Abstract
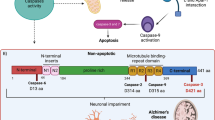
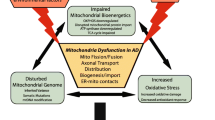
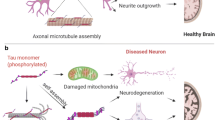
Alzheimer’s disease is the most common age-related progressive neurodegenerative disorder of brain cortex and hippocampus leading to cognitive impairment. Accumulation of extracellular amyloid plaques and intraneuronal neurofibrillary tangles are believed to be the main hallmarks of the disease. Origin of Alzheimer’s disease is not totally clear, multiple initiator factors are likely to exist. Intracellular impacts of Alzheimer’s disease include mitochondrial dysfunction, oxidative stress, ER-stress, disruption of autophagy, severe metabolic challenges leading to massive neuronal apoptosis. Mitochondria are the key players in all these processes. This formed the basis for the so-called mitochondrial cascade hypothesis. This review provides current data on the molecular mechanisms of the development of Alzheimer’s disease associated with mitochondria. Special attention was paid to the interaction between Tau protein and mitochondria, as well as to the promising therapeutic approaches aimed at preventing development of neurodegeneration.
Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- Drp1:
-
dynamin-related protein 1
- ETC:
-
electron transport chain
- hTau:
-
full-length human Tau protein
- MitoQ:
-
mitoquinone mesylate
- OPA1:
-
mitochondrial dynamin like GTPase
- PINK1:
-
PTEN-induced kinase 1
- P-Tay:
-
hyperphosphorylated Tau protein
- ROS:
-
reactive oxygen species
- SkQ1:
-
10-(6′-plastoquinonyl)decyltriphenylphosphonium
- TRAK2:
-
trafficking kinesin-binding protein 2
- UCHL-1:
-
ubiquitin carboxy-terminal hydrolase L1
References
Goleva, T., Rogov, A., and Zvyagilskaya, R. (2017) Alzheimer’s disease: molecular hall marks and yeast models, J. Alzheimer’s Dis. Parkinsonism, 7, 394-401, https://doi.org/10.4172/2161-0460.1000394.
Soria Lopez, J. A., González, H. M., and Léger, G. C. (2019) Alzheimer’s disease, Handb. Clin. Neurol., 167, 231-255, https://doi.org/10.1016/B978-0-12-804766-8.00013-3.
Eckert, A., Nisbet, R., Grimm, A., and Götz, J. (2014) March separate, strike together – role of phosphorylated TAU in mitochondrial dysfunction in Alzheimer’s disease, Biochim. Biophys. Acta, 1842, 1258-1266, https://doi.org/10.1016/j.bbadis.2013.08.013.
Manczak, M., and Reddy, P. H. (2012) Abnormal interaction between the mitochondrial fission protein Drp1 and hyperphosphorylated tau in Alzheimer’s disease neurons: implications for mitochondrial dysfunction and neuronal damage, Hum. Mol. Genet., 21, 2538-2547, https://doi.org/10.1093/hmg/dds072.
Rai, S. N., Singh, C., Singh, A., Singh, M. P., and Singh, B. K. (2020) Mitochondrial dysfunction: a potential therapeutic target to treat Alzheimer’s disease, Mol. Neurobiol., 57, 3075-3088, https://doi.org/10.1007/s12035-020-01945-y.
John, A., and Reddy, P. H. (2021) Synaptic basis of Alzheimer’s disease: focus on synaptic amyloid beta, P-tau and mitochondria, Ageing Res. Rev., 65, 101208, https://doi.org/10.1016/j.arr.2020.101208.
Briston, T., and Hicks, A. R. (2018) Mitochondrial dysfunction and neurodegenerative proteinopathies: mechanisms and prospects for therapeutic intervention, Biochem Soc Trans., 46, 829-842, https://doi.org/10.1042/BST20180025.
Amadoro, G., Corsetti, V., Stringaro, A., Colone, M., D’Aguanno, S., et al. (2010) A NH2 tau fragment targets neuronal mitochondria at AD synapses: possible implications for neurodegeneration, J. Alzheimer’s Dis., 21, 445-470, https://doi.org/10.3233/JAD-2010-100120.
David, D.C., Hauptmann, S., Scherping, I., Schuessel, K., Keil, U., et al. (2005) Proteomic and functional analyses reveal a mitochondrial dysfunction in P301L tau transgenic mice, J. Biol. Chem., 280, 23802-23814, https://doi.org/10.1074/jbc.M500356200.
