Abstract
N6-methyladenosine (m6A) is identified as the most common, abundant and conserved internal transcriptional modification, especially within eukaryotic messenger RNAs (mRNAs). M6A modification is installed by the m6A methyltransferases (METTL3/14, WTAP, RBM15/15B and KIAA1429, termed as “writers”), reverted by the demethylases (FTO and ALKBH5, termed as “erasers”) and recognized by m6A binding proteins (YTHDF1/2/3, IGF2BP1 and HNRNPA2B1, termed as “readers”). Acumulating evidence shows that, m6A RNA methylation has an outsize effect on RNA production/metabolism and participates in the pathogenesis of multiple diseases including cancers. Until now, the molecular mechanisms underlying m6A RNA methylation in various tumors have not been comprehensively clarified. In this review, we mainly summarize the recent advances in biological function of m6A modifications in human cancer and discuss the potential therapeutic strategies.
Similar content being viewed by others
Introduction
According to MODOMICS, 163 different chemical modifications in RNA have been identified in all living organisms by the end of 2017 [1]. Among these modifications, N6-methyladenosine (m6A), methylated at the N6 position of adenosine, has been considered as the most pervasive, abundant and conserved internal transcriptional modification within eukaryotic messenger RNAs (mRNAs) [2], microRNAs (miRNAs) [3] and long non-coding RNAs (lncRNAs) [4]. RNA m6A is enriched near stop codon and 3′ untranslated terminal region (UTR) [5, 6] and translated near 5′ UTR in a cap-independent manner [7], thereby affecting RNA transcription, processing, translation and metabolism.
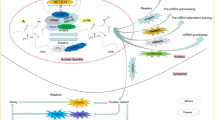
The deposition of m6A is encoded by a methyltransferase complex involving three homologous factors jargonized as ‘writers’, ‘erasers’ and ‘readers’ (Fig. 1). Methyltransferase-like 3 (METTL3) [8], METTL14 [9], Wilms tumor 1-associated protein (WTAP) [10], RBM15/15B [11] and KIAA1429 [12] are categorized as the components of ‘writers’ that catalyze the formation of m6A; ‘erasers’, fat mass and obesity-associated protein (FTO) [13] and alkB homologue 5 (ALKBH5) [14], selectively remove the methyl code from target mRNAs; ‘Readers’ are capable of decoding m6A methylation and generating a functional signal, including YT521-B homology (YTH) domain-containing protein [15], eukaryotic initiation factor (eIF) 3 [11], IGF2 mRNA binding proteins (IGF2BP) families [16] and heterogeneous nuclear ribonucleoprotein (HNRNP) protein families [17]. YTH domain can recognize m6A through a conserved aromatic cage [18] and another two proteins FMR1, LRPPRC “read” this modification [19, 20]. Contrary to the conventional ‘writer’-‘eraser’-‘reader’ paradigm, few studies reveal METTL3/16 as a m6A ‘writer’ or ‘reader’ [21].
Molecular composition of m6A RNA methylation. M6A methylation is a dynamic and reversible process coordinated by a series of methyltransferases (METTL3/14, WTAP, RBM15/15B, and KIAA1429, termed as “m6A writers”), demethylases (FTO and ALKBH5, “m6A erasers”) and identifiers (YTHDF1/2/3, YTHDC1, HNRNPA2B1, HNRNPC, eIF3, FMR1, and LRPPRC, “m6A. ‘Readers”)
M6A RNA modification is a dynamic and reversible process which was corroborated by the discovery of ‘eraser’ in 2011 [13]. It is associated with multiple diseases such as obesity, infertility and cancer [22]. In this review, we summarize the function and therapeutic advances of m6A modifications in human cancer and provide their promising applications in the treatment of these malignant tumors (Table 1).
Biological function of m6A modification in mammals
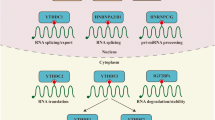
Recent years have witnessed a substantial progress of m6A post-transcriptional modification in regulating RNA transcription [23, 24], processing event [25,26,27], splicing [28,29,30,31,32,33], RNA stabilities [34,35,36,37,38,39,40] and translation [42,43,44,45,46,47,48,49] (Fig. 2).
Regulatory Functions of m6A modification in RNA splicing, processing, translation and degradation. M6A RNA modification is involved in regulating the life cycle of RNA including RNA splicing (regulated by WTAP, FTO, ALKBH5 and YTHDC1), RNA processing (regulated by METTL3/14 and ALKBH5), RNA translation (regulated by METTL3, YTHDF1/3, eIF3 and FMR1) and RNA degradation (regulated by YTHDF2)
M6A modification in RNA transcript
METTL3 and FTO are implicated in regulating transcription of CCAAT-enhancer binding protein (CEBP) family. METTL3 is localized to the starting sites of CEBPZ, which is required for recruitment of METTL3 to chromatin [23]. CEBPA is identified as an exclusive transcription factor displaying a positive correlation with FTO and regulating its transcription in acute myeloid leukemia (AML) [24].
