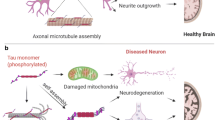
Abstract
Tau protein plays a pivotal role in the central nervous system (CNS), participating in microtubule stability, axonal transport, and synaptic communication. Research interest has focused on studying the role of post-translational tau modifications in mitochondrial failure, oxidative damage, and synaptic impairment in Alzheimer’s disease (AD). Soluble tau forms produced by its pathological cleaved induced by caspases could lead to neuronal injury contributing to oxidative damage and cognitive decline in AD. For example, the presence of tau cleaved by caspase-3 has been suggested as a relevant factor in AD and is considered a previous event before neurofibrillary tangles (NFTs) formation.
Interestingly, we and others have shown that caspase-cleaved tau in N- or C- terminal sites induce mitochondrial bioenergetics defects, axonal transport impairment, neuronal injury, and cognitive decline in neuronal cells and murine models. All these abnormalities are considered relevant in the early neurodegenerative manifestations such as memory and cognitive failure reported in AD. Therefore, in this review, we will discuss for the first time the importance of truncated tau by caspases activation in the pathogenesis of AD and how its negative actions could impact neuronal function.


Similar content being viewed by others
Data Availability
N/A.
Abbreviations
- AA :
-
Amino acids
- AD :
-
Alzheimer’s disease
- AMPA :
-
α-Amino-3-hydroxy-5-methyl-4-isoxazole propionic acid
- Apaf-1 :
-
Apoptosis protease-activating factor-1 adapter molecule
- Asp421 :
-
Asparginine 421
- AT8 :
-
Marker of tau hyperphosphorylation in Ser202/Thr305 sites
- ATP :
-
Adenosine triphosphate
- CA :
-
Cornus ammonis
- CAMKII :
-
Ca2+/calmodulin-dependent protein kinase II
- Cdk2 :
-
Cyclin-dependent kinase 2
- Cdk5 :
-
Cyclin-dependent kinase 5
- CNS :
-
Central nervous system
- CsA :
-
Cyclosporine A
- DNA :
-
Deoxyribonucleic acid
- DRP1 :
-
Dynamin-related protein 1
- fEPSPs :
-
Field excitatory post-synaptic potentials
- GSK-3β :
-
Glycogen synthase kinase-3 beta
- HEK :
-
Human embryonic kidney cells
- IMM :
-
Inner mitochondrial membrane
- LDH :
-
Lactate dehydrogenase
- LTP :
-
Long-term potentiation
- LTD :
-
Long terminal depression
- MAP :
-
Microtubule-associated protein
- MAPK :
-
Mitogen-activated protein kinase
- MCI :
-
Mild cognitive impairment
- MCU :
-
Mitochondrial calcium uniporter
- mEPSCs :
-
Miniature excitatory post-synaptic currents
- MFN :
-
Mitofusins
- mPTP :
-
Mitochondrial permeability transition pore
- N2a :
-
Neuroblastoma 2a
- NCI :
-
Non-cognitively impaired
- NFTs :
-
Neurofibrillary tangles
- NMDA :
-
N-Methyl-D-aspartate
- NPS :
-
Neurites plaques
- NPTs :
-
Neuropil threads
- NR1 :
-
N-Methyl-D-aspartic receptor 1
- NR2B :
-
N-Methyl-D-aspartic receptor 2
- Nrf2 :
-
Nuclear factor (erythroid-derived 2)-like 2
- OMM :
-
Outer mitochondrial membrane
- Opa1 :
-
Optic atrophy protein 1
- OXPHOS :
-
Oxidative phosphorylation
- PIPES :
-
Piperazine-N,N′-bis(2-ethane sulfonic acid)
- PKA :
-
Protein kinase A
- PKC :
-
Protein kinase C
- PSD95 :
-
Post-synaptic density protein 95
- ROS :
-
Reactive oxygen species
- SNAP-25 :
-
Synaptosome-associated protein
- TRAK :
-
Trafficking kinesin-binding
- zVAD-fmk :
-
Carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone
- 8oxodG :
-
8-Oxo-2′-deoxyguanosine
- ΔΨm :
-
Mitochondrial membrane potential
References
Kadavath H, Hofele RV, Biernat J et al (2015) Tau stabilizes microtubules by binding at the interface between tubulin heterodimers. Proc National Acad Sci 112:7501–7506. https://doi.org/10.1073/pnas.1504081112
Mietelska-Porowska A, Wasik U, Goras M et al (2014) Tau protein modifications and interactions: their role in function and dysfunction. Int J Mol Sci 15:4671–4713. https://doi.org/10.3390/ijms15034671
Yen S, Liu W-K, Hall FL et al (1995) Alzheimer neurofibrillary lesions: molecular nature and potential roles of different components. Neurobiol Aging 16:381–387. https://doi.org/10.1016/0197-4580(95)00022-7
Drummond E, Pires G, MacMurray C et al (2020) Phosphorylated tau interactome in the human Alzheimer’s disease brain. Brain 143:awaa223. https://doi.org/10.1093/brain/awaa223
Wang J-Z, Wang Z-H, Tian Q (2014) Tau hyperphosphorylation induces apoptotic escape and triggers neurodegeneration in Alzheimer’s disease. Neurosci Bull 30:359–366. https://doi.org/10.1007/s12264-013-1415-y
Braak H, Braak E, Grundke-Iqbal I, Iqbal K (1986) Occurrence of neuropil threads in the senile human brain and in Alzheimer’s disease: a third location of paired helical filaments outside of neurofibrillary tangles and neuritic plaques. Neurosci Lett 65:351–355. https://doi.org/10.1016/0304-3940(86)90288-0
de Calignon A, Fox LM, Pitstick R et al (2010) Caspase activation precedes and leads to tangles. Nature 464:1201–1204. https://doi.org/10.1038/nature08890
Kanno T, Tsuchiya A, Nishizaki T (2014) Hyperphosphorylation of tau at Ser396 occurs in the much earlier stage than appearance of learning and memory disorders in 5XFAD mice. Behav Brain Res 274:302–306. https://doi.org/10.1016/j.bbr.2014.08.034
del Alonso AC, Zaidi T, Novak M et al (2001) Hyperphosphorylation induces self-assembly of τ into tangles of paired helical filaments/straight filaments. Proc National Acad Sci 98:6923–6928. https://doi.org/10.1073/pnas.121119298
Bolós M, Pallas-Bazarra N, Terreros-Roncal J et al (2017) Soluble tau has devastating effects on the structural plasticity of hippocampal granule neurons. Transl Psychiat 7:1267. https://doi.org/10.1038/s41398-017-0013-6
Gamblin TC, Chen F, Zambrano A et al (2003) Caspase cleavage of tau: Linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc National Acad Sci 100:10032–10037. https://doi.org/10.1073/pnas.1630428100