Rhein, V., Song, X., Wiesner, A., Ittner, L. M., Baysang, G., et al. (2009) Amyloid-b and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer’s disease mice, Proc. Natl. Acad. Sci. USA, 106, 20057-20062, https://doi.org/10.1073/pnas.0905529106.
Zhu, H., Zhang, W., Zhao, Y., Shu, X., Wang, W., et al. (2018) GSK3β-mediated tau hyperphosphorylation triggers diabetic retinal neurodegeneration by disrupting synaptic and mitochondrial functions, Mol. Neurodegener., 13, 62, https://doi.org/10.1186/s13024-018-0295-z.
DuBoff, B., Feany, M., and Götz, J. (2013) Why size matters – balancing mitochondrial dynamics in Alzheimer’s disease, Trends Neurosci., 36, 325-335, https://doi.org/10.1016/j.tins.2013.03.002.
Li, X.C., Hu, Y., Wang, Z., Luo, Y., Zhang, Y., et al. (2016) Human wild-type full-length tau accumulation disrupts mitochondrial dynamics and the functions via increasing mitofusins, Sci. Rep., 6, 24756, https://doi.org/10.1038/srep24756.
Pérez, M. J., Vergara-Pulgar, K., Jara, C., Cabezas-Opazo, F., and Quintanilla, R. A. (2018) Caspase-cleaved tau impairs mitochondrial dynamics in Alzheimer’s disease, Mol. Neurobiol., 55, 1-15, https://doi.org/10.1007/s12035-017-0385-x.
Lopes, S., Teplytska, L., Vaz-Silva, J., Dioli, C., Trindade, R., et al. (2017) Tau deletion prevents stress-induced dendritic atrophy in prefrontal cortex: role of synaptic mitochondria, Cereb. Cortex, 27, 2580-2591, https://doi.org/10.1093/cercor/bhw057.
Vossel, K. A., Zhang, K., Brodbeck, J., Daub, A. C., Sharma, P., et al. (2010) Tau reduction prevents Abeta-induced defects in axonal transport, Science, 330, 198, https://doi.org/10.1126/science.1194653.
Yao, J., Irwin, R. W., Zhao, L., Nilsen, J., Hamilton, R. T., et al. (2009) Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease, Proc. Natl. Acad. Sci. USA, 106, 14670-14675, https://doi.org/10.1073/pnas.0903563106.
Resende, R., Moreira, P. I., Proenca, T., Deshpande, A., Busciglio, J., et al. (2008) Brain oxidative stress in a triple-transgenic mouse model of Alzheimer disease, Free Radic. Biol. Med., 44, 2051-2057, https://doi.org/10.1016/j.freeradbiomed.2008.03.012.
Sensi, S. L., Rapposelli, I. G., Frazzini, V., and Mascetra, N. (2008) Altered oxidant-mediated intraneuronal zinc mobilization in a triple transgenic mouse model of Alzheimer’s disease, Exp. Gerontol., 43, 488-492, https://doi.org/10.1016/j.exger.2007.10.018.
Chou, J. L., Shenoy, D. V., Thomas, N., Choudhary, P. K., Laferla, F. M., et al. (2011) Early dysregulation of the mitochondrial proteome in a mouse model of Alzheimer’s disease, J. Proteom., 74, 466-479, https://doi.org/10.1016/j.jprot.2010.12.012.
Kandimalla, R., Manczak, M., Fry, D., Suneetha, Y., Sesaki, H., et al. (2016) Reduced dynamin-related protein 1 protects against phosphorylated Tau-induced mitochondrial dysfunction and synaptic damage in Alzheimer’s disease, Hum. Mol. Genet., 25, 4881-4897, https://doi.org/10.1093/hmg/ddw312.
Reddy, P. H., and Oliver, D. M. (2019) Amyloid beta and phosphorylated tau-induced defective autophagy and mitophagy in Alzheimer’s disease, Cells, 8, 488, https://doi.org/10.3390/cells8050488.
Medala, V.K., Gollapelli, B., Dewanjee, S., Ogunmokun, G., Kandimalla, R., et al. (2021) Mitochondrial dysfunction, mitophagy, and role of dynamin-related protein 1 in Alzheimer’s disease, J. Neurosci. Res., 99, 1120-1135, https://doi.org/10.1002/jnr.24781.