M6A modification in RNA processing
M6A modifications promote the initiation of miRNA biogenesis [3] and regulate nuclear mRNA processing events [25]. METTL3 recognizes the pri-miRNAs by microprocessor protein DGCR8 and causes the elevation of mature miRNAs and concomitant reduction of unprocessed pri-miRNAs in breast cancer [3]. METTL14 interacts with DGCR8 to modulate pri-miR-126 and suppresses the metastatic potential of hepatocellular carcinoma (HCC) [26]. FTO can regulate poly(A) site and 3′ UTR length by interacting with METTL3 [25]. YTHDC1 knockout in oocytes exhibits massive defects and contributes to extensive alternative polyadenylation and 3′ UTR length alterations [27].
M6A modification in RNA splicing
M6A RNA modifications that overlap in space with the splicing enhancer regions affect alternative RNA splicing by acting as key pre-mRNA splicing regulators [28]. Inhibiton of m6A methyltransferase impacts gene expression and alternative splicing patterns [29]. FTO regulates nuclear mRNA alternative splicing by binding with SRSF2 [25]. FTO and ALKBH5 regulate m6A around splice sites to control the splicing of Runt-related transcription factor 1 (RUNX1T1) in exon [28], and removal of m6A by FTO reduces the recruitment of SRSF2 and prompts the skipping of exon 6, leading to a short isoform of RUNX1T1 [30]. Depletion of METTL3 is associated with RNA splicing in pancreatic cancer [31]. WTAP is enriched in some proteins involved in pre-mRNA splicing [32]. But, some studies show that, M6A is not enriched at the ends of alternatively spliced exons and METTL3 unaffects pre-mRNA splicing in embryonic stem cells [33].
M6A modification in RNA degradation
M6A is a determinant of cytoplasmic mRNA stability [34], and reduces mRNA stability [35]. A RNA decay monitoring system is adopted to investigate the effects of m6A modifications on RNA degradation [36]. Knockdown of METTL3 abolishes SOCS2 m6A modification and augments SOCS2 expression [37]. M6A-mediated SOCS2 degradation also relies on m6A ‘reader’ YTHDFs [37], which accelerate the decay of m6A-modified transcripts [38] or target mRNA [39]. Knockout of m6A methyltransferase attenuates YTHDF2 specific binding with target mRNAs and increases their stability [40]. M6A RNA methylation also controls T cell homeostasis by targeting the IL-7/STAT5/SOCS pathways [41] and decreases the stability of MYC/CEBPA transcripts [24].
M6A modification in RNA translation
M6A modifications occur in mRNA and noncoding RNA (ncRNAs) to regulate gene expression in its 5′ or 3′ UTR [7, 42]. METTL3 enhances mRNA translation [8], while depletion of METTL3 selectively inhibits mRNAs translation in 5′UTR [43] and reduces AFF4 and MYC translation in bladder cancer [44] but increase that of zinc finger protein 750 and fibroblast growth factor 14 in nasopharyngeal carcinoma [45].
M6A modifications facilitate the initiated translation through interacting with the initiation factors eIF3, CBP80 and eIF4E in an RNA-independent manner [46]. Heat-shock-induced translation of heat-shock protein 70 (HSP70) alters the transcriptome-wide distribution of m6A [7] and affects DNA repair [47]. ABCF1-sensitive transcripts largely overlaps with METTL3-modified mRNAs and are critical for m6A-regulated mRNA translation [43]. In addition, FMR1 binds to hundreds of mRNAs to negatively regulate their translation [20]. YTHDF1 facilitates the translation of m6A-modified mRNAs in protein-synthesis and YTHDF3 acts in the initial stage of m6A-driven translation from circular RNAs (circRNAs) [38, 48, 49].
M6A RNA modification in metabolic and developmental diseases
The methyltransferases and demethylases of m6A are associated with a variety of diseases, such as obesity [13, 50], type 2 diabetes mellitus (T2DM) [51], growth retardation, developmental delay, facial dysmorphism [52]. Besides, m6A modification affects infertility [14], developmental arrest [22], neuronal disorder [53] and infectious diseases [54, 55].
M6A modification in metabolic and infectious diseases
M6A modification is involved in metabolic abnormalities in patients with T2DM and obesity [56]. FTO regulates the energy homeostasis and dopaminergic pathway through FTO-dependent m6A demethylation [50, 51], and it is ubiquitous in adipose and muscle tissues, influencing RUNX1T1 splicing in adipogenesis [28, 30]. METTL3/14 reduce the abundance of Hepatitis C virus replication, but FTO promotes its production through YTHDF proteins [54]. M6A is also identified as a conserved modulatory symbol across Flaviviridae genomes, including dengue, Zika virus and West Nile virus [55].
M6A modification in infertility
Deficiency of demethylase ALKBH5 leads to the aberrant spermatogenesis and apoptosis with impaired fertility in testes and striking changes in DNA methyltransferase 1 (Dnmt1) and ubiquitin-like with PHD and RING finger domains 1 (Uhrf1) [14]. YTHDF2 is required for maternal transcriptome during oocyte maturation [57]. YTHDC1/2 determine the germline development in mouse [58], and YTHDC1 is essential for spermatogonia in males and oocyte maturation in females [27].
M6A modification in nervous system development
M6A modification regulates the pace of cerebral cortex development [59] and m6A-regulated histone modifications enhances self-renewal of neural stem cells by METTL3/14 [60]. M6A has dual effects on delaying tempo of corticogenesis by two distinct pathways: increased cell-cycle length and decreased mRNA decay [59]. M6A depletion decreases the decay of radial glia cells associated with stem cell maintenance, neurogenesis and differentiation [61].
