Abstract
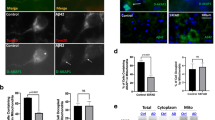
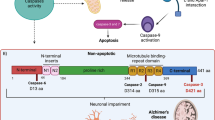
Alzheimer’s disease (AD) is characterized by the presence of aggregates of tau protein. Tau truncated by caspase-3 (D421) or tau hyperphosphorylated at Ser396/S404 might play a role in the pathogenesis of AD. Mitochondria are dynamic organelles that modify their size and function through mitochondrial dynamics. Recent studies have shown that alterations of mitochondrial dynamics affect synaptic communication. Therefore, we studied the effects of pathological forms of tau on the regulation of mitochondrial dynamics. We used primary cortical neurons from tau(−/−) knockout mice and immortalized cortical neurons (CN1.4) that were transfected with plasmids containing green fluorescent protein (GFP) or GFP with different tau forms: full-length (GFP-T4), truncated (GFP-T4C3), pseudophosphorylated (GFP-T42EC), or both truncated and pseudophosphorylated modifications of tau (GFP-T4C3-2EC). Cells expressing truncated tau showed fragmented mitochondria compared to cells that expressed full-length tau. These findings were corroborated using primary neurons from tau(−/−) knockout mice that expressed the truncated and both truncated and pseudophosphorylated forms of tau. Interestingly, mitochondrial fragmentation was accompanied by a significant reduction in levels of optic atrophy protein 1 (Opa1) in cells expressing the truncated form of tau. In addition, treatment with low concentrations of amyloid-beta (Aβ) significantly reduced mitochondrial membrane potential, cell viability, and mitochondrial length in cortical cells and primary neurons from tau(−/−) mice that express truncated tau. These results indicate that the presence of tau pathology impairs mitochondrial dynamics by reducing Opa1 levels, an event that could lead to mitochondrial impairment observed in AD.








Similar content being viewed by others
References
Querfurth HW, LaFerla FM (2010) Alzheimer’s disease. N Engl J Med 362(4):329–344. doi:10.1056/NEJMra0909142
Rissman RA, Poon WW, Blurton-Jones M, Oddo S, Torp R, Vitek MP, LaFerla FM, Rohn TT et al (2004) Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J Clin Invest 114(1):121–130. doi:10.1172/JCI20640
Cho JH, Johnson GV (2004) Glycogen synthase kinase 3 beta induces caspase-cleaved tau aggregation in situ. J Biol Chem 279(52):54716–54723. doi:10.1074/jbc.M403364200
Lee S, Shea TB (2012) Caspase-mediated truncation of tau potentiates aggregation. Int J Alzheimers Dis 2012:731063. doi:10.1155/2012/731063
Stokin GB, Lillo C, Falzone TL, Brusch RG, Rockenstein E, Mount SL, Raman R, Davies P et al (2005) Axonopathy and transport deficits early in the pathogenesis of Alzheimer’s disease. Science 307(5713):1282–1288. doi:10.1126/science.1105681
Cowan CM, Bossing T, Page A, Shepherd D, Mudher A (2010) Soluble hyper-phosphorylated tau causes microtubule breakdown and functionally compromises normal tau in vivo. Acta Neuropathol 120(5):593–604. doi:10.1007/s00401-010-0716-8
Plouffe V, Mohamed NV, Rivest-McGraw J, Bertrand J, Lauzon M, Leclerc N (2012) Hyperphosphorylation and cleavage at D421 enhance tau secretion. PLoS One 7(5):e36873. doi:10.1371/journal.pone.0036873
Zhang Y, Chen L, Shen G, Zhao Q, Shangguan L, He M (2014) GRK5 dysfunction accelerates tau hyperphosphorylation in APP (swe) mice through impaired cholinergic activity. Neuroreport 25(7):542–547. doi:10.1097/WNR.0000000000000142
Dixit R, Ross JL, Goldman YE, Holzbaur EL (2008) Differential regulation of dynein and kinesin motor proteins by tau. Science 319(5866):1086–1089. doi:10.1126/science.1152993
Dolan PJ, Johnson GV (2010) The role of tau kinases in Alzheimer’s disease. Curr Opin Drug Discov Dev 13(5):595–603
Quintanilla RA, Dolan PJ, Jin YN, Johnson GV (2012) Truncated tau and Abeta cooperatively impair mitochondria in primary neurons. Neurobiol Aging 33(3):e625–e635. doi:10.1016/j.neurobiolaging.2011.02.007 619