Fuente-Muñoz, C. E., Rosas-Lemus, M., Moreno-Castilla, P., Bermúdez-Rattoni, F., Uribe-Carvajal, S., et al. (2020) Age-dependent decline in synaptic mitochondrial function is exacerbated in vulnerable brain regions of female 3xTg-AD mice, Int. J. Mol. Sci., 21, 8727, https://doi.org/10.3390/ijms21228727.
Shefa, U., Jeong, N. Y., Song, I. O., Chung, H. J., Kim, D., et al. (2019) Mitophagy links oxidative stress conditions and neurodegenerative diseases, Neural Regen. Res., 14, 749-756, https://doi.org/10.4103/1673-5374.249218.
Morton, H., Kshirsagar, S., Orlov, E., Bunquin, L. E., Sawant, N., et al. (2021) Defective mitophagy and synaptic degeneration in Alzheimer’s disease: focus on aging, mitochondria and synapse, Free Radic. Biol. Med., 172, 652-667, https://doi.org/10.1016/j.freeradbiomed.2021.07.013.
Reiss, A. B., Arain, H. A., Stecker, M. M., Siegart, N. M., and Kasselman, L. J. (2018) Amyloid toxicity in Alzheimer’s disease, Rev. Neurosci., 29, 613-627, https://doi.org/10.1515/revneuro-2017-0063.
Camilleri, A., Ghio, S., Caruana, M., Weckbecker, D., Schmidt, F., et al. (2020) Tau-induced mitochondrial membrane perturbation is dependent upon cardiolipin, Biochim. Biophys. Acta Biomembr., 1862, 183064, https://doi.org/10.1016/j.bbamem.2019.183064.
Tang, Z., Ioja, E., Bereczki, E., Hultenby, K., Li, C., et al. (2015) mTor mediates tau localization and secretion: implication for Alzheimer’s disease, Biochim. Biophys. Acta, 1853, 1646-1657, https://doi.org/10.1016/j.bbamcr.2015.03.003.
Amorim, J. A., Canas, P. M., Tome, A. R., Rolo, A. P., Agostinho, P., et al. (2017) Mitochondria in excitatory and inhibitory synapses have similar susceptibility to amyloid-beta peptides modeling Alzheimer’s disease, J. Alzheimer’s Dis., 60, 525-536, https://doi.org/10.3233/JAD-170356.
Camilleri, A., Zarb, C., Caruana, M., Ostermeier, U., Ghio, S., et al. (2013) Mitochondrial membrane perermeabilization by amyloid aggregates and protection by polyphenols, Biochim. Biophys. Acta, 1828, 2532-2543, https://doi.org/10.1016/j.bbamem.2013.06.026.
Ardail, D., Privat, J. P., Egret-Charlier, M., Levrat, C., Lerme, F., et al. (1990) Mitochondrial contact sites. Lipid composition and dynamics, J. Biol. Chem., 265, 18797-18802.
Paradies, G., Paradies, V., De Benedictis, V., Ruggiero, F. M., and Petrosillo, G. (2014) Functional role of cardiolipin in mitochondrial bioenergetics, Biochim. Biophys. Acta, 1837, 408-417, https://doi.org/10.1016/j.bbabio.2013.10.006.
Suga, K., Hamasaki, A., Chinzaka, J., and Umakoshi, H. (2016) Liposomes modified with cardiolipin can act as a platform to regulate the potential flux of NADP+-dependent isocitrate dehydrogenase, Metab. Eng. Commun., 3, 8-14, https://doi.org/10.1016/j.meteno.2015.11.002.
Schug, Z. T., and Gottlieb, E. (2009) Cardiolipin acts as a mitochondrial signalling platform to launch apoptosis, Biochim. Biophys. Acta, 1788, 2022-2031, https://doi.org/10.1016/j.bbamem.2009.05.004.
Lasagna-Reeves, C. A., Castillo-Carranza, D. L., Sengupta, U., Clos, A. L., Jackson, G. R., et al. (2011) Tau oligomers impair memory and induce synaptic and mitochondrial dysfunction in wild-type mice, Mol. Neurodegener., 6, 39, https://doi.org/10.1186/1750-1326-6-39.
Du, H., Guo, L., Yan, S., Sosunov, A. A., McKhann, G. M., and Yan, S. S. (2010) Early deficits in synaptic mitochondria in an Alzheimer’s disease mouse model, Proc. Natl. Acad. Sci. USA, 107, 18670-18675, https://doi.org/10.1073/pnas.1006586107.