Rohn TT, Rissman RA, Davis MC et al (2002) Caspase-9 Activation and caspase cleavage of tau in the Alzheimer’s disease brain. Neurobiol Dis 11:341–354. https://doi.org/10.1006/nbdi.2002.0549
Horowitz PM, Patterson KR, Guillozet-Bongaarts AL et al (2004) Early N-terminal changes and caspase-6 cleavage of tau in Alzheimer’s Disease. J Neurosci 24:7895–7902. https://doi.org/10.1523/jneurosci.1988-04.2004
Ozcelik S, Sprenger F, Skachokova Z et al (2016) Co-expression of truncated and full-length tau induces severe neurotoxicity. pdf. Nature. https://doi.org/10.1038/mp.2015.228
Fox LM, William CM, Adamowicz DH et al (2011) Soluble tau species, not neurofibrillary aggregates, disrupt neural system integration in a tau transgenic model. pdf. OXFORD Academic. https://doi.org/10.1097/nen.0b013e318220a658
Sokolow S, Henkins KM, Bilousova T et al (2015) Pre-synaptic C-terminal truncated tau is released from cortical synapses in Alzheimer’s disease. J Neurochem 133:368–379. https://doi.org/10.1111/jnc.12991
Liu P, Smith BR, Montonye ML et al (2020) A soluble truncated tau species related to cognitive dysfunction is elevated in the brain of cognitively impaired human individuals. Sci Rep-uk 10:3869. https://doi.org/10.1038/s41598-020-60777-x
Biundo F, d’Abramo C, Tambini MD et al (2008) Abolishing tau cleavage by caspases at Aspartate421 causes memory:synaptic plasticity deficits and pre-pathological tau alterations. pdf. Trans Psych Nat. https://doi.org/10.1038/tp.2017.165
Kang HJ, Yoon WJ, Moon GJ et al (2005) Caspase-3-mediated cleavage of PHF-1 tau during apoptosis irrespective of excitotoxicity and oxidative stress: an implication to Alzheimer’s disease. Neurobiol Dis 18:450–458. https://doi.org/10.1016/j.nbd.2004.12.004
Matthews-Roberson TA, Quintanilla RA, Ding H, Johnson GVW (2008) Immortalized cortical neurons expressing caspase-cleaved tau are sensitized to endoplasmic reticulum stress induced cell death. Brain Res 1234:206–212. https://doi.org/10.1016/j.brainres.2008.07.111
Quintanilla RA, Matthews-Roberson TA, Dolan PJ, Johnson GVW (2009) Caspase-cleaved tau expression induces mitochondrial dysfunction in immortalized cortical neurons: implications for the pathogenesis of Alzheimer’disease. J Biol Chem 284:18754–18766. https://doi.org/10.1074/jbc.m808908200
Quntanilla RA, Tapia-Monsalves C (2020) The role of mitochondrial impairment in Alzheimer’s disease neurodegeneration: the tau connection. Curr Neuropharmacol 18:1076–1091. https://doi.org/10.2174/1570159x18666200525020259
Pérez MJ, Jara C, Quintanilla RA (2018) Contribution of tau pathology to mitochondrial impairment in neurodegeneration. Front Neurosci-switz 12:441. https://doi.org/10.3389/fnins.2018.00441
Javadov S, Kozlov AV, Camara AKS (2020) Mitochondria in health and diseases. pdf. MDPI. https://doi.org/10.3390/cells9051177
Morio B, Panthu B, Bassot A, Rieusset J (2021) Role of mitochondria in liver metabolic health and diseases. Cell Calcium 94:102336. https://doi.org/10.1016/j.ceca.2020.102336
Nguyen BY, Ruiz-Velasco A, Bui T et al (2019) Mitochondrial function in the heart: the insight into mechanisms and therapeutic potentials. Brit J Pharmacol 176:4302–4318. https://doi.org/10.1111/bph.14431
de Caldeira DAF, Weiss DJ, Rocco PRM et al (2021) Mitochondria in focus: from function to therapeutic strategies in chronic lung diseases. Front Immunol 12:782074. https://doi.org/10.3389/fimmu.2021.782074
Martin LJ (2010) Mitochondrial and cell death mechanisms in neurodegenerative diseases. Pharm 3:839–915. https://doi.org/10.3390/ph3040839
Pérez MJ, Vergara-Pulgar K, Jara C et al (2018) Caspase-cleaved tau impairs mitochondrial dynamics in Alzheimer’s disease. Mol Neurobiol 55:1004–1018. https://doi.org/10.1007/s12035-017-0385-x
Quintanilla RA, Tapia-Monsalves C, Vergara EH et al (2020) Truncated tau induces mitochondrial transport failure through the impairment of TRAK2 protein and bioenergetics decline in neuronal cells. Front Cell Neurosci 14:175. https://doi.org/10.3389/fncel.2020.00175
Briel N, Pratsch K, Roeber S et al (2021) Contribution of the astrocytic tau pathology to synapse loss in progressive supranuclear palsy and corticobasal degeneration. Brain Pathol 31:e12914. https://doi.org/10.1111/bpa.12914
Binder LI, Frankfurter A, Rebhun LI (1985) The distribution of tau in the mammalian central nervous system pdf. J Cell Biol. https://doi.org/10.1083/jcb.101.4.1371
Hanger DP, Goniotaki D, Noble W (2019) synaptic localization of tau. Adv Exp Med Biol. https://doi.org/10.1007/978-981-32-9358-8_9
Tapia-Rojas C, Cabezas-Opazo F, Deaton CA et al (2018) It’s all about tau. Prog Neurobiol 175:54–76. https://doi.org/10.1016/j.pneurobio.2018.12.005
Takemura R, Kanai Y, Hirokawa N (1991) In situ localization of tau mRNA in developing rat brain. Neuroscience 44:393–407. https://doi.org/10.1016/0306-4522(91)90064-u
Neve RL, Harris P, Kosik KS et al (1986) Identification of cDNA clones for the human microtubule-associated protein tau and chromosomal localization of the genes for tau and microtubule-associated protein 2. Mol Brain Res 1:271–280. https://doi.org/10.1016/0169-328x(86)90033-1
Götz J, Halliday G, Nisbet RM (2019) Molecular pathogenesis of the tauopathies pdf. Ann Rev. https://doi.org/10.1146/annurev-pathmechdis-012418-012936
Johnson GVW, Stoothoff WH (2004) Tau phosphorylation in neuronal cell function and dysfunction. J Cell Sci 117:5721–5729. https://doi.org/10.1242/jcs.01558
Billingsley ML, Kincaid RL (1997) Regulated phosphorylation and dephosphorylation of tau protein: effects on microtubule interaction, intracellular trafficking and neurodegeneration. Biochem J 323:577–591. https://doi.org/10.1042/bj3230577
Goedert M, Jakes R (1990) Expression of separate isoforms of human tau protein: correlation with the tau pattern in brain and effects on tubulin polymerization. Embo J 9:4225–4230. https://doi.org/10.1002/j.1460-2075.1990.tb07870.x