Zhang Q, Zhang X, Sun A (2009) Truncated tau at D421 is associated with neurodegeneration and tangle formation in the brain of Alzheimer transgenic models. Acta Neuropathol 117(6):687–697. doi:10.1007/s00401-009-0491-6
Quintanilla RA, Matthews-Roberson TA, Dolan PJ, Johnson GV (2009) Caspase-cleaved tau expression induces mitochondrial dysfunction in immortalized cortical neurons: implications for the pathogenesis of Alzheimer disease. J Biol Chem 284(28):18754–18766. doi:10.1074/jbc.M808908200
Kandimalla R, Reddy PH (2016) Multiple faces of dynamin-related protein 1 and its role in Alzheimer’s disease pathogenesis. Biochim Biophys Acta 1862(4):814–828. doi:10.1016/j.bbadis.2015.12.018
Reddy PH, Reddy TP, Manczak M, Calkins MJ, Shirendeb U, Mao P (2011) Dynamin-related protein 1 and mitochondrial fragmentation in neurodegenerative diseases. Brain Res Rev 67(1–2):103–118. doi:10.1016/j.brainresrev.2010.11.004
R Kandimalla, Manczak M, Fry D, Suneetha Y, Sesaki H, Reddy PH (2016) Reduced dynamin-related protein 1 protects against phosphorylated tau-induced mitochondrial dysfunction and synaptic damage in Alzheimer’s disease. Human Molecular Genetics
Hauptmann S, Scherping DS, Brandt U, Schulz KL, Jendrach M, Leuner K, Eckert A, Müller WE (2009) Mitochondrial dysfunction: an early event in Alzheimer pathology accumulates with age in AD transgenic mice. Neurobiol Aging 30(10):1574–1586. doi:10.1016/j.neurobiolaging.2007.12.005
Rhein V, Song X, Wiesner A, Ittner LM, Baysang G, Meier F, Ozmen L, Bluethmann H et al (2009) Amyloid-beta and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer’s disease mice. Proc Natl Acad Sci U S A 106(47):20057–20062. doi:10.1073/pnas.0905529106
Trushina E, Nemutlu E, Zhang S, Christensen T, Camp J, Mesa J, Siddiqui A, Tamura Y et al (2012) Defects in mitochondrial dynamics and metabolomic signatures of evolving energetic stress in mouse models of familial Alzheimer’s disease. PLoS One 7(2):e32737. doi:10.1371/journal.pone.0032737
Cabezas-Opazo FA, Vergara-Pulgar K, Perez MJ, Jara C, Osorio-Fuentealba C, Quintanilla RA (2015) Mitochondrial dysfunction contributes to the pathogenesis of Alzheimer’s disease. Oxidative Med Cell Longev 2015:509654. doi:10.1155/2015/509654
Quintanilla RA, von Bernhardi R, Godoy JA, Inestrosa NC, Johnson GV (2014) Phosphorylated tau potentiates Abeta-induced mitochondrial damage in mature neurons. Neurobiol Dis 71:260–269. doi:10.1016/j.nbd.2014.08.016
Takuma H, Tomiyama T, Kuida K, Mori H (2004) Amyloid beta peptide-induced cerebral neuronal loss is mediated by caspase-3 in vivo. J Neuropathol Exp Neurol 63(3):255–261
David DC, Hauptmann S, Scherping I, Schuessel K, Keil U, Rizzu P, Ravid R, Drose S et al (2005) Proteomic and functional analyses reveal a mitochondrial dysfunction in P301L tau transgenic mice. J Biol Chem 280(25):23802–23814. doi:10.1074/jbc.M500356200
Lasagna-Reeves CA, Glabe CG, Kayed R (2011) Amyloid-beta annular protofibrils evade fibrillar fate in Alzheimer disease brain. J Biol Chem 286(25):22122–22130. doi:10.1074/jbc.M111.236257
Ding H, Matthews TA, Johnson GV (2006) Site-specific phosphorylation and caspase cleavage differentially impact tau-microtubule interactions and tau aggregation. J Biol Chem 281(28):19107–19114. doi:10.1074/jbc.M511697200
Matthews-Roberson TA, Quintanilla RA, Ding H, Johnson GV (2008) Immortalized cortical neurons expressing caspase-cleaved tau are sensitized to endoplasmic reticulum stress induced cell death. Brain Res 1234:206–212. doi:10.1016/j.brainres.2008.07.111
Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E (2008) Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci 9(7):505–518. doi:10.1038/nrn2417
Rapoport M, Dawson HN, Binder LI, Vitek MP, Ferreira A (2002) Tau is essential to beta-amyloid-induced neurotoxicity. Proc Natl Acad Sci U S A 99(9):6364–6369. doi:10.1073/pnas.092136199
