lin X, Lin L, Jiang W, Xie C. Survival outcomes for patients with four treatments in stages I-III esophageal squamous cell carcinoma: a SEER analysis. Transl Cancer Res. 2021;10(5):2144–52. https://doi.org/10.21037/tcr-20-2995.
Kontomanolis EN, Koutras A, Syllaios A, Schizas D, Mastoraki A, Garmpis N, et al. Role of oncogenes and tumor-suppressor genes in carcinogenesis: a review. Anticancer Res. 2020;40(11):6009–15. https://doi.org/10.21873/anticanres.14622.
Balmain A, Brown K. Oncogene activation in chemical carcinogenesis. Amsterdam: Elsevier; 1988. p. 147–82.
Gebhart E, Liehr T. Patterns of genomic imbalances in human solid tumors (Review). Int J Oncol. 2000;16(2):383–99. https://doi.org/10.3892/ijo.16.2.383.
Wang L, Shan L, Zhang S, Ying J, Xue L, Yuan Y, et al. PIK3CA gene mutations and overexpression: implications for prognostic biomarker and therapeutic target in Chinese esophageal squamous cell carcinoma. PLoS ONE. 2014;9(7):e103021. https://doi.org/10.1371/journal.pone.0103021.
Munari FF, Cruvinel-Carloni A, Lacerda CF, de Oliveira ATT, Scapulatempo-Neto C, da Silva SRM, et al. PIK3CA mutations are frequent in esophageal squamous cell carcinoma associated with chagasic megaesophagus and are associated with a worse patient outcome. Infect Agent Cancer. 2018;13:43. https://doi.org/10.1186/s13027-018-0216-3.
Gu Y, Lin X, Kapoor A, Chow MJ, Jiang Y, Zhao K, et al. The oncogenic potential of the centromeric border protein FAM84B of the 8q24.21 gene desert. Genes. 2020. https://doi.org/10.3390/genes11030312.
Zhang Q, Zhao X, Zhang C, Wang W, Li F, Liu D, et al. Overexpressed PKMYT1 promotes tumor progression and associates with poor survival in esophageal squamous cell carcinoma. Cancer Manag Res. 2019;11:7813–24. https://doi.org/10.2147/CMAR.S214243.
Dong D, Zhang W, Xiao W, Wu Q, Cao Y, Gao X, et al. A GRN autocrine-dependent FAM135B/AKT/mTOR feedforward loop promotes esophageal squamous cell carcinoma progression. Cancer Res. 2021;81(4):910–22. https://doi.org/10.1158/0008-5472.CAN-20-0912.
Peng C, Song Y, Chen W, Wang X, Liu X, Wang F, et al. FLVCR1 promotes the proliferation and tumorigenicity of synovial sarcoma through inhibiting apoptosis and autophagy. Int J Oncol. 2018;52(5):1559–68. https://doi.org/10.3892/ijo.2018.4312.
Kong L, Yu Y, Guan H, Jiang L, Sun F, Li X, et al. TGIF1 plays a carcinogenic role in esophageal squamous cell carcinoma through the Wnt/β-catenin and Akt/mTOR signaling pathways. Int J Mol Med. 2021. https://doi.org/10.3892/ijmm.2021.4910.
Zhang H, Qin G, Zhang C, Yang H, Liu J, Hu H, et al. TRAIL promotes epithelial-to-mesenchymal transition by inducing PD-L1 expression in esophageal squamous cell carcinomas. J Exp Clin Cancer Res. 2021;40(1):209. https://doi.org/10.1186/s13046-021-01972-0.
Wang X, Ge X, Wang H, Huang J, Song Q, Xu C, et al. SOX2 amplification and chromosome 3 gain significantly impact prognosis in esophageal squamous cell carcinoma. Ann Transl Med. 2021;9(4):321. https://doi.org/10.21037/atm-20-1290.
Bi Y, Kong P, Zhang L, Cui H, Xu X, Chang F, et al. EP300 as an oncogene correlates with poor prognosis in esophageal squamous carcinoma. J Cancer. 2019;10(22):5413–26. https://doi.org/10.7150/jca.34261.
Abudureheman A, Ainiwaer J, Hou Z, Niyaz M, Turghun A, Hasim A, et al. High MLL2 expression predicts poor prognosis and promotes tumor progression by inducing EMT in esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 2018;144(6):1025–35. https://doi.org/10.1007/s00432-018-2625-5.
Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2013;38(1):23–38. https://doi.org/10.1038/npp.2012.112.
Teng H, Xue M, Liang J, Wang X, Wang L, Wei W, et al. Inter- and intratumor DNA methylation heterogeneity associated with lymph node metastasis and prognosis of esophageal squamous cell carcinoma. Theranostics. 2020;10(7):3035–48. https://doi.org/10.7150/thno.42559.
Zhang L, Lu Q, Chang C. Epigenetics in health and disease. Adv Exp Med Biol. 2020;1253:3–55. https://doi.org/10.1007/978-981-15-3449-2_1.
Lin L, Cheng X, Yin D. Aberrant DNA methylation in esophageal squamous cell carcinoma: biological and clinical implications. Front Oncol. 2020;10:549850. https://doi.org/10.3389/fonc.2020.549850.
Morin RD, Mendez-Lago M, Mungall AJ, Goya R, Mungall KL, Corbett RD, et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature. 2011;476(7360):298–303. https://doi.org/10.1038/nature10351.
Pasqualucci L, Trifonov V, Fabbri G, Ma J, Rossi D, Chiarenza A, et al. Analysis of the coding genome of diffuse large B-cell lymphoma. Nat Genet. 2011;43(9):830–7. https://doi.org/10.1038/ng.892.
Stephens PJ, Tarpey PS, Davies H, Van Loo P, Greenman C, Wedge DC, et al. The landscape of cancer genes and mutational processes in breast cancer. Nature. 2012;486(7403):400–4. https://doi.org/10.1038/nature11017.
Li H, Li Q, Lian J, Chu Y, Fang K, Xu A, et al. MLL2 promotes cancer cell lymph node metastasis by interacting with RelA and facilitating STC1 transcription. Cell Signal. 2020;65:109457. https://doi.org/10.1016/j.cellsig.2019.109457.
van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254(5038):1643–7. https://doi.org/10.1126/science.1840703.
Sang M, Wang L, Ding C, Zhou X, Wang B, Wang L, et al. Melanoma-associated antigen genes—an update. Cancer Lett. 2011;302(2):85–90. https://doi.org/10.1016/j.canlet.2010.10.021.
Sang M, Lian Y, Zhou X, Shan B. MAGE-A family: attractive targets for cancer immunotherapy. Vaccine. 2011;29(47):8496–500. https://doi.org/10.1016/j.vaccine.2011.09.014.
Gu L, Sang M, Li J, Liu F, Wu Y, Liu S, et al. Demethylation-mediated upregulation of melanoma-associated antigen-A11 correlates with malignant progression of esophageal squamous cell carcinoma. Dig Liver Dis. 2019;51(10):1475–82. https://doi.org/10.1016/j.dld.2019.04.018.
Liu S, Liu F, Huang W, Gu L, Meng L, Ju Y, et al. MAGE-A11 is activated through TFCP2/ZEB1 binding sites de-methylation as well as histone modification and facilitates ESCC tumor growth. Oncotarget. 2018;9(3):3365–78. https://doi.org/10.18632/oncotarget.22973.
Sang M, Gu L, Liu F, Lian Y, Yin D, Fan X, et al. Prognostic significance of MAGE-A11 in esophageal squamous cell carcinoma and identification of related genes based on DNA microarray. Arch Med Res. 2016;47(3):151–61. https://doi.org/10.1016/j.arcmed.2016.06.001.
Boccaletto P, Baginski B. MODOMICS: an operational guide to the use of the RNA modification pathways database. Methods Mol Biol. 2021;2284:481–505. https://doi.org/10.1007/978-1-0716-1307-8_26.
Chen XY, Zhang J, Zhu JS. The role of m(6)A RNA methylation in human cancer. Mol Cancer. 2019;18(1):103. https://doi.org/10.1186/s12943-019-1033-z.
Wang T, Kong S, Tao M, Ju S. The potential role of RNA N6-methyladenosine in cancer progression. Mol Cancer. 2020;19(1):88. https://doi.org/10.1186/s12943-020-01204-7.
Mendel M, Delaney K, Pandey RR, Chen KM, Wenda JM, Vagbo CB, et al. Splice site m(6)A methylation prevents binding of U2AF35 to inhibit RNA splicing. Cell. 2021;184(12):3125-3142 e25.
Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3’ UTRs and near stop codons. Cell. 2012;149(7):1635–46. https://doi.org/10.1016/j.cell.2012.05.003.
Kataoka H, Sekiguchi M. Molecular cloning and characterization of the alkB gene of Escherichia coli. Mol Gen Genet. 1985;198(2):263–9. https://doi.org/10.1007/bf00383004.
Liu Y, Yuan Q, Xie L. The AlkB family of Fe (II)/alpha-ketoglutarate-dependent dioxygenases modulates embryogenesis through epigenetic regulation. Curr Stem Cell Res Ther. 2018. https://doi.org/10.2174/1574888x12666171027105532.
Kurowski MA, Bhagwat AS, Papaj G, Bujnicki JM. Phylogenomic identification of five new human homologs of the DNA repair enzyme AlkB. BMC Genomics. 2003;4(1):48. https://doi.org/10.1186/1471-2164-4-48.
Xue J, Xiao P, Yu X, Zhang X. A positive feedback loop between AlkB homolog 5 and miR-193a-3p promotes growth and metastasis in esophageal squamous cell carcinoma. Hum Cell. 2021;34(2):502–14. https://doi.org/10.1007/s13577-020-00458-z.
Liu S, Huang M, Chen Z, Chen J, Chao Q, Yin X, et al. FTO promotes cell proliferation and migration in esophageal squamous cell carcinoma through up-regulation of MMP13. Exp Cell Res. 2020;389(1):111894. https://doi.org/10.1016/j.yexcr.2020.111894.
Cui Y, Zhang C, Ma S, Li Z, Wang W, Li Y, et al. RNA m6A demethylase FTO-mediated epigenetic up-regulation of LINC00022 promotes tumorigenesis in esophageal squamous cell carcinoma. J Exp Clin Cancer Res. 2021;40(1):294. https://doi.org/10.1186/s13046-021-02096-1.
Gao R, Ye M, Liu B, Wei M, Ma D, Dong K. m6A Modification: a double-edged sword in tumor development. Front Oncol. 2021;11:679367. https://doi.org/10.3389/fonc.2021.679367.
Han H, Yang C, Zhang S, Cheng M, Guo S, Zhu Y, et al. METTL3-mediated m(6)A mRNA modification promotes esophageal cancer initiation and progression via notch signaling pathway. Mol Ther Nucleic Acids. 2021;26:333–46. https://doi.org/10.1016/j.omtn.2021.07.007.
Chen X, Huang L, Yang T, Xu J, Zhang C, Deng Z, et al. METTL3 promotes esophageal squamous cell carcinoma metastasis through enhancing GLS2 expression. Front Oncol. 2021;11:667451. https://doi.org/10.3389/fonc.2021.667451.
Liu Z, Wu K, Gu S, Wang W, Xie S, Lu T, et al. A methyltransferase-like 14/miR-99a-5p/tribble 2 positive feedback circuit promotes cancer stem cell persistence and radioresistance via histone deacetylase 2-mediated epigenetic modulation in esophageal squamous cell carcinoma. Clin Transl Med. 2021;11(9):e545. https://doi.org/10.1002/ctm2.545.
Xu LC, Pan JX, Pan HD. Construction and validation of an m6A RNA methylation regulators-based prognostic signature for esophageal cancer. Cancer Manag Res. 2020;12:5385–94. https://doi.org/10.2147/CMAR.S254870.
Zhang X, Lu N, Wang L, Wang Y, Li M, Zhou Y, et al. Recent advances of m(6)A methylation modification in esophageal squamous cell carcinoma. Cancer Cell Int. 2021;21(1):421. https://doi.org/10.1186/s12935-021-02132-2.
Lee EY, Muller WJ. Oncogenes and tumor suppressor genes. Cold Spring Harb Perspect Biol. 2010;2(10):a003236. https://doi.org/10.1101/cshperspect.a003236.
Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–93. https://doi.org/10.1016/j.cell.2012.03.017.
Aoki M, Fujishita T. Oncogenic Roles of the PI3K/AKT/mTOR Axis. Curr Top Microbiol Immunol. 2017;407:153–89. https://doi.org/10.1007/82_2017_6.