Chen Q, Zhou Z, Zhang L et al (2012) Tau protein is involved in morphological plasticity in hippocampal neurons in response to BDNF. Neurochem Int 60:233–242. https://doi.org/10.1016/j.neuint.2011.12.013
Mandelkow E-M, Stamer K, Vogel R et al (2003) Clogging of axons by tau, inhibition of axonal traffic and starvation of synapses. Neurobiol Aging 24:1079–1085. https://doi.org/10.1016/j.neurobiolaging.2003.04.007
Kimura T, Whitcomb DJ, Jo J et al (2014) Microtubule-associated protein tau is essential for long-term depression in the hippocampus. Philosophical Trans Royal Soc B Biol Sci 369:20130144. https://doi.org/10.1098/rstb.2013.0144
Briner A, Götz J, Polanco JC (2020) Fyn kinase controls tau aggregation in vivo. Cell Reports 32:108045. https://doi.org/10.1016/j.celrep.2020.108045
Mondragón-Rodríguez S, Trillaud-Doppia E, Dudilot A et al (2012) Interaction of endogenous tau protein with synaptic proteins is regulated by N-methyl-d-aspartate receptor-dependent tau phosphorylation*. J Biol Chem 287:32040–32053. https://doi.org/10.1074/jbc.m112.401240
Jara C, Aránguiz A, Cerpa W et al (2018) Genetic ablation of tau improves mitochondrial function and cognitive abilities in the hippocampus. Redox Biol 18:279–294. https://doi.org/10.1016/j.redox.2018.07.010
Means JC, Gerdes BC, Kaja S et al (2016) Caspase-3-dependent proteolytic cleavage of tau causes neurofibrillary tangles and results in cognitive impairment during normal aging. Neurochem Res 41:2278–2288. https://doi.org/10.1007/s11064-016-1942-9
Jarero-Basulto JJ, Luna-Muñoz J, Mena R et al (2013) Proteolytic cleavage of polymeric tau protein by caspase-3: implications for Alzheimer disease. J Neuropathol Exp Neurol 72:1145–1161. https://doi.org/10.1097/nen.0000000000000013
Zhang Q, Zhang X, Sun A (2009) Truncated tau at D421 is associated with neurodegeneration and tangle formation in the brain of Alzheimer transgenic models. Acta Neuropathol 117:687–697. https://doi.org/10.1007/s00401-009-0491-6
Guillozet-Bongaarts AL, Garcia-Sierra F, Reynolds MR et al (2005) Tau truncation during neurofibrillary tangle evolution in Alzheimer’s disease. Neurobiol Aging 26:1015–1022. https://doi.org/10.1016/j.neurobiolaging.2004.09.019
Mroczko B, Groblewska M, Litman-Zawadzka A (2019) The role of protein misfolding and tau oligomers (TauOs) in Alzheimer’s disease (AD). Int J Mol Sci 20:4661. https://doi.org/10.3390/ijms20194661
He H, Liu Y, Sun Y, Ding F (2021) Misfolding and self-assembly dynamics of microtubule-binding repeats of the Alzheimer-related protein tau. J Chem Inf Model 61:2916–2925. https://doi.org/10.1021/acs.jcim.1c00217
Kovacech B, Novak M (2010) Tau truncation is a productive posttranslational modification of neurofibrillary degeneration in Alzheimer’s disease.pdf. Curr Alzheimer Res 7:708–716. https://doi.org/10.2174/156720510793611556
Kopeikina KJ, Hyman BT, Spires-Jones TL (2013) Soluble forms of tau are toxic in Alzheimer’s disease pdf. Trans Neurosci 3:223–233. https://doi.org/10.2478/s13380-012-0032-y
Barghorn S, Mandelkow E (2002) Toward a unified scheme for the aggregation of tau into Alzheimer paired helical filaments †. Biochemistry-us 41:14885–14896. https://doi.org/10.1021/bi026469j
Sahara N, Maeda S, Murayama M et al (2007) Assembly of two distinct dimers and higher-order oligomers from full-length tau. Eur J Neurosci 25:3020–3029. https://doi.org/10.1111/j.1460-9568.2007.05555.x
Patterson KR, Remmers C, Fu Y et al (2011) Characterization of prefibrillar tau oligomers in vitro and in Alzheimer disease*. J Biol Chem 286:23063–23076. https://doi.org/10.1074/jbc.m111.237974
Schneider A, Biernat J, von Bergen M et al (1999) Phosphorylation that detaches tau protein from microtubules (Ser262, Ser214) also protects it against aggregation into Alzheimer paired helical filaments †. Biochemistry-us 38:3549–3558. https://doi.org/10.1021/bi981874p
Zilka N, Filipcik P, Koson P et al (2006) Truncated tau from sporadic Alzheimer’s disease suffices to drive neurofibrillary degeneration in vivo. Febs Lett 580:3582–3588. https://doi.org/10.1016/j.febslet.2006.05.029
Morsch R, Simon W, Coleman PD (1999) Neurons may live for decades with neurofibrillary tangles.pdf. Journal neuropathology Experimental Neurology 58:188–197. https://doi.org/10.1097/00005072-199902000-00008
SantaCruz K, Lewis J, Spires T et al (2005) Tau suppression in a neurodegenerative mouse model improves memory function. J Frankl Inst 309:476–481. https://doi.org/10.1126/science.1113694
Eldadah BA, Faden AI (2000) Caspase pathways, neuronal apoptosis, and CNS injury. J Neurotraum 17:811–829. https://doi.org/10.1089/neu.2000.17.811
Fernández DJ, Lamkanfi M (2015) Inflammatory caspases: key regulators of inflammation and cell death. Biol Chem 396:193–203. https://doi.org/10.1515/hsz-2014-0253
Cotman CW, Poon WW, Rissman RA, Blurton-Jones M (2001) The role of caspase cleavage of tau in Alzheimer disease neuropathology. pdf. J Neuropathol Exp Neurol. https://doi.org/10.1093/jnen/64.2.104
Dhage PA, Sharbidre AA, Magdum SM (2023) Interlacing the relevance of caspase activation in the onset and progression of Alzheimer’s disease. Brain Res Bull 192:83–92. https://doi.org/10.1016/j.brainresbull.2022.11.008
Thornberry NA (1997) The caspase family of cysteine proteases. Brit Med Bull 53:478–490. https://doi.org/10.1093/oxfordjournals.bmb.a011625
McIlwain DR, Berger T, Mak TW (2013) Caspase functions in cell death and disease. Csh Perspect Biol 5:a008656. https://doi.org/10.1101/cshperspect.a008656
Troy CM, Akpan N, Jean YY (2011) Chapter 7 regulation of caspases in the nervous system implications for functions in health and disease. Prog Mol Biol Transl 99:265–305. https://doi.org/10.1016/b978-0-12-385504-6.00007-5