Pallo SP, Johnson GV (2015) Tau facilitates Abeta-induced loss of mitochondrial membrane potential independent of cytosolic calcium fluxes in mouse cortical neurons. Neurosci Lett 597:32–37. doi:10.1016/j.neulet.2015.04.021
Pallo SP, DiMaio J, Cook A, Nilsson B, Johnson GV (2016) Mechanisms of tau and Abeta-induced excitotoxicity. Brain Res 1634:119–131. doi:10.1016/j.brainres.2015.12.048
Vossel KA, Xu JC, Fomenko V, Miyamoto T, Suberbielle E, Knox JA, Ho K, Kim DH et al (2015) Tau reduction prevents Abeta-induced axonal transport deficits by blocking activation of GSK3beta. J Cell Biol 209(3):419–433. doi:10.1083/jcb.201407065
Selkoe DJ (2008) Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res 192(1):106–113. doi:10.1016/j.bbr.2008.02.016
Verstreken P, Ly CV, Venken KJ, Koh TW, Zhou Y, Bellen HJ (2005) Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron 47(3):365–378. doi:10.1016/j.neuron.2005.06.018
Ly CV, Verstreken P (2006) Mitochondria at the synapse. Neuroscientist 12(4):291–299. doi:10.1177/1073858406287661
Ivannikov MV, Macleod GT (2013) Mitochondrial free Ca(2)(+) levels and their effects on energy metabolism in Drosophila motor nerve terminals. Biophys J 104(11):2353–2361. doi:10.1016/j.bpj.2013.03.064
Rangaraju V, Calloway N, Ryan TA (2014) Activity-driven local ATP synthesis is required for synaptic function. Cell 156(4):825–835. doi:10.1016/j.cell.2013.12.042
Reyes RC, Parpura V (2008) Mitochondria modulate Ca2+-dependent glutamate release from rat cortical astrocytes. J Neurosci 28(39):9682–9691. doi:10.1523/JNEUROSCI.3484-08.2008
Li Z, Okamoto K, Hayashi Y, Sheng M (2004) The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell 119(6):873–887. doi:10.1016/j.cell.2004.11.003
Chen H, McCaffery JM, Chan DC (2007) Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell 130(3):548–562. doi:10.1016/j.cell.2007.06.026
Kageyama Y, Zhang Z, Roda R, Fukaya M, Wakabayashi J, Wakabayashi N, Kensler TW, Reddy PH et al (2012) Mitochondrial division ensures the survival of postmitotic neurons by suppressing oxidative damage. J Cell Biol 197(4):535–551. doi:10.1083/jcb.201110034
Bertholet AM, Millet AM, Guillermin O, Daloyau M, Davezac N, Miquel MC, Belenguer P (2013) OPA1 loss of function affects in vitro neuronal maturation. Brain 136(Pt 5):1518–1533. doi:10.1093/brain/awt060
Williams PA, Piechota M, von Ruhland C, Taylor E, Morgan JE, Votruba M (2012) Opa1 is essential for retinal ganglion cell synaptic architecture and connectivity. Brain 135(Pt 2):493–505. doi:10.1093/brain/awr330
Kushnareva YE, Gerencser AA, Bossy B, Ju WK, White AD, Waggoner J, Ellisman MH, Perkins G et al (2013) Loss of OPA1 disturbs cellular calcium homeostasis and sensitizes for excitotoxicity. Cell Death Differ 20(2):353–365. doi:10.1038/cdd.2012.128
Pritchard SM, Dolan PJ, Vitkus A, Johnson GV (2011) The toxicity of tau in Alzheimer disease: turnover, targets and potential therapeutics. J Cell Mol Med 15(8):1621–1635. doi:10.1111/j.1582-4934.2011.01273.x
Kosik KS, Joachim CL, Selkoe DJ (1986) Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci U S A 83(11):4044–4048
Basurto-Islas G, Luna-Munoz J, Guillozet-Bongaarts AL, Binder LI, Mena R, Garcia-Sierra F (2008) Accumulation of aspartic acid421- and glutamic acid391-cleaved tau in neurofibrillary tangles correlates with progression in Alzheimer disease. J Neuropathol Exp Neurol 67(5):470–483. doi:10.1097/NEN.0b013e31817275c7
Guillozet-Bongaarts AL, Garcia-Sierra F, Reynolds MR, Horowitz PM, Fu Y, Wang T, Cahill ME, Bigio EH et al (2005) Tau truncation during neurofibrillary tangle evolution in Alzheimer’s disease. Neurobiol Aging 26(7):1015–1022. doi:10.1016/j.neurobiolaging.2004.09.019