Esteras, N., Rohrer, J. D., Hardy, J., Wray, S., and Abramov, A. Y. (2017) Mitochondrial hyperpolarization in iPSC-derived neurons from patients of FTDP-17 with 10+16 MAPT mutation leads to oxidative stress and neurodegeneration, Redox Biol., 12, 410-422, https://doi.org/10.1016/j.redox.2017.03.008.
Eckert, A., Schulz, K.L., Rhein, V., and Gotz, J. (2010) Convergence of amyloid-beta and tau pathologies on mitochondria in vivo, Mol. Neurobiol., 41, 107-114, https://doi.org/10.1007/s12035-010-8109-5.
Tracy, T. E., Madero-Pérez, J., Swaney, D. L., Chang, T. S., Moritz, M., et al. (2022) Tau interactome maps synaptic and mitochondrial processes associated with neurodegeneration, Cell, 185, 712-728, https://doi.org/10.1016/j.cell.2021.12.041.
Sanz-Blasco, S., Valero, R. A., Rodriguez-Crespo, I., Villalobos, C., and Nunez, L. (2008) Mitochondrial Ca2+ overload underlies Abeta oligomers neurotoxicity providing an unexpected mechanism of neuroprotection by NSAIDs, PLoS One, 3, e2718, https://doi.org/10.1371/journal.pone.0002718.
Pallo, S. P., and Johnson, G. V. W. (2015) Tau facilitates Aβ-induced loss of mitochondrial membrane potential independent of cytosolic calcium fluxes in mouse cortical neurons, Neurosci. Lett., 597, 32-37, https://doi.org/10.1016/j.neulet.2015.04.021.
Palikaras, K., Achanta, K., Choi, S., Akbari, M., and Bohr, V. A. (2021) Alteration of mitochondrial homeostasis is an early event in a C. elegans model of human tauopathy, Aging (Albany NY), 13, 23876-23894, https://doi.org/10.18632/aging.203683.
Zheng, J., Akbari, M., Schirmer, C., Reynaert, M. L., Loyens, A., et al. (2020) Hippocampal tau oligomerization early in tau pathology coincides with a transient alteration of mitochondrial homeostasis and DNA repair in a mouse model of tauopathy, Acta Neuropathol. Commun., 8, 25, https://doi.org/10.1186/s40478-020-00896-8.
Jara, C., Aránguiz, A., Cerpa, W., Tapia-Rojas, C., and Quintanilla, R. A. (2018) Genetic ablation of tau improves mitochondrial function and cognitive abilities in the hippocampus, Redox Biol., 18, 279-294, https://doi.org/10.1016/j.redox.2018.07.010.
Jara, C., Cerpa, W., Tapia-Rojas, C., and Quintanilla, R. A. (2021) Tau deletion prevents cognitive impairment and mitochondrial dysfunction age associated by a mechanism dependent on cyclophilin-D, Front. Neurosci., 14, 586710, https://doi.org/10.3389/fnins.2020.586710.
Jagasia, R., Grote, P., Westermann, B., and Conradt, B. (2005) DRP-1-mediated mitochondrial fragmentation during EGL-1-induced cell death in C. elegans, Nature, 433, 754-760, https://doi.org/10.1038/nature03316.
Malka, F., Guillery, O., Cifuentes-Diaz, C., Guillou, E., Belenguer, P., et al. (2006) Separate fusion of outer and inner mitochondrial membranes, EMBO Rep., 6, 853-859, https://doi.org/10.1038/sj.embor.7400488.
Chen, H., Detmer, S. A., Ewald, A. J., Griffin, E. E., Fraser, S. E., et al. (2003) Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development, J. Cell Biol., 160, 189-200, https://doi.org/10.1083/jcb.200211046.
Ishihara, N., Fujita, Y., Oka, T., and Mihara, K. (2006) Regulation of mitochondrial morphology through proteolytic cleavage of OPA1, EMBO J., 25, 2966-2977, https://doi.org/10.1038/sj.emboj.7601184.
Otara, H., Wang, C., Cleland, M. M., Setoguchi, K., Yokota, S., et al. (2010) Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells, J. Cell Biol., 191, 1141-1158, https://doi.org/10.1083/jcb.201007152.
Chan, D. C. (2006) Mitochondria: dynamic organelles in disease, aging, and development, Cell, 125, 1241-1252, https://doi.org/10.1016/j.cell.2006.06.010.