Wang L, Zhang Z, Yu X, Li Q, Wang Q, Chang A, et al. SOX9/miR-203a axis drives PI3K/AKT signaling to promote esophageal cancer progression. Cancer Lett. 2020;468:14–26. https://doi.org/10.1016/j.canlet.2019.10.004.
Gen Y, Yasui K, Nishikawa T, Yoshikawa T. SOX2 promotes tumor growth of esophageal squamous cell carcinoma through the AKT/mammalian target of rapamycin complex 1 signaling pathway. Cancer Sci. 2013;104(7):810–6. https://doi.org/10.1111/cas.12155.
Arechavaleta-Velasco F, Perez-Juarez CE, Gerton GL, Diaz-Cueto L. Progranulin and its biological effects in cancer. Med Oncol. 2017;34(12):194. https://doi.org/10.1007/s12032-017-1054-7.
Yang D, Li R, Wang H, Wang J, Han L, Pan L, et al. Clinical implications of progranulin in gastric cancer and its regulation via a positive feedback loop involving AKT and ERK signaling pathways. Mol Med Rep. 2017;16(6):9685–91. https://doi.org/10.3892/mmr.2017.7796.
Feng T, Zheng L, Liu F, Xu X, Mao S, Wang X, et al. Growth factor progranulin promotes tumorigenesis of cervical cancer via PI3K/Akt/mTOR signaling pathway. Oncotarget. 2016;7(36):58381–95. https://doi.org/10.18632/oncotarget.11126.
Wang C, Cheng L, Song S, Wu S, Sun G. Gli1 interacts with YAP1 to promote tumorigenesis in esophageal squamous cell carcinoma. J Cell Physiol. 2020;235(11):8224–35. https://doi.org/10.1002/jcp.29477.
Rizvi S, Demars CJ, Comba A, Gainullin VG, Rizvi Z, Almada LL, et al. Combinatorial chemoprevention reveals a novel smoothened-independent role of GLI1 in esophageal carcinogenesis. Cancer Res. 2010;70(17):6787–96. https://doi.org/10.1158/0008-5472.Can-10-0197.
Wei L, Xu Z. Cross-signaling among phosphinositide-3 kinase, mitogen-activated protein kinase and sonic hedgehog pathways exists in esophageal cancer. Int J Cancer. 2011;129(2):275–84. https://doi.org/10.1002/ijc.25673.
Yang Z, Cui Y, Ni W, Kim S, Xuan Y. Gli1, a potential regulator of esophageal cancer stem cell, is identified as an independent adverse prognostic factor in esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 2017;143(2):243–54. https://doi.org/10.1007/s00432-016-2273-6.
Maehama T, Nishio M, Otani J, Mak TW, Suzuki A. The role of Hippo-YAP signaling in squamous cell carcinomas. Cancer Sci. 2021;112(1):51–60. https://doi.org/10.1111/cas.14725.
Yu F, Yu C, Li F, Zuo Y, Wang Y, Yao L, et al. Wnt/beta-catenin signaling in cancers and targeted therapies. Signal Transduct Target Ther. 2021;6(1):307. https://doi.org/10.1038/s41392-021-00701-5.
Koni M, Pinnaro V, Brizzi MF. The Wnt signalling pathway: a tailored target in cancer. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21207697.
Zhang Y, Wang X. Targeting the Wnt/beta-catenin signaling pathway in cancer. J Hematol Oncol. 2020;13(1):165. https://doi.org/10.1186/s13045-020-00990-3.
Lv Z, Liu RD, Chen XQ, Wang B, Li LF, Guo YS, et al. HIF1alpha promotes the stemness of oesophageal squamous cell carcinoma by activating the Wnt/betacatenin pathway. Oncol Rep. 2019;42(2):726–34. https://doi.org/10.3892/or.2019.7203.
Gou Y, Jin D, He S, Han S, Bai Q. RNF168 is highly expressed in esophageal squamous cell carcinoma and contributes to the malignant behaviors in association with the Wnt/β-catenin signaling pathway. Aging. 2021;13(4):5403–14. https://doi.org/10.18632/aging.202471.
Tang Y, Yang P, Zhu Y, Su Y. LncRNA TUG1 contributes to ESCC progression via regulating miR-148a-3p/MCL-1/Wnt/β-catenin axis in vitro. Thorac Cancer. 2020;11(1):82–94. https://doi.org/10.1111/1759-7714.13236.