Means JC, Gerdes BC, Kaja S, Sumien N, Payne AJ, Stark DA, Borden PK, Price JL et al (2016) Caspase-3-dependent proteolytic cleavage of tau causes neurofibrillary tangles and results in cognitive impairment during normal aging. Neurochem Res 41(9):2278–2288. doi:10.1007/s11064-016-1942-9
Kim Y, Choi H, Lee W, Park H, Kam TI, Hong SH, Nah J, Jung S et al (2016) Caspase-cleaved tau exhibits rapid memory impairment associated with tau oligomers in a transgenic mouse model. Neurobiol Dis 87:19–28. doi:10.1016/j.nbd.2015.12.006
Ozcelik S, Sprenger F, Skachokova Z, Fraser G, Abramowski D, Clavaguera F, Probst A, Frank S et al (2016) Co-expression of truncated and full-length tau induces severe neurotoxicity. Mol Psychiatry. doi:10.1038/mp.2015.228
Wang X, Su B, Fujioka H, Zhu X (2008) Dynamin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer’s disease patients. Am J Pathol 173(2):470–482. doi:10.2353/ajpath.2008.071208
Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, Zhu X (2009) Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J Neurosci 29(28):9090–9103. doi:10.1523/JNEUROSCI.1357-09.2009
Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T, Bartoli D, Polishuck RS et al (2006) OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 126(1):177–189. doi:10.1016/j.cell.2006.06.025
Varanita T, Soriano ME, Romanello V, Zaglia T, Quintana-Cabrera R, Semenzato M, Menabo R, Costa V et al (2015) The OPA1-dependent mitochondrial cristae remodeling pathway controls atrophic, apoptotic, and ischemic tissue damage. Cell Metab 21(6):834–844. doi:10.1016/j.cmet.2015.05.007
de Calignon A, Fox LM, Pitstick R, Carlson GA, Bacskai BJ, Spires-Jones TL, Hyman BT (2010) Caspase activation precedes and leads to tangles. Nature 464(7292):1201–1204. doi:10.1038/nature08890
Dumont M, Stack C, Elipenahli C, Jainuddin S, Gerges M, Starkova NN, Yang L, Starkov AA et al (2011) Behavioral deficit, oxidative stress, and mitochondrial dysfunction precede tau pathology in P301S transgenic mice. FASEB J 25(11):4063–4072. doi:10.1096/fj.11-186650
Acknowledgements
This work was supported by FONDECYT #1140968 and Anillo ACT1411 (RAQ).
Author information
Authors and Affiliations
Corresponding author
Additional information
María José Pérez and Katiana Vergara-Pulgar contributed equally to this work.
Electronic supplementary material
Figure supplementary 1
Caspase-cleaved tau expression did not affect pDrp1 and Mfn1 regulation in immortalized cortical neurons. (A) CN1.4 cells were transfected with GFP and tau constructs [GFP-T4, GFP-T42EC, GFP-T4C3, and GFP-T4C3-2EC] to determine the expression of pDrp1 using anti-pDrp1 antibody that detects Drp1 phosphorylation at S616. Representative western blot analysis from 3 independent experiments that show no significant differences in Drp1 activation by the presence of tau pathology. (B) Representative western blot image from 3 experiments of Mfn1 expression in CN1.4 cells transfected with pathological forms of tau. As well as, Mfn2, Mfn1 levels were not affected by the presence of tau pathology in these cells. In both western blot analysis the levels of β-actin were evaluated as a loading control. (GIF 857 kb)
Figure supplementary 2
Expression levels of Drp1 and Mfn2 in CN 1.4 cells transfected with pathological forms of tau. (A), (B) are representative western blots images from CN 1.4 cells transfected with GFP and GFP-tau forms in where the levels of total Drp1 and Mfn2 were evaluated. Images includes molecular weight standard to corroborate Drp1 and Mnf2 expression in CN 1.4 cells. β-actin levels were evaluated as a loading control. Images are representative of 4 independent experiments. (GIF 918 kb)
Rights and permissions
About this article
Cite this article
Pérez, M.J., Vergara-Pulgar, K., Jara, C. et al. Caspase-Cleaved Tau Impairs Mitochondrial Dynamics in Alzheimer’s Disease. Mol Neurobiol 55, 1004–1018 (2018). https://doi.org/10.1007/s12035-017-0385-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0385-x