Tait SWG, Green DR (2013) Mitochondrial regulation of cell death. Csh Perspect Biol 5:a008706. https://doi.org/10.1101/cshperspect.a008706
Yeh W-C, Itie A, Elia AJ et al (2000) Requirement for Casper (c-FLIP) in regulation of death receptor–induced apoptosis and embryonic development. Immunity 12:633–642. https://doi.org/10.1016/s1074-7613(00)80214-9
Annunziato L, Amoroso S, Pannaccione A et al (2003) Apoptosis induced in neuronal cells by oxidative stress: role played by caspases and intracellular calcium ions. Toxicol Lett 139:125–133. https://doi.org/10.1016/s0378-4274(02)00427-7
Keramaris E, Stefanis L, MacLaurin J et al (2000) Involvement of caspase 3 in apoptotic death of cortical neurons evoked by DNA damage. Mol Cell Neurosci 15:368–379. https://doi.org/10.1006/mcne.2000.0838
Doyle KM, Kennedy D, Gorman AM et al (2011) Unfolded proteins and endoplasmic reticulum stress in neurodegenerative disorders. J Cell Mol Med 15:2025–2039. https://doi.org/10.1111/j.1582-4934.2011.01374.x
Ho FY, Tsang WP, Kong SK, Kwok TT (2006) The critical role of caspases activation in hypoxia/reoxygenation induced apoptosis. Biochem Bioph Res Co 345:1131–1137. https://doi.org/10.1016/j.bbrc.2006.04.178
Jiang X, Wang X (2000) Cytochrome c promotes caspase-9 activation by inducing nucleotide binding to Apaf-1*. J Biol Chem 275:31199–31203. https://doi.org/10.1074/jbc.c000405200
Lamkanfi M, Kanneganti T-D (2010) Caspase-7: a protease involved in apoptosis and inflammation. Int J Biochem Cell Biology 42:21–24. https://doi.org/10.1016/j.biocel.2009.09.013
Oakley DH, Klickstein N, Commins C et al (2021) Continuous monitoring of tau-induced neurotoxicity in patient-derived iPSC-neurons. J Neurosci 41:4335–4348. https://doi.org/10.1523/jneurosci.2590-20.2021
D’Amelio M, Cavallucci V, Cecconi F (2010) Neuronal caspase-3 signaling: not only cell death. Cell Death Differ 17:1104–1114. https://doi.org/10.1038/cdd.2009.180
Wang J-Y, Luo Z-G (2014) Non-apoptotic role of caspase-3 in synapse refinement. Neurosci Bull 30:667–670. https://doi.org/10.1007/s12264-014-1454-4
Li Z, Sheng M (2012) Caspases in synaptic plasticity. Mol. Brain 5:15. https://doi.org/10.1186/1756-6606-5-15
Villavicencio-Tejo F, Olesen MA, Aránguiz A, Quintanilla RA (2022) Activation of the Nrf2 pathway prevents mitochondrial dysfunction induced by caspase-3 cleaved tau: implications for Alzheimer’s disease. Antioxidants 11:515. https://doi.org/10.3390/antiox11030515
Zheng J, Akbari M, Schirmer C et al (2020) Hippocampal tau oligomerization early in tau pathology coincides with a transient alteration of mitochondrial homeostasis and DNA repair in a mouse model of tauopathy. Acta Neuropathologica Commun 8:25. https://doi.org/10.1186/s40478-020-00896-8
Bravarenko NI, Onufriev MV, Stepanichev MY et al (2006) Caspase-like activity is essential for long-term synaptic plasticity in the terrestrial snail Helix. Eur J Neurosci 23:129–140. https://doi.org/10.1111/j.1460-9568.2005.04549.x
Huesmann GR, Clayton DF (2006) Dynamic role of postsynaptic caspase-3 and BIRC4 in zebra finch song-response habituation. Neuron 52:1061–1072. https://doi.org/10.1016/j.neuron.2006.10.033
Zhang A, Lorke DE, Wu S-X, Yew DT (2006) Caspase-3 immunoreactivity in different cortical areas of young and aging macaque (Macaca mulatta) monkeys. Neurosignals 15:64–73. https://doi.org/10.1159/000094602
Seaman JE, Julien O, Lee PS et al (2016) Cacidases: caspases can cleave after aspartate, glutamate and phosphoserine residues. Cell Death Differ 23:1717–1726. https://doi.org/10.1038/cdd.2016.62
Zhao Y, Tseng I-C, Heyser CJ et al (2015) Appoptosin-mediated caspase cleavage of tau contributes to progressive supranuclear palsy pathogenesis. Neuron 87:963–975. https://doi.org/10.1016/j.neuron.2015.08.020
Friedrich MG, Skora A, Hancock SE et al (2021) Tau is truncated in five regions of the normal adult human brain. Int J Mol Sci 22:3521. https://doi.org/10.3390/ijms22073521
Gu J, Xu W, Jin N et al (2020) Truncation of Tau selectively facilitates its pathological activities. J Biol Chem 295:13812–13828. https://doi.org/10.1074/jbc.ra120.012587
Nunez WA, Combs B, Gamblin TC, Ackley BD (2022) Age-dependent accumulation of tau aggregation in Caenorhabditis elegans. Frontiers Aging 3:928574. https://doi.org/10.3389/fragi.2022.928574
García-Sierra F, Mondragón-Rodríguez S, Basurto-Islas G (2008) Truncation of tau protein and its pathological significance in Alzheimer’s disease. J Alzheimers Dis 14:401–409. https://doi.org/10.3233/jad-2008-14407
Fasulo L, Ugolini G, Visintin M et al (2000) The neuronal microtubule-associated protein tau is a substrate for caspase-3 and an effector of apoptosis. J Neurochem 75:624–633. https://doi.org/10.1046/j.1471-4159.2000.0750624.x
Chung C-W, Song Y-H, Kim I-K et al (2001) Proapoptotic effects of tau cleavage product generated by caspase-3. Neurobiol Dis 8:162–172. https://doi.org/10.1006/nbdi.2000.0335
Yogev S, Cooper R, Fetter R et al (2016) Microtubule organization determines axonal transport dynamics. Neuron 92:449–460. https://doi.org/10.1016/j.neuron.2016.09.036
Venkatramani A, Panda D (2019) Regulation of neuronal microtubule dynamics by tau: implications for tauopathies. Int J Biol Macromol 133:473–483. https://doi.org/10.1016/j.ijbiomac.2019.04.120
Ding H, Matthews TA, Johnson GVW (2006) Site-specific phosphorylation and caspase cleavage differentially impact tau-microtubule interactions and tau aggregation*. J Biol Chem 281:19107–19114. https://doi.org/10.1074/jbc.m511697200
Kandel ER, Dudai Y, Mayford MR (2014) The molecular and systems biology of memory. Cell 157:163–186. https://doi.org/10.1016/j.cell.2014.03.001
Di J, Cohen LS, Corbo CP et al (2016) Abnormal tau induces cognitive impairment through two different mechanisms: synaptic dysfunction and neuronal loss. Sci Rep-uk 6:20833. https://doi.org/10.1038/srep20833