Nieto MA, Huang RY, Jackson RA, Thiery JP. Emt: 2016. Cell. 2016;166(1):21–45. https://doi.org/10.1016/j.cell.2016.06.028.
Kahlert UD, Maciaczyk D, Doostkam S, Orr BA, Simons B, Bogiel T, et al. Activation of canonical WNT/β-catenin signaling enhances in vitro motility of glioblastoma cells by activation of ZEB1 and other activators of epithelial-to-mesenchymal transition. Cancer Lett. 2012;325(1):42–53. https://doi.org/10.1016/j.canlet.2012.05.024.
De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13(2):97–110. https://doi.org/10.1038/nrc3447.
Puisieux A, Brabletz T, Caramel J. Oncogenic roles of EMT-inducing transcription factors. Nat Cell Biol. 2014;16(6):488–94. https://doi.org/10.1038/ncb2976.
Jiang Y, Zhan H. Communication between EMT and PD-L1 signaling: new insights into tumor immune evasion. Cancer Lett. 2020;468:72–81. https://doi.org/10.1016/j.canlet.2019.10.013.
Pastushenko I, Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019;29(3):212–26. https://doi.org/10.1016/j.tcb.2018.12.001.
Li Y, Yang HX, Luo RZ, Zhang Y, Li M, Wang X, et al. High expression of p300 has an unfavorable impact on survival in resectable esophageal squamous cell carcinoma. Ann Thorac Surg. 2011;91(5):1531–8. https://doi.org/10.1016/j.athoracsur.2010.12.012.
Razzaque MS, Atfi A. TGIF function in oncogenic Wnt signaling. Biochim Biophys Acta. 2016;1865(2):101–4. https://doi.org/10.1016/j.bbcan.2015.10.003.
Zhang MZ, Ferrigno O, Wang Z, Ohnishi M, Prunier C, Levy L, et al. TGIF governs a feed-forward network that empowers Wnt signaling to drive mammary tumorigenesis. Cancer Cell. 2015;27(4):547–60. https://doi.org/10.1016/j.ccell.2015.03.002.
Wang L, Liu G, Bolor-Erdene E, Li Q, Mei Y, Zhou L. Identification of KIF4A as a prognostic biomarker for esophageal squamous cell carcinoma. Aging. 2021;13(21):24050–70. https://doi.org/10.18632/aging.203585.
Guo X, Zhu R, Luo A, Zhou H, Ding F, Yang H, et al. EIF3H promotes aggressiveness of esophageal squamous cell carcinoma by modulating snail stability. J Exp Clin Cancer Res. 2020;39(1):175. https://doi.org/10.1186/s13046-020-01678-9.
Ma S, Meng Z, Chen R, Guan KL. The hippo pathway: biology and pathophysiology. Annu Rev Biochem. 2019;88:577–604. https://doi.org/10.1146/annurev-biochem-013118-111829.
Sun X, Chen P, Chen X, Yang W, Chen X, Zhou W, et al. KIF4A enhanced cell proliferation and migration via Hippo signaling and predicted a poor prognosis in esophageal squamous cell carcinoma. Thorac Cancer. 2021;12(4):512–24. https://doi.org/10.1111/1759-7714.13787.
Zhou X, Li Y, Wang W, Wang S, Hou J, Zhang A, et al. Regulation of hippo/YAP signaling and esophageal squamous carcinoma progression by an E3 ubiquitin ligase PARK2. Theranostics. 2020;10(21):9443–57. https://doi.org/10.7150/thno.46078.
Pang D, Wang W, Zhou X, Lu K, Zhang J, Chen Z, et al. RACO-1 modulates hippo signalling in oesophageal squamous cell carcinoma. J Cell Mol Med. 2020;24(20):11912–21. https://doi.org/10.1111/jcmm.15811.
Zhang J, Wang JL, Zhang CY, Ma YF, Zhao R, Wang YY. The prognostic role of FZD6 in esophageal squamous cell carcinoma patients. Clin Transl Oncol. 2020;22(7):1172–9. https://doi.org/10.1007/s12094-019-02243-3.
Jia J, Li H, Chu J, Sheng J, Wang C, Jia Z, et al. LncRNA FAM83A-AS1 promotes ESCC progression by regulating miR-214/CDC25B axis. J Cancer. 2021;12(4):1200–11. https://doi.org/10.7150/jca.54007.