M6A modification in inflammation and metabolism-related cancer
Cacinogenesis is characterized by stepwise accumulation of genetic/epigenetic alterations of different proto-oncogenes and tumor-suppressor genes following other diseases including chronic inflammation and metabolic diseases. METTL3/14 and FTO influence Hepatitis C virus replication and production, and endogenous mediators of inflammatory responses (proinflammatory cytokines, reactive oxygen, et al) can promote genetic/epigenetic alterations [62]. FTO affects RUNX1T1 splicing in adipogenesis [28, 30], and RUNX1T1 is essential for pancreas development [63]. Transcription factor forkhead box protein O1 (FOXO1) as another direct substrate of FTO, regulates gluconeogenesis in liver [64] and promotes the growth of pancreatic ductal adenocarcinoma [65].
M6A RNA modification in human cancer
Emerging evidence suggests that, m6A modification is associated with the tumor proliferation, differentiation, tumorigenesis [46], proliferation [66], invasion [46] and metastasis [26] and functions as oncogenes or anti-oncogenes in malignant tumors (Table 1 and Fig. 3).
Acute myeloid leukemia (AML)
FTO is highly expressed in AML with t(11q23)/MLL rearrangements, t(15;17)/PML-RARA, FLT3-ITD and/or NPM1 mutations and promotes leukemic cell transformation and tumorigenesis [67]. METTL3/14 are expressed in hematopoietic stem/progenitor cells (HSPCs) and AML cells with t(11q23), t(15;17), or t(8;21), control the terminal myeloid differentiation of HSPCs and promote the survival and proliferation of AML [68]. WTAP acts in cell proliferation and arrests the differentiation of leukemia [69].
M6A promotes the translation of c-MYC, BCL2 and PTEN in AML [70]. METTL14 acts an oncogenic role by regulating its targets MYB/MYC through m6A modification [68]. YTHDF2, responsible for the decay of m6A-modified mRNA transcripts [40], is also associated with MYC in leukemia [71]. Besides, YTHDF2 stabilizes Tal1 mRNAs and increases its expansion in AML [72].
Collectively, these studies corroborate the functional importance of m6A modifications in leukemia, such as METTL3 [23, 70], METTL14 [68], FTO [24, 67] and YTHDF2 [24, 40] and they provide profound insights into development and maintenance of AML and self-renewal of leukemia stem/initiation cells through the downstream MYC and Tal1 pathways.
Glioblastoma (GBM)
METTL3/14 inhibit GSC growth, self-renewal and tumorigenesis, but FTO and ALKBH5 indicate poor survival in GBM by regulating ADAM19 and transcription factor FOXM1 [73, 74]. LncRNA antisense to FOXM1 (FOXM1-AS) promotes the interaction of ALKBH5 with FOXM1 nascent transcripts in the tumorigenesis of GSCs [73].
Lung cancer
M6A demethylase FTO is identified as a prognostic factor in lung squamous cell carcinoma (LUSC) and facilitates cell proliferation and invasion, but inhibits cell apoptosis by regulating MZF1 expression [75]. METTL3 acts as a oncogene in lung cancer by increasing EGFR and TAZ expression and promoting cell growth, survival and invasion [46]. METTL3-eIF3 caused mRNA circularization promotes the translation and oncogenesis of lung adenocarcinoma [46]. Besides, SUMOylation of METTL3 is of importance for the promotion of tumor growth at lysine residues K177, K211, K212 and K215 in non-small cell lung carcinoma (NSCLC) [76]. These studies provide insights into the critical roles of METTL3 and FTO in lung carcinoma.
Hepatocellular carcinoma (HCC)
METTL3 is related to a poor prognosis in HCC patients and promotes HCC cell proliferation, migration and colony formation by YTHDF2-dependent posttranscriptional silencing of SOCS2 [37]. But, METTL14 is an anti-metastatic factor and serves as a favorable factor in HCC by regulating m6A-dependent miRNA processing [26]. MiR-145 down-regulates YTHDF2 through targeting its mRNA 3′ UTR [77]. In conclusion, METTL3 upregulation or METTL14 downregulation predicts poor prognosis in patients with HCC and contributes to HCC progression and metastasis [26, 37]. METTL3 suppresses SOCS2 expression in HCC via the miR-145/m6A/YTHDF2 dependent axis [37, 77]. Thus, these studies suggest a new dimension of epigenetic alteration in liver carcinogenesis.
Breast cancer and colorectal cancer (CRC)
METTL3 is associated with the expression of mammalian hepatitis B X-interacting protein (HBXIP), displaying an aggressiveness in breast cancer. HBXIP-induced METTL3 promotes the proliferation of breast cancer via inhibiting tumor suppressor let-7 g [78]. Besides, ALKBH5 decreases the levels of m6A in NANOG mRNA and enhances its stability, leading to an increase of NANOG mRNA and protein levels in breast cancer stem cells (BCSCs) [79]. Another m6A eraser ‘FTO’ polymorphism has no association with the risk of CRC [80], but the m6A ‘writer’ WTAP is associated with carbonic anhydrase IV (CA4), which inhibits the proliferation and induces apoptosis and cycle arrest by repressing the Wnt signaling through targeting the WTAP-WT1-TBL1 axis [81].