Usenovic M, Niroomand S, Drolet RE et al (2015) Internalized tau oligomers cause neurodegeneration by inducing accumulation of pathogenic tau in human neurons derived from induced pluripotent stem cells. J Neurosci 35:14234–14250. https://doi.org/10.1523/jneurosci.1523-15.2015
Cowan CM, Quraishe S, Hands S et al (2015) Rescue from tau-induced neuronal dysfunction produces insoluble tau oligomers. Sci Rep-uk 5:17191. https://doi.org/10.1038/srep17191
Hoover BR, Reed MN, Su J et al (2010) Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron 68:1067–1081. https://doi.org/10.1016/j.neuron.2010.11.030
Zhou L, McInnes J, Wierda K et al (2017) Tau association with synaptic vesicles causes presynaptic dysfunction. Nat Commun 8:15295. https://doi.org/10.1038/ncomms15295
Teravskis PJ, Ashe KH, Liao D (2020) The accumulation of tau in postsynaptic structures: a common feature in multiple neurodegenerative diseases? Neurosci 26:503–520. https://doi.org/10.1177/1073858420916696
Jadhav S, Katina S, Kovac A et al (2015) Truncated tau deregulates synaptic markers in rat model for human tauopathy. Front Cell Neurosci 9:24. https://doi.org/10.3389/fncel.2015.00024
Loon A, Zamudio F, Sanneh A et al (2022) Accumulation of C-terminal cleaved tau is distinctly associated with cognitive deficits, synaptic plasticity impairment, and neurodegeneration in aged mice. Geroscience 44:173–194. https://doi.org/10.1007/s11357-021-00408-z
Kim Y, Choi H, Lee W et al (2016) Caspase-cleaved tau exhibits rapid memory impairment associated with tau oligomers in a transgenic mouse model. Neurobiol Dis 87:19–28. https://doi.org/10.1016/j.nbd.2015.12.006
Tapia-Monsalves C, Olesen MA, Villavicencio-Tejo F, Quintanilla RA (2023) Cyclosporine A (CsA) prevents synaptic impairment caused by truncated tau by caspase-3. Mol Cell Neurosci 125:103861. https://doi.org/10.1016/j.mcn.2023.103861
LeBlanc A, Liu H, Goodyer C et al (1999) Caspase-6 role in apoptosis of human neurons, amyloidogenesis, and Alzheimer’s disease*. J Biol Chem 274:23426–23436. https://doi.org/10.1074/jbc.274.33.23426
Louneva N, Cohen JW, Han L-Y et al (2008) Caspase-3 is enriched in postsynaptic densities and increased in Alzheimer’s disease. Am J Pathology 173:1488–1495. https://doi.org/10.2353/ajpath.2008.080434
Kudryashov IE, Yakovlev AA, Kudryashova IV, Gulyaeva NV (2004) Inhibition of caspase-3 blocks long-term potentiation in hippocampal slices. Neurosci Behav Physiol 34:877–880. https://doi.org/10.1023/b:neab.0000042571.86110.28
Conze C, Rierola M, Trushina NI et al (2022) Caspase-cleaved tau is senescence-associated and induces a toxic gain of function by putting a brake on axonal transport. Mol Psychiatr 27:3010–3023. https://doi.org/10.1038/s41380-022-01538-2
Noël A, Foveau B, LeBlanc AC (2021) Caspase-6-cleaved tau fails to induce tau hyperphosphorylation and aggregation, neurodegeneration, glial inflammation, and cognitive deficits. Cell Death Dis 12:227. https://doi.org/10.1038/s41419-021-03506-0
Guo H, Albrecht S, Bourdeau M et al (2004) Active caspase-6 and caspase-6-cleaved tau in neuropil threads, neuritic plaques, and neurofibrillary tangles of Alzheimer’s disease.pdf. Am J Pathol. 165:523–531. https://doi.org/10.1016/s0002-9440(10)63317-2
Theofilas P, Ambrose AJ, Butler D, Wang C, Morales DO, Petersen C, Chin B, Yang et al (2021) Caspase inhibition mitigates tau cleavage and neurotoxicity in iPSC-induced neurons with the V337M MAPT mutation. Alzheimer's Dement 17: e051471. https://doi.org/10.1002/alz.051471
Ramcharitar J, Afonso VM, Albrecht S et al (2013) Caspase-6 activity predicts lower episodic memory ability in aged individuals. Neurobiol Aging 34:1815–1824. https://doi.org/10.1016/j.neurobiolaging.2013.01.007
Ramcharitar J, Albrecht S, Afonso VM et al (2013) Cerebrospinal fluid tau cleaved by caspase-6 reflects brain levels and cognition in aging and Alzheimer disease.pdf. J Neuropathol Exp Neurol 72:824–832. https://doi.org/10.1097/nen.0b013e3182a0a39f
Guo T, Noble W, Hanger DP (2017) Roles of tau protein in health and disease. Acta Neuropathol 133:665–704. https://doi.org/10.1007/s00401-017-1707-9
Zhao X, Kotilinek LA, Smith B et al (2016) Caspase-2 cleavage of tau reversibly impairs memory. Nat Med 22:1268–1276. https://doi.org/10.1038/nm.4199
Tamayev R, Akpan N, Arancio O et al (2012) Caspase-9 mediates synaptic plasticity and memory deficits of Danish dementia knock-in mice: caspase-9 inhibition provides therapeutic protection. Mol Neurodegener 7:60. https://doi.org/10.1186/1750-1326-7-60
Bekris LM, Yu C-E, Bird TD, Tsuang DW (2010) Review Article: Genetics of Alzheimer disease. J Geriatr Psych Neur 23:213–227. https://doi.org/10.1177/0891988710383571
DeKosky ST, Scheff SW (1990) Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol 27:457–464. https://doi.org/10.1002/ana.410270502
Tönnies E, Trushina E (2017) Oxidative stress, synaptic dysfunction, and Alzheimer’s disease.pdf. Journal of Alzheimer’s Disease 57:1105–1121. https://doi.org/10.3233/jad-161088
Gomez-Nicola D, Boche D (2015) Post-mortem analysis of neuroinflammatory changes in human Alzheimer’s disease. Alzheimer’s Res Ther 7:42. https://doi.org/10.1186/s13195-015-0126-1
Medeiros R, Baglietto-Vargas D, LaFerla FM (2011) The role of tau in Alzheimer’s disease and related disorders. Cns Neurosci Ther 17:514–524. https://doi.org/10.1111/j.1755-5949.2010.00177.x
Matthews K (2006) Tau protein abnormalities correlate with the severity of dementia in Alzheimer’s disease. Nat Clin Pract Neuro 2:178–178. https://doi.org/10.1038/ncpneuro0139
Moloney CM, Lowe VJ, Murray ME (2021) Visualization of neurofibrillary tangle maturity in Alzheimer’s disease: a clinicopathologic perspective for biomarker research. Alzheimer’s Dementia 17:1554–1574. https://doi.org/10.1002/alz.12321