Zhang H, Wang Y, Zhang W, Wu Q, Fan J, Zhan Q. BAALC-AS1/G3BP2/c-Myc feedback loop promotes cell proliferation in esophageal squamous cell carcinoma. Cancer Commun. 2021;41(3):240–57. https://doi.org/10.1002/cac2.12127.
Zhao Z, Yang S, Zhou A, Li X, Fang R, Zhang S, et al. Small extracellular vesicles in the development, diagnosis, and possible therapeutic application of esophageal squamous cell carcinoma, front. Oncol. 2021;11: 732702. https://doi.org/10.3389/fonc.2021.732702.
Tong Y, Yang L, Yu C, Zhu W, Zhou X, Xiong Y, et al. Tumor-secreted exosomal lncRNA POU3F3 promotes cisplatin resistance in ESCC by inducing fibroblast differentiation into CAFs. Mol Ther Oncolytics. 2020;18:1–13. https://doi.org/10.1016/j.omto.2020.05.014.
Shi S, Huang X, Ma X, Zhu X, Zhang Q. Research of the mechanism on miRNA193 in exosomes promotes cisplatin resistance in esophageal cancer cells. PLoS ONE. 2020;15(5):e0225290. https://doi.org/10.1371/journal.pone.0225290.
Yang YC, Liu GJ, Yuan DF, Li CQ, Xue M, Chen LJ. Influence of exosome-derived miR-21on chemotherapy resistance of esophageal cancer. Eur Rev Med Pharmacol Sci. 2019;23(4):1513–9. https://doi.org/10.26355/eurrev_201902_17109.
Liu T, Li P, Li J, Qi Q, Sun Z, Shi S, et al. Exosomal and intracellular miR-320b promotes lymphatic metastasis in esophageal squamous cell carcinoma. Molecular Therapy - Oncolytics. 2021;23:163–80. https://doi.org/10.1016/j.omto.2021.09.003.
Goto M, Liu M. Chemokines and their receptors as biomarkers in esophageal cancer. Esophagus. 2020;17(2):113–21. https://doi.org/10.1007/s10388-019-00706-8.
Inoue S, Yoshida T, Nishino T, Goto M, Aoyama M, Kawakita N, et al. Biomarkers predicting the response to chemotherapy and the prognosis in patients with esophageal squamous cell carcinoma. Gen Thorac Cardiovasc Surg. 2021;69(3):525–33. https://doi.org/10.1007/s11748-021-01586-5.
Murugan AK, Munirajan AK, Tsuchida N. Genetic deregulation of the PIK3CA oncogene in oral cancer. Cancer Lett. 2013;338(2):193–203. https://doi.org/10.1016/j.canlet.2013.04.005.
Suzuki H, Wang L, Shan L, Zhang S, Ying J, Xue L, et al. PIK3CA gene mutations and overexpression: implications for prognostic biomarker and therapeutic target in chinese esophageal squamous cell carcinoma. PLoS ONE. 2014. https://doi.org/10.1371/journal.pone.0103021.
Zheng S, Yang C, Lu M, Liu Q, Liu T, Dai F, et al. PIK3CA promotes proliferation and motility but is unassociated with lymph node metastasis or prognosis in esophageal squamous cell carcinoma. Hum Pathol. 2016;53:121–9. https://doi.org/10.1016/j.humpath.2015.11.013.
Chen P, Zhang Z, Chen X. Overexpression of PKMYT1 facilitates tumor development and is correlated with poor prognosis in clear cell renal cell carcinoma. Med Sci Monit. 2020;26:e926755. https://doi.org/10.12659/msm.926755.
Asquith CRM, Laitinen T, East MP. PKMYT1: a forgotten member of the WEE1 family. Nat Rev Drug Discov. 2020;19(3):157. https://doi.org/10.1038/d41573-019-00202-9.
Wiel C, Le Gal K, Ibrahim MX, Jahangir CA, Kashif M, Yao H, et al. BACH1 stabilization by antioxidants stimulates lung cancer metastasis. Cell. 2019;178(2):330-345.e22. https://doi.org/10.1016/j.cell.2019.06.005.
Sohoni S, Ghosh P, Wang T, Kalainayakan SP, Vidal C, Dey S, et al. Elevated heme synthesis and uptake underpin intensified oxidative metabolism and tumorigenic functions in non-small cell lung cancer cells. Cancer Res. 2019;79(10):2511–25. https://doi.org/10.1158/0008-5472.Can-18-2156.