Brief summary of m6A modification-related carcinogenesis
M6A RNA modifications regulate RNA production/metabolism and take part in the carcinogenesis. On the one hand, m6A-modified genes usually act a oncogenic role in cancer, leading to alterations of mRNA translation and acceleration of tumor progression, and decreasing m6A modification results in tumor development. On the other hand, given that SUMOylation of METTL3 represses its m6A methyltransferase capacity and results in tumor growth of NSCLC, modification of m6A methylase can determine the tumor development.
M6A modification in cancer treatment
M6A modification indicates new directions for the treatment of various cancers. Regulators or inhibitors of m6A modifications may provide the potential therapeutic strategies for cancers, such as MA2 in GBM [74], R-2HG/SPI1/FB23–2 in AML [24, 68, 82] and CA4 in CRC [81]. Meclofenamic acid (MA) as one of the selective FTO inhibitors is a non-steroidal anti-inflammatory drug by competing with FTO binding sites [83]. MA2, the ethyl ester derivative of MA, increases m6A modification, leading to the suppression of tumor progression [74, 83]. The expression of ASB2 and RARA is increased in hematopoiesis and they act as key regulators of ATRA-induced differentiation of leukemia cells [84]. FTO enhances the leukemogenesis of AML by inhibition of the ASB2 and RARA expression [67]. FB23–2, as another inhibitor of m6A demethylase FTO suppresses AML cell proliferation and promotes the cell differentiation and apoptosis [82].
ALKBH5 and FTO are α-ketoglutarate (α-KG)-dependent dioxygenases [85], which are competitively inhibited by D2-hydorxyglutarate (D2-HG) and elevated in isocitrate de-hydrogenases (IDH)-mutant cancers for transferring isocitrate to α-KG [86]. R-2-hydroxyglutarate (R-2HG), an metabolite by mutating IDH1/2 enzyme, exhibits anti-leukemia effects through increasing m6A levels in R-2HG-sensitive AML [24].
S-adenosylmethionine (SAM) serves as a cofactor substrate in METTL3/14 complex and its product S-adenosylhomocysteine (SAH) inhibits the methyltransferases by competing with adenosylmethionine [87]. 3-deazaadenosine (DAA) inhibits SAH hydrolase and interrupts insertion of m6A into mRNA substrates [88] and its analogs suppress the replication of various viruses editing m6A- mRNA in cancers [89, 90].
METTL14 acts an oncogenic role by regulating MYB/MYC axis through m6A modification [68]. SPI1, a hematopoietic transcription factor, directly inhibits METTL14 expression in malignant hematopoietic cells [68] and may be a potential therapeutic target for AML. CA4 inhibits the tumorigenicity of CRC by suppressing the WTAP-WT1-TBL1 axis [81].
Future prospect
M6A RNA modifications act by regulating RNA transcript, splicing, processing, translation and decay and participate in the tumorigenesis and metastasis of multiple malignancies. However, the underlying mechanisms of m6A modifications in cancer should be further addressed.. Besides FMR1 and LRPPRC, the function of ALKBH family in m6A RNA methylation is undetermined. METTL14 has different expression levels in various tumor tissues. Given a dual role of METTL14 either as a tumor suppressor [26] or an oncogene in cancer [68], its role in other cancers need be further elucidated. Though some inhibitors of m6A methylation have shown promising effects on cancer development [68, 81], novel therapeutic strategies for m6A RNA methylation should be further explored in the treatment of cancer.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its additional files.
Abbreviations
- ALKBH5:
-
Alkb homologue 5
- AML:
-
Acute myeloid leukemia
- BCLAF1:
-
BCL2-associated transcription factor 1
- BCSCs:
-
Breast cancer stem cells
- CA4:
-
Carbonic anhydrase IV
- CEBP:
-
CCAAT-enhancer binding protein
- circRNAs:
-
Circular RNAs
- CRC:
-
Colorectal cancer
- D2-HG:
-
D2-hydorxyglutarate
- DAA:
-
3-deazaadenosine
- Dnmt1:
-
DNA methyltransferase 1
- eIF:
-
eukaryotic initiation factor
- FGF14:
-
Fibroblast growth factor 14
- FOXM1-AS:
-
Antisense to FOXM1
- FOXO1:
-
Forkhead box protein O1
- FTO:
-
Fat mass and obesity-associated protein
- GSCs:
-
Glioblastoma stem-like cells
- HBXIP:
-
Hepatitis B X-interacting protein
- HCC:
-
Hepatocellular carcinoma
- HNRNP:
-
Heterogeneous nuclear ribonucleoprotein
- HSP70:
-
Heat-shock protein 70
- HSPCs:
-
Hematopoietic stem/progenitor cells
- IDH:
-
Isocitrate de-hydrogenases
- IGF2BP:
-
IGF2 mRNA binding proteins
- lncRNAs:
-
Long non-coding RNAs
- LUSC:
-
Lung squamous cell carcinoma
- m6A:
-
N6-methyladenosine
- MA:
-
Meclofenamic acid
- METTL3:
-
Methyltransferase-like 3
- miRNAs:
-
Micro RNAs
- mRNAs:
-
Messenger RNAs
- ncRNAs:
-
Noncoding RNAs
- NSCLC:
-
Non-small cell lung carcinoma
- R-2HG:
-
R-2-hydroxyglutarate
- RUNX1T1:
-
Runt-related transcription factor 1
- SAH:
-
S-adenosylhomocysteine
- SAM:
-
S-adenosylmethionine
- SOCS2:
-
Suppressor of cytokine signaling 2
- SRSF:
-
serine/arginine-rich splicing factor
- T2DM:
-
Type 2 diabetes mellitus
- THRAP3:
-
Thyroid hormone receptor-associated protein 3
- Uhrf1:
-
Ubiquitin-like with PHD and RING finger domains 1
- UTR:
-
Untranslated terminal region
- WTAP:
-
Wilms tumour 1-associated protein
- YTHDF:
-
YT521-B homology domain-containing protein family
- ZNF750:
-
Zinc finger protein 750
- α-KG:
-
Α-ketoglutarate
References
Boccaletto P, Machnicka MA, Purta E, Piątkowski P, Bagiński B, Wirecki TK, et al. MODOMICS: a database of RNA modification pathways 2017 update. Nucleic Acids Res. 2018;46(Database issue):D303–7.