Brion J-P (2006) Immunological demonstration of tau protein in neurofibrillary tangles of Alzheimer’s disease. J Alzheimer’s Dis 9:177–185. https://doi.org/10.3233/jad-2006-9s321
Polydoro M, Dzhala VI, Pooler AM et al (2014) Soluble pathological tau in the entorhinal cortex leads to presynaptic deficits in an early Alzheimer’s disease model. Acta Neuropathol 127:257–270. https://doi.org/10.1007/s00401-013-1215-5
Rissman RA, Poon WW, Blurton-Jones M et al (2004) Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J Clin Invest 114:121–130. https://doi.org/10.1172/jci20640
Basurto-Islas G, Luna-Muñoz J, Guillozet-Bongaarts AL et al (2008) Accumulation of aspartic acid421- and glutamic acid391-cleaved tau in neurofibrillary tangles correlates with progression in Alzheimer disease. J Neuropathol Exp Neurol 67:470–483. https://doi.org/10.1097/nen.0b013e31817275c7
Albrecht S, Bourdeau M, Bennett D et al (2007) Activation of caspase-6 in aging and mild cognitive impairment. Am J Pathol 170:1200–1209. https://doi.org/10.2353/ajpath.2007.060974
Klaiman G, Petzke TL, Hammond J, LeBlanc AC (2008) Targets of caspase-6 activity in human neurons and Alzheimer disease. Mol Cell Proteomics 7:1541–1555. https://doi.org/10.1074/mcp.m800007-mcp200
Theofilas P, Piergies AMH, Oh I et al (2022) Caspase-6-cleaved tau is relevant in Alzheimer’s disease and marginal in four-repeat tauopathies: diagnostic and therapeutic implications. Neuropath Appl Neuro 48:e12819. https://doi.org/10.1111/nan.12819
LeBlanc AC, Ramcharitar J, Afonso V et al (2014) Caspase-6 activity in the CA1 region of the hippocampus induces age-dependent memory impairment. Cell Death Differ 21:696–706. https://doi.org/10.1038/cdd.2013.194
Shimohama S, Tanino H, Fujimoto S (1999) Changes in caspase expression in Alzheimer’s disease: comparison with development and aging. Biochem Bioph Res Co 256:381–384. https://doi.org/10.1006/bbrc.1999.0344
Hlynialuk C, Kemper L, Leinonen-Wright K et al (2022) Caspase-2 mRNA levels are not elevated in mild cognitive impairment, Alzheimer’s disease, Huntington’s disease, or Lewy Body dementia.pdf. Plos One 17:e0274784. https://doi.org/10.1371/journal.pone.0274784
Sheng Z-H (2014) Mitochondrial trafficking and anchoring in neurons: new insight and implications. J Cell Biol 204:1087–1098. https://doi.org/10.1083/jcb.201312123
Wang L, Liu M, Gao J et al (2021) Mitochondrial fusion suppresses tau pathology-induced neurodegeneration and cognitive decline. J Alzheimer’s Dis 84:1057–1069. https://doi.org/10.3233/jad-215175
Chang DTW, Honick AS, Reynolds IJ (2006) Mitochondrial trafficking to synapses in cultured primary cortical neurons. J Neurosci Official J Soc Neurosci 26:7035–7045. https://doi.org/10.1523/jneurosci.1012-06.2006
Attwell D, Laughlin SB (2001) An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metabolism 21:1133–1145. https://doi.org/10.1097/00004647-200110000-00001
Harris JJ, Jolivet R, Attwell D (2012) Synaptic energy use and supply. Neuron 75:762–777. https://doi.org/10.1016/j.neuron.2012.08.019
Kühlbrandt W (2015) Structure and function of mitochondrial membrane protein complexes. Bmc Biol 13:89. https://doi.org/10.1186/s12915-015-0201-x
van der Bliek AM, Shen Q, Kawajiri S (2013) Mechanisms of mitochondrial fission and fusion. Cold Spring Harb Perspect Biol 5:a011072. https://doi.org/10.1101/cshperspect.a011072
Castora FJ, Kerns KA, Pflanzer HK et al (2022) Expression changes in mitochondrial genes affecting mitochondrial morphology, transmembrane potential, fragmentation, amyloidosis, and neuronal cell death found in brains of Alzheimer’s disease patients. J Alzheimer’s Dis 90:119–137. https://doi.org/10.3233/jad-220161
Wang X, Su B, Lee HG et al (2009) Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J Neurosci 29:9090–9103. https://doi.org/10.1523/jneurosci.1357-09.2009
Manczak M, Calkins MJ, Reddy PH (2011) Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer’s disease: implications for neuronal damage. Hum Mol Genet 20:2495–2509. https://doi.org/10.1093/hmg/ddr139
Calkins MJ, Manczak M, Mao P et al (2011) Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer’s disease. Hum Mol Genet 20:4515–4529. https://doi.org/10.1093/hmg/ddr381
Sena LA, Chandel NS (2012) Physiological roles of mitochondrial reactive oxygen species. Mol Cell 48:158–167. https://doi.org/10.1016/j.molcel.2012.09.025
Yin Z, Burger N, Kula-Alwar D et al (2021) Structural basis for a complex I mutation that blocks pathological ROS production. Nat Commun 12:707. https://doi.org/10.1038/s41467-021-20942-w
Muller FL, Liu Y, Remmen HV (2004) Complex III releases superoxide to both sides of the inner mitochondrial membrane*. J Biol Chem 279:49064–49073. https://doi.org/10.1074/jbc.m407715200
Marchi ED, Bonora M, Giorgi C, Pinton P (2014) The mitochondrial permeability transition pore is a dispensable element for mitochondrial calcium efflux. Cell Calcium 56:1–13. https://doi.org/10.1016/j.ceca.2014.03.004
Stefani DD, Patron M, Rizzuto R (2015) Structure and function of the mitochondrial calcium uniporter complex. Biochimica Et Biophysica Acta Bba - Mol Cell Res 1853:2006–2011. https://doi.org/10.1016/j.bbamcr.2015.04.008
Luongo TS, Lambert JP, Gross P et al (2017) The mitochondrial Na+/Ca2+ exchanger is essential for Ca2+ homeostasis and viability. Nature 545:93–97. https://doi.org/10.1038/nature22082
Natarajan GK, Glait L, Mishra J et al (2020) Total matrix Ca2+ modulates Ca2+ efflux via the Ca2+/H+ exchanger in cardiac mitochondria. Front Physiol 11:510600. https://doi.org/10.3389/fphys.2020.510600
Vos M, Lauwers E, Verstreken P (2010) Synaptic mitochondria in synaptic transmission and organization of vesicle pools in health and disease. Front Synaptic Neurosci 2:139. https://doi.org/10.3389/fnsyn.2010.00139