Trease, A. J., George, J. W., Roland, N. J., Lichter, E. Z., Emanuel, K., et al. (2022) Hyperphosphorylated human tau accumulates at the synapse, localizing on synaptic mitochondrial outer membranes and disrupting respiration in a mouse model of tauopathy, Front. Mol. Neurosci., 15, 852368, https://doi.org/10.3389/fnmol.2022.852368.
Quintanilla, R. A., Tapia-Monsalves, C., Vergara, E. H., Pérez, M. J., and Aranguiz, A. (2020) Truncated tau induces mitochondrial transport failure through the impairment of TRAK2 protein and bioenergetics decline in neuronal cells, Front. Cell Neurosci., 14, 175, https://doi.org/10.3389/fncel.2020.00175.
Jeong, Y. Y., Jia, N., Ganesan, D., and Cai, Q. (2022) Broad activation of the PRKN pathway triggers synaptic failure by disrupting synaptic mitochondrial supply in early tauopathy, Autophagy, 18, 1472-1474, https://doi.org/10.1080/15548627.2022.2039987.
Jarero-Basulto, J. J., Luna-Munoz, J., Mena, R., Kristofikova, Z., Ripova, D., et al. (2013) Proteolytic cleavage of polymeric tau protein by caspase-3: implications for Alzheimer’s disease, J. Neuropathol. Exp. Neurol., 72, 1145-1161, https://doi.org/10.1097/NEN.0000000000000013.
Kopeikina, K. J., Carlson, G. A., Pitstick, R., Ludvigson, A. E., Peters, A., et al. (2011) Tau accumulation causes mitochondrial distribution deficits in neurons in a mouse model of tauopathy and in human Alzheimer’s disease brain, Am. J. Pathol., 179, 2071-2082, https://doi.org/10.1016/j.ajpath.2011.07.004.
Plucinska, G., Paquet, D., Hruscha, A., Godinho, L., Haass, C., et al. (2012) In vivo imaging of disease-related mitochondrial dynamics in a vertebrate model system, J. Neurosci., 32, 16203-16212, https://doi.org/10.1523/JNEUROSCI.1327-12.2012.
Shahpasand, K., Uemura, I., Saito, T., Asano, T., Hata, K., et al. (2012) Regulation of mitochondrial transport and inter-microtubule spacing by tau phosphorylation at the sites hyperphosphorylated in Alzheimer’s disease, J. Neurosci., 32, 2430-2441, https://doi.org/10.1523/JNEUROSCI.5927-11.2012.
Kandimalla, R., Manczak, M., Pradeepkiran, J. A., Morton, H., and Reddy, P. H. (2022) A partial reduction of Drp1 improves cognitive behavior and enhances mitophagy, autophagy and dendritic spines in a transgenic Tau mouse model of Alzheimer disease, Hum. Mol. Genet., 31, 1788-1805, https://doi.org/10.1093/hmg/ddab360.
Abtahi, S. L., Masoudi, R., and Haddadi, M. (2020) The distinctive role of tau and amyloid beta in mitochondrial dysfunction through alteration in Mfn2 and Drp1 mRNA levels: a comparative study in Drosophila melanogaster, Gene, 754, 144854, https://doi.org/10.1016/j.gene.2020.144854.
Alavi, M. V. (2021) Tau phosphorylation and OPA1 proteolysis are unrelated events: implications for Alzheimer’s disease, Biochim. Biophys. Acta Mol. Cell. Res., 1868, 119116, https://doi.org/10.1016/j.bbamcr.2021.119116.
Kerr, J. S., Adriaanse, B. A., Greig, N. H., Mattson, M. P., Cader, M. Z., et al. (2017) Mitophagy and Alzheimer’s disease: cellular and molecular mechanisms, Trends Neurosci., 40, 151-166, https://doi.org/10.1016/j.tins.2017.01.002.
Hu, Y., Li, X. C., Wang, Z. H., Luo, Y., Zhang, X., et al. (2016) Tau accumulation impairs mitophagy via increasing mitochondrial membrane potential and reducing mitochondrial Parkin, Oncotarget, 7, 17356-17368, https://doi.org/10.18632/oncotarget.7861.
Cummins, N., Tweedie, A., Zuryn, S., Bertran-Gonzalez, J., and Götz, J. (2019) Disease-associated tau impairs mitophagy by inhibiting Parkin translocation to mitochondria, EMBO J., 38, e99360, https://doi.org/10.15252/embj.201899360.