Desrosiers R, Friderici K, Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci U S A. 1974;71(10):3971–5.
Alarcón CR, Lee H, Goodarzi H, Halberg N, Tavazoie SF. N6-methyladenosine marks primary microRNAs for processing. Nature. 2015;519(7544):482–5.
Patil DP, Chen CK, Pickering BF, Chow A, Jackson C, Guttman M, et al. m6A RNA methylation promotes XIST-mediated transcriptional repression. Nature. 2016;537(7620):369–73.
Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell. 2012;149(7):1635–46.
Ke S, Alemu EA, Mertens C, Gantman EC, Fak JJ, Mele A, et al. A majority of m6A residues are in the last exons, allowing the potential for 3′ UTR regulation. Genes Dev. 2015;29(19):2037–53.
Meyer KD, Patil DP, Zhou J, Zinoviev A, Skabkin MA, Elemento O, et al. 5′ UTR m6A promotes cap-independent translation. Cell. 2015;163(4):999–1010.
Schumann U, Shafik A, Preiss T. METTL3 gains R/W access to the Epitranscriptome. Mol Cell. 2016;62(3):323–4.
Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10(2):93–5.
Ping XL, Sun BF, Wang L, Xiao W, Yang X, Wang W-J, et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24(2):177–89.
Meyer KD, Jaffrey SR. Rethinking m(6)a readers, writers, and erasers. Annu Rev Cell Dev Biol. 2017;33:319–42.
Schwartz S, Mumbach MR, Jovanovic M, Wang T, Maciag K, Bushkin GG, et al. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5′ sites. Cell Rep. 2014;8(1):284–96.
Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, et al. N6-Methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7(12):885–7.
Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang C-M, Li CJ, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49(1):18–29.
Haussmann IU, Bodi Z, Sanchez-Moran E, Mongan NP, Archer N, Fray RG, et al. m6A potentiates Sxl alternative pre-mRNA splicing for robust Drosophila sex determination. Nature. 2016;540(7632):301–4.
Müller S, Glaß M, Singh AK, Haase J, Bley N, Fuchs T, et al. IGF2BP1 promotes SRF-dependent transcription in cancer in a m6A- and miRNA-dependent manner. Nucleic Acids Res. 2019;47(1):375–90.
Zhao BS, Roundtree IA, He C. Post-transcriptional gene regulation by mRNA modifications. Nat Rev Mol Cell Biol. 2017;18(1):31–42.
Xu C, Liu K, Ahmed H, Loppnau P, Schapira M, Min J. Structural basis for the discriminative recognition of N6-Methyladenosine RNA by the human YT521-B homology domain family of proteins. J Biol Chem. 2015;290(41):24902–13.
Arguello AE, DeLiberto AN, Kleiner RE. RNA chemical proteomics reveals the N6-Methyladenosine (m6A)-regulated protein-RNA Interactome. J Am Chem Soc. 2017;139(48):17249–52.
Edupuganti RR, Geiger S, Lindeboom RGH, Shi H, Hsu PJ, Lu Z, et al. N(6)-methyladenosine (m(6)a) recruits and repels proteins to regulate mRNA homeostasis. Nat Struct Mol Biol. 2017;24(10):870–8.
Pendleton KE, Chen B, Liu K, Hunter OV, Xie Y, Tu BP, et al. The U6 snRNA m(6)a methyltransferase METTL16 regulates SAM Synthetase intron retention. Cell. 2017;169(5):824–835.e14.
Wei W, Ji X, Guo X, Ji S. Regulatory role of N6-Methyladenosine (m6A) methylation in RNA processing and human diseases. J Cell Biochem. 2017:2534–43.
Barbieri I, Tzelepis K, Pandolfini L, Shi J, Millán-Zambrano G, Robson SC, et al. Promoter-bound METTL3 maintains myeloid leukaemia by m6A-dependent translation control. Nature. 2017;552(7683):126–31.
Su R, Dong L, Li C, Nachtergaele S, Wunderlich M, Qing Y, et al. R-2HG exhibits anti-tumor activity by targeting FTO/m6A/MYC/CEBPA signaling. Cell. 2018;172(1–2):90–105.e23.