Zhou S, Zhang M, Zhou C, Meng Y, Yang H, Ye W. FLVCR1 predicts poor prognosis and promotes malignant phenotype in esophageal squamous cell carcinoma via upregulating CSE1L. Front Oncol. 2021;11:660955. https://doi.org/10.3389/fonc.2021.660955.
Hsu FM, Cheng JC, Chang YL, Lee JM, Koong AC, Chuang EY. Circulating mRNA Profiling in esophageal squamous cell carcinoma identifies FAM84B As A biomarker in predicting pathological response to neoadjuvant chemoradiation. Sci Rep. 2015;5:10291. https://doi.org/10.1038/srep10291.
Ghoussaini M, Song H, Koessler T, Al Olama AA, Kote-Jarai Z, Driver KE, et al. Multiple loci with different cancer specificities within the 8q24 gene desert. J Natl Cancer Inst. 2008;100(13):962–6. https://doi.org/10.1093/jnci/djn190.
Huang XP, Rong TH, Wang JY, Tang YQ, Li BJ, Xu DR, et al. Negative implication of C-MYC as an amplification target in esophageal cancer. Cancer Genet Cytogenet. 2006;165(1):20–4. https://doi.org/10.1016/j.cancergencyto.2005.07.009.
Cheng C, Cui H, Zhang L, Jia Z, Song B, Wang F, et al. Genomic analyses reveal FAM84B and the NOTCH pathway are associated with the progression of esophageal squamous cell carcinoma. Gigascience. 2016;5:1. https://doi.org/10.1186/s13742-015-0107-0.
Zheng YJ, Liang TS, Wang J, Zhao JY, Zhai SN, Yang DK, et al. MicroRNA-155 acts as a diagnostic and prognostic biomarker for oesophageal squamous cell carcinoma. Artif Cells Nanomed Biotechnol. 2020;48(1):977–82. https://doi.org/10.1080/21691401.2020.1773479.
Shiromoto Y, Sakurai M, Minakuchi M, Ariyoshi K, Nishikura K. ADAR1 RNA editing enzyme regulates R-loop formation and genome stability at telomeres in cancer cells. Nat Commun. 2021;12(1):1654. https://doi.org/10.1038/s41467-021-21921-x.
Qiao JJ, Chan TH, Qin YR, Chen L. ADAR1: a promising new biomarker for esophageal squamous cell carcinoma? Expert Rev Anticancer Ther. 2014;14(8):865–8. https://doi.org/10.1586/14737140.2014.928595.
Wang T, Lv X, Jiang S, Han S, Wang Y. Expression of ADAM29 and FAM135B in the pathological evolution from normal esophageal epithelium to esophageal cancer: their differences and clinical significance. Oncol Lett. 2020;19(3):1727–34. https://doi.org/10.3892/ol.2020.11272.
Lu YF, Yu JR, Yang Z, Zhu GX, Gao P, Wang H, et al. Promoter hypomethylation mediated upregulation of MicroRNA-10b-3p targets FOXO3 to promote the progression of esophageal squamous cell carcinoma (ESCC). J Exp Clin Cancer Res. 2018;37(1):301. https://doi.org/10.1186/s13046-018-0966-1.
Hirano H, Kato K. Systemic treatment of advanced esophageal squamous cell carcinoma: chemotherapy, molecular-targeting therapy and immunotherapy. Jpn J Clin Oncol. 2019;49(5):412–20. https://doi.org/10.1093/jjco/hyz034.
Acknowledgements
This work is supported by the Key Project of the Health Commission of Hunan Province (202201043124), the Innovation Platform Open Fund Project of the Department of Education of Hunan Province (20K110). We thank Prof. Qian Tao of the Department of Clinical Oncology, Chinese University of Hong Kong, for the comments on the manuscript.
Author information
Authors and Affiliations
Contributions
XZ and JX designed the project. SX wrote the paper. SX draw the figure of the article. GH, WZ, TF, JZ and YL, XL also read and agree to release versions of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiao, S., Huang, G., Zeng, W. et al. Roles of oncogenes in esophageal squamous cell carcinoma and their therapeutic potentials. Clin Transl Oncol 25, 578–591 (2023). https://doi.org/10.1007/s12094-022-02981-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-022-02981-x