Corsetti, V., Florenzano, F., Atlante, A., Bobba, A., Ciotti, M. T., et al. (2015) NH2-truncated human tau induces deregulated mitophagy in neurons by aberrant recruitment of Parkin and UCHL-1: implications in Alzheimer’s disease, Hum. Mol. Genet., 24, 3058-3081, https://doi.org/10.1093/hmg/ddv059.
Escobar-Henriques, M., and Langer, T. (2014) Dynamic survey of mitochondria by ubiquitin, EMBO Rep., 15, 231-243, https://doi.org/10.1002/embr.201338225.
Hegde, A. N., and DiAntonio, A. (2002) Ubiquitin and the synapse, Nat. Rev. Neurosci., 3, 854-861, https://doi.org/10.1038/nrn961.
Zhu, X., Perry, G., Smith, M. A., and Wang, X. (2013) Abnormal mitochondrial dynamics in the pathogenesis of Alzheimer’s disease, J. Alzheimer’s Dis., 33, 253-262, https://doi.org/10.3233/JAD-2012-129005.
Amadoro, G., Corsetti, V., Florenzano, F., Atlante, A., Bobba, A., et al. (2014) Morphological and bioenergetic demands underlying the mitophagy in post-mitotic neurons: the pink-parkin pathway, Front. Aging Neurosci., 6, 18, https://doi.org/10.3389/fnagi.2014.00018.
Reddy, P. H., Tripathi, R., Troung, Q., Tirumala, K., Reddy, T. P., et al. (2012) Abnormal mitochondrial dynamics and synaptic degeneration as early events in Alzheimer’s disease: implications to mitochondria-targeted antioxidant therapeutics, Biochim. Biophys. Acta, 1822, 639-649, https://doi.org/10.1016/j.bbadis.2011.10.011.
Koyano, F., Okatsu, K., Ishigaki, S., Fujioka, Y., Kimura, M., et al. (2013) The principal PINK1 and Parkin cellular events triggered in response to dissipation of mitochondrial membrane potential occur in primary neurons, Genes Cells., 18, 672-681, https://doi.org/10.1111/gtc.12066.
Bingol, B., Tea, J. S., Phu, L., Reichelt, M., Bakalarski, C. E., et al. (2014) The mitochondrial deubiquitinase USP30 opposes parkin-mediated mitophagy, Nature, 510, 370-375, https://doi.org/10.1038/nature13418.
Osaka, H., Wang, Y.L., Takada, K., Takizawa, S., Setsuie, R., et al. (2003) Ubiquitin carboxy-terminal hydrolase L1 binds to and stabilizes monoubiquitin in neuron, Hum. Mol. Genet., 12, 1945-1958, https://doi.org/10.1093/hmg/ddg211.
Liu, Y., Fallon, L., Lashuel, H. A., Liu, Z., and Lansbury, P. T., Jr. (2002) The UCH-L1 gene encodes two opposing enzymatic activities that affect alpha-synuclein degradation and Parkinson’s disease susceptibility, Cell, 111, 209-218, https://doi.org/10.1016/s0092-8674(02)01012-7.
Cartier, A. E., Djakovic, S. N., Salehi, A., Wilson, S. M., Masliah, E., et al. (2009) Regulation of synaptic structure by ubiquitin C-terminal hydrolase L1, J. Neurosci., 29, 7857-7868, https://doi.org/10.1523/JNEUROSCI.1817-09.2009.
Chen, F., Sugiura, Y., Myers, K. G., Liu, Y., and Lin, W. (2010) Ubiquitin carboxyl-terminal hydrolase L1 is required for maintaining the structure and function of the neuromuscular junction, Proc. Natl. Acad. Sci. USA, 107, 1636-1641, https://doi.org/10.1073/pnas.0911516107.
Guglielmotto, M., Monteleone, D., Boido, M., Piras, A., Giliberto, L., et al. (2012) Aβ1-42-mediated down-regulation of Uch-L1 is dependent on NF-κB activation and impaired BACE1 lysosomal degradation, Aging Cell, 11, 834-844, https://doi.org/10.1111/j.1474-9726.2012.00854.x.