Bartosovic M, Molares HC, Gregorova P, Hrossova D, Kudla G, Vanacova S. N6-methyladenosine demethylase FTO targets pre-mRNAs and regulates alternative splicing and 3′-end processing. Nucleic Acids Res. 2017;45(19):11356–70.
Ma JZ, Yang F, Zhou CC, Liu F, Yuan J-H, Wang F, et al. METTL14 suppresses the metastatic potential of hepatocellular carcinoma by modulating N(6) -methyladenosine-dependent primary MicroRNA processing. Hepatol Baltim Md. 2017;65(2):529–43.
Kasowitz SD, Ma J, Anderson SJ, Leu NA, Xu Y, Gregory BD, et al. Nuclear m6A reader YTHDC1 regulates alternative polyadenylation and splicing during mouse oocyte development. PLoS Genet. 2018;14(5):e1007412.
Zhao X, Yang Y, Sun BF, Shi Y, Yang X, Xiao W, et al. FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res. 2014;24(12):1403–19.
Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485(7397):201–6.
Ben-Haim MS, Moshitch-Moshkovitz S, Rechavi G. FTO: linking m6A demethylation to adipogenesis. Cell Res. 2015;25(1):3–4.
Taketo K, Konno M, Asai A, Koseki J, Toratani M, Satoh T, et al. The epitranscriptome m6A writer METTL3 promotes chemo- and radioresistance in pancreatic cancer cells. Int J Oncol. 2018;52(2):621–9.
Horiuchi K, Kawamura T, Iwanari H, Ohashi R, Naito M, Kodama T, et al. Identification of Wilms’ tumor 1-associating protein complex and its role in alternative splicing and the cell cycle. J Biol Chem. 2013;288(46):33292–302.
Rosa-Mercado NA, Withers JB, Steitz JA. Settling the m(6)a debate: methylation of mature mRNA is not dynamic but accelerates turnover. Genes Dev. 2017;31(10):957–8.
Ke S, Pandya-Jones A, Saito Y, Fak JJ, Vågbø CB, Geula S, et al. m6A mRNA modifications are deposited in nascent pre-mRNA and are not required for splicing but do specify cytoplasmic turnover. Genes Dev. 2017;31(10):990–1006.
Geula S, Moshitch-Moshkovitz S, Dominissini D, Mansour AA, Kol N, Salmon-Divon M, et al. m6A mRNA methylation facilitates resolution of naïve pluripotency toward differentiation. Science. 2015;347(6225):1002–6.
Du H, Zhao Y, He J, Zhang Y, Xi H, Liu M, et al. YTHDF2 destabilizes m(6)A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat Commun. 2016;7:12626.
Chen M, Wei L, Law CT, Tsang FH-C, Shen J, Cheng CL-H, et al. RNA N6-methyladenosine methyltransferase-like 3 promotes liver cancer progression through YTHDF2-dependent posttranscriptional silencing of SOCS2. Hepatol Baltim Md. 2017;67(6):2254–70.
Shi H, Wang X, Lu Z, Zhao BS, Ma H, Hsu PJ, et al. YTHDF3 facilitates translation and decay of N(6)-methyladenosine-modified RNA. Cell Res. 2017;27(3):315–28.
Wang X, He C. Dynamic RNA modifications in posttranscriptional regulation. Mol Cell. 2014;56(1):5–12.
Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505(7481):117–20.
Li HB, Tong J, Zhu S, Batista PJ, Duffy EE, Zhao J, et al. M(6)a mRNA methylation controls T cell homeostasis by targeting the IL-7/STAT5/SOCS pathways. Nature. 2017;548(7667):338–42.
Kennedy EM, Bogerd HP, Kornepati AVR, Kang D, Ghoshal D, Marshall JB, et al. Posttranscriptional m(6)a editing of HIV-1 mRNAs enhances viral gene expression. Cell Host Microbe. 2016;19(5):675–85.
Coots RA, Liu XM, Mao Y, Dong L, Zhou J, Wan J, et al. m6A facilitates eIF4F-independent mRNA translation. Mol Cell. 2017;68(3):504–514.e7.
Cheng M, Sheng L, Gao Q, Xiong Q, Zhang H, Wu M, et al. The m6A methyltransferase METTL3 promotes bladder cancer progression via AFF4/NF-κB/MYC signaling network. Oncogene. 2019;38(19):3667–80.
Zhang P, He Q, Lei Y, Li Y, Wen X, Hong M, et al. m6A-mediated ZNF750 repression facilitates nasopharyngeal carcinoma progression. Cell Death Dis. 2018;9(12):1169.
Lin S, Choe J, Du P, Triboulet R, Gregory RI. The m6A methyltransferase METTL3 promotes translation in human Cancer cells. Mol Cell. 2016;62(3):335–45.
Xiang Y, Laurent B, Hsu CH, Nachtergaele S, Lu Z, Sheng W, et al. RNA m(6)a methylation regulates the ultraviolet-induced DNA damage response. Nature. 2017;543(7646):573–6.
Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, et al. N6-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161(6):1388–99.
Yang Y, Fan X, Mao M, Song X, Wu P, Zhang Y, et al. Extensive translation of circular RNAs driven by N(6)-methyladenosine. Cell Res. 2017;27(5):626–41.