Sanganahalli BG, Herman P, Hyder F, Kannurpatti SS (2013) Mitochondrial calcium uptake capacity modulates neocortical excitability. J Cereb Blood Flow Metabolism 33:1115–1126. https://doi.org/10.1038/jcbfm.2013.61
Reiss AB, Ahmed S, Dayaramani C et al (2022) The role of mitochondrial dysfunction in Alzheimer’s disease: a potential pathway to treatment. Exp Gerontol 164:111828. https://doi.org/10.1016/j.exger.2022.111828
Sharma C, Kim S, Nam Y et al (2021) Mitochondrial dysfunction as a driver of cognitive impairment in Alzheimer’s disease. Int J Mol Sci 22:4850. https://doi.org/10.3390/ijms22094850
Hirai K, Aliev G, Nunomura A et al (2001) Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci 21:3017–3023. https://doi.org/10.1523/jneurosci.21-09-03017.2001
Yao J, Irwin RW, Zhao L et al (2009) Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proc National Acad Sci 106:14670–14675. https://doi.org/10.1073/pnas.0903563106
Blass JP, Sheu RK, Gibson GE (2000) Inherent abnormalities in energy metabolism in Alzheimer disease: interaction with cerebrovascular compromise. Ann Ny Acad Sci 903:204–221. https://doi.org/10.1111/j.1749-6632.2000.tb06370.x
Ishii K, Sasaki M, Kitagaki H et al (1997) Reduction of cerebellar glucose metabolism in advanced Alzheimer’s disease. J Nucl Med Off Publ Soc Nucl Med 38:925–928
David DC, Hauptmann S, Scherping I et al (2005) Proteomic and functional analyses reveal a mitochondrial dysfunction in P301L tau transgenic mice*. J Biol Chem 280:23802–23814. https://doi.org/10.1074/jbc.m500356200
Brooks WM, Lynch PJ, Ingle CC et al (2007) Gene expression profiles of metabolic enzyme transcripts in Alzheimer’s disease. Brain Res 1127:127–135. https://doi.org/10.1016/j.brainres.2006.09.106
Xie H, Guan J, Borrelli LA et al (2013) Mitochondrial alterations near amyloid plaques in an Alzheimer’s disease mouse model. J Neurosci 33:17042–17051. https://doi.org/10.1523/jneurosci.1836-13.2013
Dragicevic N, Mamcarz M, Zhu Y et al (2010) Mitochondrial amyloid-β levels are associated with the extent of mitochondrial dysfunction in different brain regions and the degree of cognitive impairment in Alzheimer’s transgenic mice. J Alzheimer’s Dis 20:S535–S550. https://doi.org/10.3233/jad-2010-100342
Manczak M, Park BS, Jung Y, Reddy PH (2004) Differential expression of oxidative phosphorylation genes in patients with Alzheimer’s disease: implications for early mitochondrial dysfunction and oxidative damage. Neuromol Med 5:147–162. https://doi.org/10.1385/nmm:5:2:147
Jadiya P, Kolmetzky DW, Tomar D et al (2019) Impaired mitochondrial calcium efflux contributes to disease progression in models of Alzheimer’s disease. Nat Commun 10:3885. https://doi.org/10.1038/s41467-019-11813-6
Mattson MP, Barger SW, Cheng B et al (1993) β-Amyloid precursor protein metabolites and loss of neuronal Ca2+ homeostasis in Alzheimer’s disease. Trends Neurosci 16:409–414. https://doi.org/10.1016/0166-2236(93)90009-b
Baumgartner HK, Gerasimenko JV, Thorne C et al (2009) Calcium elevation in mitochondria is the main Ca2+ requirement for mitochondrial permeability transition pore (mPTP) opening*. J Biol Chem 284:20796–20803. https://doi.org/10.1074/jbc.m109.025353
Schinder AF, Olson EC, Spitzer NC, Montal M (1996) Mitochondrial dysfunction is a primary event in glutamate neurotoxicity. J Neurosci 16:6125–6133. https://doi.org/10.1523/jneurosci.16-19-06125.1996
Moreira PI, Santos MS, Moreno A et al (2002) Effect of amyloid ?-peptide on permeability transition pore: a comparative study. J Neurosci Res 69:257–267. https://doi.org/10.1002/jnr.10282
Du H, Guo L, Fang F et al (2008) Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat Med 14:1097–1105. https://doi.org/10.1038/nm.1868
Jara C, Cerpa W, Tapia-Rojas C, Quintanilla RA (2021) Tau deletion prevents cognitive impairment and mitochondrial dysfunction age associated by a mechanism dependent on cyclophilin-D. Front Neurosci-switz 14:586710. https://doi.org/10.3389/fnins.2020.586710
Lopes S, Teplytska L, Vaz-Silva J et al (2016) Tau deletion prevents stress-induced dendritic atrophy in prefrontal cortex: role of synaptic mitochondria. Cereb Cortex 27:2580–2591. https://doi.org/10.1093/cercor/bhw057
Bertholet AM, Delerue T, Millet AM et al (2016) Mitochondrial fusion:fission dynamics in neurodegeneration and neuronal plasticity.pdf. Neurobiol Dis 90:3–19. https://doi.org/10.1016/j.nbd.2015.10.011
de la Cueva M, Antequera D, Ordoñez-Gutierrez L et al (2022) Amyloid-β impairs mitochondrial dynamics and autophagy in Alzheimer’s disease experimental models. Sci Rep-uk 12:10092. https://doi.org/10.1038/s41598-022-13683-3
Szabo L, Eckert A, Grimm A (2020) Insights into disease-associated tau impact on mitochondria. Int J Mol Sci 21:6344. https://doi.org/10.3390/ijms21176344
Kandimalla R, Manczak M, Pradeepkiran JA et al (2021) A partial reduction of Drp1 improves cognitive behavior and enhances mitophagy, autophagy and dendritic spines in a transgenic Tau mouse model of Alzheimer disease. Hum Mol Genet 31:1788–1805. https://doi.org/10.1093/hmg/ddab360
Bevan RJ, Williams PA, Waters CT et al (2020) OPA1 deficiency accelerates hippocampal synaptic remodelling and age-related deficits in learning and memory. Brain Commun 2:fcaa101. https://doi.org/10.1093/braincomms/fcaa101
Quintanilla RA, Dolan PJ, Jin YN, Johnson GVW (2012) Truncated tau and Aβ cooperatively impair mitochondria in primary neurons. Neurobiol Aging 33:619.e25-619.e35. https://doi.org/10.1016/j.neurobiolaging.2011.02.007
Pathak D, Shields LY, Mendelsohn BA et al (2015) The role of mitochondrially derived ATP in synaptic vesicle recycling* ♦. J Biol Chem 290:22325–22336. https://doi.org/10.1074/jbc.m115.656405
Marland JRK, Hasel P, Bonnycastle K, Cousin MA (2016) Mitochondrial calcium uptake modulates synaptic vesicle endocytosis in central nerve terminals*. J Biol Chem 291:2080–2086. https://doi.org/10.1074/jbc.m115.686956