Poon, W. W., Carlos, A. J., Aguilar, B. L., Berchtold, N. C., Kawano, C. K., et al. (2013) β-Amyloid (Aβ) oligomers impair brain-derived neurotrophic factor retrograde trafficking by down-regulating ubiquitin C-terminal hydrolase, UCH-L1, J. Biol. Chem., 288, 16937-16948, https://doi.org/10.1074/jbc.M113.463711.
Zanon, A., Rakovic, A., Blankenburg, H., Doncheva, N. T., Schwienbacher, C., et al. (2013) Profiling of Parkin- binding partners using tandem affinity purification, PLoS One, 8, e78648, https://doi.org/10.1371/journal.pone.0078648.
Shevtsova, E. F., Maltsev, A. V., Vinogradova, D. V., Shevtsov, P. N., and Bachurin, S. O. (2021) Mitochondria as a promising target for developing novel agents for treating Alzheimer’s disease, Med. Res. Rev., 41, 803-827, https://doi.org/10.1002/med.21715.
Johri, A. (2021) Disentangling mitochondria in Alzheimer’s disease, Int. J. Mol. Sci., 22, 11520, https://doi.org/10.3390/ijms222111520.
Mary, A., Eysert, F., Checler, F., and Chami, M. (2022) Mitophagy in Alzheimer’s disease: molecular defects and therapeutic approaches, Mol. Psychiatry, https://doi.org/10.1038/s41380-022-01631-6.
Kshirsagar, S., Sawant, N., Morton, H., Reddy, A. P., and Reddy, P. H. (2021) Mitophagy enhancers against phosphorylated Tau-induced mitochondrial and synaptic toxicities in Alzheimer’s disease, Pharmacol. Res., 174, 105973, https://doi.org/10.1016/j.phrs.2021.105973.
Feniouk, B. A., and Skulachev, V. P. (2017) Cellular and molecular mechanisms of action of mitochondria-targeted antioxidants, Curr. Aging Sci., 10, 41-48, https://doi.org/10.2174/1874609809666160921113706.
Plotnikov, E. Y., Silachev, D. N., Jankauskas, S. S., Rokitskaya, T. I., Chupyrkina, A. A., et al. (2012) Mild uncoupling of respiration and phosphorylation as a mechanism providing nephro- and neuroprotective effects of penetrating cations of the SkQ family, Biochemistry (Moscow), 77, 1029-1037, https://doi.org/10.1134/s0006297912090106.
Lukashev, A. N., Skulachev, M. V., Ostapenko, V., Savchenko, A. Y., Pavshintsev, V. V., et al. (2014) Advances in development of rechargeable mitochondrial antioxidants, Prog. Mol. Biol. Transl. Sci., 127, 251-265, https://doi.org/10.1016/b978-0-12-394625-6.00010-6.
Isaev, N. K., Stelmashook, E. V., Genrikhs, E. E., Korshunova, G. A., Sumbatyan, N. V., et al. (2016) Neuroprotective properties of mitochondria-targeted antioxidants of the SkQ-type, Rev. Neurosci., 27, 849-855, https://doi.org/10.1515/revneuro-2016-0036.
Oddo, S., Caccamo, A., Shepherd, J. D., Murphy, M. P., Golde, T. E., et al. (2003) Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Aβ and synaptic dysfunction, Neuron, 39, 409-421, https://doi.org/10.1016/s0896-6273(03)00434-3.
Young, M. L., and Franklin, J. L. (2019) The mitochondria-targeted antioxidant MitoQ inhibits memory loss, neuropathology, and extends lifespan in aged 3xTg-AD mice, Mol. Cell Neurosci., 101, 103409, https://doi.org/10.1016/j.mcn.2019.103409.
Samluk, L., Ostapczuk, P., and Dziembowska, M. (2022) Long-term mitochondrial stress induces early steps of Tau aggregation by increasing reactive oxygen species levels and affecting cellular proteostasis, Mol. Biol. Cell, 33, ar67, https://doi.org/10.1091/mbc.E21-11-0553.
Stefanova, N. A., Muraleva, N. A., Maksimova, K. Y., Rudnitskaya, E. A., Kiseleva, E., et al. (2016) An antioxidant specifically targeting mitochondria delays progression of Alzheimer’s disease-like pathology, Aging (Albany NY), 8, 2713-2733, https://doi.org/10.18632/aging.101054.
Trushina, E., Trushin, S., and Hasan, F. (2022) Mitochondrial complex I as a therapeutic target for Alzheimer’s disease, Acta Pharm. Sin. B, 12, 483-495, https://doi.org/10.1016/j.apsb.2021.11.003.