Church C, Moir L, McMurray F, Girard C, Banks GT, Teboul L, et al. Overexpression of Fto leads to increased food intake and results in obesity. Nat Genet. 2010;42(12):1086–92.
Xiao S, Zeng X, Fan Y, Su Y, Ma Q, Zhu J, et al. Gene polymorphism association with type 2 diabetes and related Gene-gene and Gene-environment interactions in a Uyghur population. Med Sci Monit Int Med J Exp Clin Res. 2016;22:474–87.
Boissel S, Reish O, Proulx K, Kawagoe-Takaki H, Sedgwick B, Yeo GSH, et al. Loss-of-function mutation in the dioxygenase-encoding FTO gene causes severe growth retardation and multiple malformations. Am J Hum Genet. 2009;85(1):106–11.
Maity A, Das B. N6-methyladenosine modification in mRNA: machinery, function and implications for health and diseases. FEBS J. 2016;283(9):1607–30.
Gokhale NS, McIntyre ABR, McFadden MJ, Roder AE, Kennedy EM, Gandara JA, et al. N6-Methyladenosine in Flaviviridae viral RNA genomes regulates infection. Cell Host Microbe. 2016;20(5):654–65.
Lichinchi G, Zhao BS, Wu Y, Lu Z, Qin Y, He C, et al. Dynamics of human and viral RNA methylation during Zika virus infection. Cell Host Microbe. 2016;20(5):666–73.
Gallagher EJ, LeRoith D. Obesity and diabetes: the increased risk of Cancer and Cancer-related mortality. Physiol Rev. 2015;95(3):727–48.
Ivanova I, Much C, Di Giacomo M, Azzi C, Morgan M, Moreira PN, et al. The RNA m(6)a reader YTHDF2 is essential for the post-transcriptional regulation of the maternal transcriptome and oocyte competence. Mol Cell. 2017;67(6).
Hsu PJ, Zhu Y, Ma H, Guo Y, Shi X, Liu Y, et al. Ythdc2 is an N(6)-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res. 2017;27(9):1115–27.
Boles NC, Temple S. Epimetronomics: m6A Marks the tempo of Corticogenesis. Neuron. 2017;96(4):718–20.
Wang Y, Li Y, Yue M, Wang J, Kumar S, Wechsler-Reya RJ, et al. N6-methyladenosine RNA modification regulates embryonic neural stem cell self-renewal through histone modifications. Nat Neurosci. 2018;21(2):195–206.
Yoon K-J, Ringeling FR, Vissers C, Jacob F, Pokrass M, Jimenez-Cyrus D, et al. Temporal control of mammalian cortical neurogenesis by m6A methylation. Cell. 2017;171(4):877–889.e17.
Chiba T, Marusawa H, Ushijima T. Inflammation-associated cancer development in digestive organs: mechanisms and roles for genetic and epigenetic modulation. Gastroenterology. 2012;143(3):550–63.
Benitez CM, Qu K, Sugiyama T, Pauerstein PT, Liu Y, Tsai J, et al. An integrated cell purification and genomics strategy reveals multiple regulators of pancreas development. PLoS Genet. 2014;10(10):e1004645.
Peng S, Xiao W, Ju D, Sun B, Hou N, Liu Q, et al. Identification of entacapone as a chemical inhibitor of FTO mediating metabolic regulation through FOXO1. Sci Transl Med. 2019;(488):11.
Song W, Li Q, Wang L, Wang L. Modulation of FoxO1 expression by miR-21 to promote growth of pancreatic ductal adenocarcinoma. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2015;35(1):184–90.
Liu J, Eckert MA, Harada BT, Liu S-M, Lu Z, Yu K, et al. m6A mRNA methylation regulates AKT activity to promote the proliferation and tumorigenicity of endometrial cancer. Nat Cell Biol. 2018;20(9):1074–83.
Li Z, Weng H, Su R, Weng X, Zuo Z, Li C, et al. FTO plays an oncogenic role in acute myeloid leukemia as a N6-Methyladenosine RNA demethylase. Cancer Cell. 2017;31(1):127–41.
Weng H, Huang H, Wu H, Qin X, Zhao BS, Dong L, et al. METTL14 inhibits hematopoietic stem/progenitor differentiation and promotes Leukemogenesis via mRNA m6A modification. Cell Stem Cell. 2017;22(2):191–205 e9.
Bansal H, Yihua Q, Iyer S, Ganapathy S, Proia D, Penalva L, et al. WTAP is a novel oncogenic protein in acute myeloid leukemia. Leukemia. 2014;28(5):1171–4.
Vu LP, Pickering BF, Cheng Y, Zaccara S, Nguyen D, Minuesa G, et al. The N(6)-methyladenosine (m(6)a)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat Med. 2017;23(11):1369–76.
Wang H, Zuo H, Liu J, Wen F, Gao Y, Zhu X, et al. Loss of YTHDF2-mediated m6A-dependent mRNA clearance facilitates hematopoietic stem cell regeneration. Cell Res. 2018;28(10):1035–8.
Li Z, Qian P, Shao W, Shi H, He XC, Gogol M, et al. Suppression of m6A reader Ythdf2 promotes hematopoietic stem cell expansion. Cell Res. 2018;28(9):904–17.