Li Z, Okamoto K-I, Hayashi Y, Sheng M (2004) The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell 119:873–887. https://doi.org/10.1016/j.cell.2004.11.003
Cheng A, Hou Y, Mattson MP (2010) Mitochondria and neuroplasticity. Asn Neuro 2:e00045. https://doi.org/10.1042/an20100019
Pilling AD, Horiuchi D, Lively CM, Saxton WM (2006) Kinesin-1 and dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol Biol Cell 17:2057–2068. https://doi.org/10.1091/mbc.e05-06-0526
Fenton AR, Jongens TA, Holzbaur ELF (2021) Mitochondrial adaptor TRAK2 activates and functionally links opposing kinesin and dynein motors. Nat Commun 12:4578. https://doi.org/10.1038/s41467-021-24862-7
Cai Q, Davis ML, Sheng Z-H (2011) Regulation of axonal mitochondrial transport and its impact on synaptic transmission. Neurosci Res 70:9–15. https://doi.org/10.1016/j.neures.2011.02.005
Fransson Å, Ruusala A, Aspenström P (2003) Atypical Rho GTPases have roles in mitochondrial homeostasis and apoptosis*. J Biol Chem 278:6495–6502. https://doi.org/10.1074/jbc.m208609200
Shahpasand K, Uemura I, Saito T et al (2012) Regulation of mitochondrial transport and inter-microtubule spacing by tau phosphorylation at the sites hyperphosphorylated in Alzheimer’s disease. J Neurosci Off J Soc Neurosci 32:2430–2441. https://doi.org/10.1523/jneurosci.5927-11.2012
Kopeikina KJ, Carlson GA, Pitstick R et al (2011) Tau accumulation causes mitochondrial distribution deficits in neurons in a mouse model of tauopathy and in human Alzheimer’s disease brain. Am J Pathol 179:2071–2082. https://doi.org/10.1016/j.ajpath.2011.07.004
Ebneth A, Godemann R, Stamer K et al (1998) Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: implications for Alzheimer’s disease. J Cell Biol 143:777–794. https://doi.org/10.1083/jcb.143.3.777
Quintanilla RA, von Bernhardi R, Godoy JA et al (2014) Phosphorylated tau potentiates Aβ-induced mitochondrial damage in mature neurons. Neurobiol Dis 71:260–269. https://doi.org/10.1016/j.nbd.2014.08.016
van Spronsen M, Mikhaylova M, Lipka J et al (2013) TRAK/Milton motor-adaptor proteins steer mitochondrial trafficking to axons and dendrites. Neuron 77:485–502. https://doi.org/10.1016/j.neuron.2012.11.027
Jash K, Gondaliya P, Kirave P et al (2020) Cognitive dysfunction: a growing link between diabetes and Alzheimer’s disease. Drug Dev Res 81:144–164. https://doi.org/10.1002/ddr.21579
Dolan C, Glynn R, Griffin S et al (2018) Brain complications of diabetes mellitus: a cross-sectional study of awareness among individuals with diabetes and the general population in Ireland. Diabet Med 35:871–879. https://doi.org/10.1111/dme.13639
Gaspar JM, Baptista FI, Macedo MP, Ambrósio AF (2016) Inside the Diabetic Brain: Role of Different Players Involved in Cognitive Decline. Acs Chem Neurosci 7:131–142. https://doi.org/10.1021/acschemneuro.5b00240
Jayaraj RL, Azimullah S, Beiram R (2020) Diabetes as a risk factor for Alzheimer’s disease in the Middle East and its shared pathological mediators. Saudi J Biol Sci 27:736–750. https://doi.org/10.1016/j.sjbs.2019.12.028
Macauley SL, Stanley M, Caesar EE et al (2015) Hyperglycemia modulates extracellular amyloid-β concentrations and neuronal activity in vivo. J Clin Invest 125:2463–2467. https://doi.org/10.1172/jci79742
Silzer TK, Phillips NR (2018) Etiology of type 2 diabetes and Alzheimer’s disease: exploring the mitochondria. Mitochondrion 43:16–24. https://doi.org/10.1016/j.mito.2018.04.004
Hobday AL, Parmar MS (2021) The link between diabetes mellitus and tau hyperphosphorylation: implications for risk of Alzheimer’s disease. Cureus 13:e18362. https://doi.org/10.7759/cureus.18362
Clodfelder-Miller BJ, Zmijewska AA, Johnson GVW, Jope RS (2006) Tau is hyperphosphorylated at multiple sites in mouse brain in vivo after streptozotocin-induced insulin deficiency. Diabetes 55:3320–3325. https://doi.org/10.2337/db06-0485
Reagan LP, Magariños AM, Yee DK et al (2000) Oxidative stress and HNE conjugation of GLUT3 are increased in the hippocampus of diabetic rats subjected to stress. Brain Res 862:292–300. https://doi.org/10.1016/s0006-8993(00)02212-5
Moreira PI, Santos MS, Seiça R, Oliveira CR (2007) Brain mitochondrial dysfunction as a link between Alzheimer’s disease and diabetes. J Neurol Sci 257:206–214. https://doi.org/10.1016/j.jns.2007.01.017
Kim B, Backus C, Oh S, Feldman EL (2013) Hyperglycemia-induced tau cleavage in vitro and in vivo: a possible link between diabetes and Alzheimer’s disease. J Alzheimer’s Dis 34:727–739. https://doi.org/10.3233/jad-121669
Kim B, Backus C, Oh S et al (2009) Increased tau phosphorylation and cleavage in mouse models of type 1 and type 2 diabetes. Endocrinology 150:5294–5301. https://doi.org/10.1210/en.2009-0695
Latina V, Giacovazzo G, Calissano P et al (2021) Tau cleavage contributes to cognitive dysfunction in strepto-zotocin-induced sporadic Alzheimer’s disease (sAD) mouse model. Int J Mol Sci 22:12158. https://doi.org/10.3390/ijms222212158
Funding
This work was supported by ANID, GRANT FONDECYT # 1200178, Santiago, Chile. MAO thanked Universidad Autónoma de Chile (PhD fellowship).
Author information
Authors and Affiliations
Contributions
MAO made the figures and conceived, wrote, and edited manuscript; RAQ conceived, edited, and financed this research.
Corresponding author
Ethics declarations
Ethics Approval
N/A.
Consent to Participate
N/A
Consent for Publication
N/A
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Olesen, M.A., Quintanilla, R.A. Pathological Impact of Tau Proteolytical Process on Neuronal and Mitochondrial Function: a Crucial Role in Alzheimer’s Disease. Mol Neurobiol 60, 5691–5707 (2023). https://doi.org/10.1007/s12035-023-03434-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03434-4