Zhang S, Zhao BS, Zhou A, Lin K, Zheng S, Lu Z, et al. M(6)a demethylase ALKBH5 maintains Tumorigenicity of glioblastoma stem-like cells by sustaining FOXM1 expression and cell proliferation program. Cancer Cell. 2017;31(4):591–606.e6.
Cui Q, Shi H, Ye P, Li L, Qu Q, Sun G, et al. M(6)a RNA methylation regulates the self-renewal and tumorigenesis of glioblastoma stem cells. Cell Rep. 2017;18(11):2622–34.
Liu J, Ren D, Du Z, Wang H, Zhang H, Jin Y. m6A demethylase FTO facilitates tumor progression in lung squamous cell carcinoma by regulating MZF1 expression. Biochem Biophys Res Commun. 2018;502(4):456–64.
Du Y, Hou G, Zhang H, Dou J, He J, Guo Y, et al. SUMOylation of the m6A-RNA methyltransferase METTL3 modulates its function. Nucleic Acids Res. 2018;46(10):5195–208.
Yang Z, Li J, Feng G, Gao S, Wang Y, Zhang S, et al. MicroRNA-145 modulates N(6)-Methyladenosine levels by targeting the 3′-untranslated mRNA region of the N(6)-Methyladenosine binding YTH domain family 2 protein. J Biol Chem. 2017;292(9):3614–23.
Cai X, Wang X, Cao C, Gao Y, Zhang S, Yang Z, et al. HBXIP-elevated methyltransferase METTL3 promotes the progression of breast cancer via inhibiting tumor suppressor let-7g. Cancer Lett. 2018;415:11–9.
Zhang C, Samanta D, Lu H, Bullen JW, Zhang H, Chen I, et al. Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m6A-demethylation of NANOG mRNA. Proc Natl Acad Sci. 2016;113(14):E2047–56.
Yang B, Thrift AP, Figueiredo JC, Jenkins MA, Schumacher FR, Conti DV, et al. Common variants in the obesity-associated genes FTO and MC4R are not associated with risk of colorectal cancer. Cancer Epidemiol. 2016;44:1–4.
Zhang J, Tsoi H, Li X, Wang H, Gao J, Wang K, et al. Carbonic anhydrase IV inhibits colon cancer development by inhibiting the Wnt signalling pathway through targeting the WTAP–WT1–TBL1 axis. Gut. 2016;65(9):1482–93.
Huang Y, Su R, Sheng Y, Dong L, Dong Z, Xu H, et al. Small-molecule targeting of oncogenic FTO demethylase in acute myeloid leukemia. Cancer Cell. 2019;35(4):677–91 e10.
Huang Y, Yan J, Li Q, Li J, Gong S, Zhou H, et al. Meclofenamic acid selectively inhibits FTO demethylation of m6A over ALKBH5. Nucleic Acids Res. 2015;43(1):373–84.
Wang S, Sun C, Li J, Zhang E, Ma Z, Xu W, et al. Roles of RNA methylation by means of N(6)-methyladenosine (m(6)a) in human cancers. Cancer Lett. 2017;408:112–20.
Fedeles BI, Singh V, Delaney JC, Li D, Essigmann JM. The AlkB family of Fe(II)/α-ketoglutarate-dependent dioxygenases: repairing nucleic acid alkylation damage and beyond. J Biol Chem. 2015;290(34):20734–42.
Lin AP, Abbas S, Kim S-W, Ortega M, Bouamar H, Escobedo Y, et al. D2HGDH regulates alpha-ketoglutarate levels and dioxygenase function by modulating IDH2. Nat Commun. 2015;6:7768.
Kloor D, Osswald H. S-Adenosylhomocysteine hydrolase as a target for intracellular adenosine action. Trends Pharmacol Sci. 2004;25(6):294–7.
Bader JP, Brown NR, Chiang PK, Cantoni GL. 3-Deazaadenosine, an inhibitor of adenosylhomocysteine hydrolase, inhibits reproduction of Rous sarcoma virus and transformation of chick embryo cells. Virology. 1978;89(2):494–505.
Mayers DL, Mikovits JA, Joshi B, Hewlett IK, Estrada JS, Wolfe AD, et al. Anti-human immunodeficiency virus 1 (HIV-1) activities of 3-deazaadenosine analogs: increased potency against 3′-azido-3′-deoxythymidine-resistant HIV-1 strains. Proc Natl Acad Sci U S A. 1995;92(1):215–9.
Gordon RK, Ginalski K, Rudnicki WR, Rychlewski L, Pankaskie MC, Bujnicki JM, et al. Anti-HIV-1 activity of 3-deaza-adenosine analogs inhibition of S-adenosylhomocysteine hydrolase and nucleotide congeners. Eur J Biochem. 2003;270(17):3507–17.
Acknowledgements
Not applicable.
Funding
Our work was supported by the grants from National Natural Science Foundation of China (No. 81873143; 81573747), and Shanghai Science and Technology Commission Western Medicine Guide project (No. 17411966500).
Author information
Authors and Affiliations
Contributions
JZ and JSZ designed this study and XYC drafted the manuscript. JZ revised this manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All of the authors are aware of and agree to the content of the paper and their being listed as a co-author of the paper.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Chen, XY., Zhang, J. & Zhu, JS. The role of m6A RNA methylation in human cancer. Mol Cancer 18, 103 (2019). https://doi.org/10.1186/s12943-019-1033-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12943-019-1033-z