Stojakovic, A., Chang, S. Y., Nesbitt, J., Pichurin, N. P., Ostroot, M. A., et al. (2021) Partial inhibition of mitochondrial complex I reduces tau pathology and improves energy homeostasis and synaptic function in 3xTg-AD mice, J. Alzheimer’s Dis., 79, 335-353, https://doi.org/10.3233/JAD-201015.
Singulani, M. P., De Paula, V. J. R., and Forlenza, O. V. (2021) Mitochondrial dysfunction in Alzheimer’s disease: therapeutic implications of lithium, Neurosci. Lett., 760, 136078, https://doi.org/10.1016/j.neulet.2021.136078.
Tayanloo-Beik, A., Kiasalari, Z., and Roghani, M. (2022) Paeonol ameliorates cognitive deficits in streptozotocin murine model of sporadic Alzheimer’s disease via attenuation of oxidative stress, inflammation, and mitochondrial dysfunction, J. Mol. Neurosci., 72, 336-348, https://doi.org/10.1007/s12031-021-01936-1.
Guo, W., Zeng, Z., Xing, C., Zhang, J., Bi, W., et al. (2022) Stem cells from human exfoliated deciduous teeth affect mitochondria and reverse cognitive decline in a senescence-accelerated mouse prone 8 model, Cytotherapy, 24, 59-71, https://doi.org/10.1016/j.jcyt.2021.07.018.
Salehi, P., Shahmirzadi, Z. Y., Mirrezaei, F. S., Boushehri, F. S., Mayahi, F., et al. (2019) A hypothetic role of minocycline as a neuroprotective agent against methylphenidate-induced neuronal mitochondrial dysfunction and tau protein hyper-phosphorylation: possible role of PI3/Akt/GSK3β signaling pathway, Med. Hypotheses, 128, 6-10, https://doi.org/10.1016/j.mehy.2019.04.017.
Colman, R. J., Anderson, R. M., Johnson, S. C., Kastman, E. K., Kosmatka, K. J., et al. (2009) Caloric restriction delays disease onset and mortality in rhesus monkeys, Science, 325, 201-204, https://doi.org/10.1126/science.1173635.
Sohal, R. S., and Weindruch, R. (1996) Oxidative stress, caloric restriction, and aging, Science, 273, 59-63, https://doi.org/10.1126/science.273.5271.59.
Sanz, A., Caro, P., Ibanez, J., Gomez, J., Gredilla, R., et al. (2005) Dietary restriction at old age lowers mitochondrial oxygen radical production and leak at complex I and oxidative DNA damage in rat brain, J. Bioenerg. Biomembr., 37, 83-90, https://doi.org/10.1007/s10863-005-4131-0.
Singh, R., Lakhanpal, D., Kumar, S., Sharma, S., Kataria, H., et al. (2012) Late-onset intermittent fasting dietary restriction as a potential intervention to retard age-associated brain function impairments in male rats, Age (Dordr), 34, 917-933, https://doi.org/10.1007/s11357-011-9289-2.
Cerqueira, F. M., Cunha, F. M., Laurindo, F. R., and Kowaltowski, A. J. (2012) Calorie restriction increases cerebral mitochondrial respiratory capacity in a NO•-mediated mechanism: impact on neuronal survival, Free Radic. Biol. Med., 52, 1236-1241, https://doi.org/10.1016/j.freeradbiomed.2012.01.011.
Lambert, A. J., Wang, B., Yardley, J., Edwards, J., and Merry, B. J. (2004) The effect of aging and caloric restriction on mitochondrial protein density and oxygen consumption, Exp. Gerontol., 39, 289-295, https://doi.org/10.1016/j.exger.2003.12.009.
Halagappa, V. K. M., Guo, Z., Pearson, M., Matsuoka, Y., Cutler, R. G., et al. (2007) Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease, Neurobiol. Dis., 26, 212-220, https://doi.org/10.1016/j.nbd.2006.12.019.
Kang, K., Xu, P., Wang, M., Chunyu, J., Sun, X., et al. (2020) FGF21 attenuates neurodegeneration through modulating neuroinflammation and oxidant-stress, Biomed. Pharmacother., 129, 110439, https://doi.org/10.1016/j.biopha.2020.110439.
Mohamed, T. M., Youssef, M. A. M., Bakry, A. A., and El-Keiy, M. M. (2021) Alzheimer's disease improved through the activity of mitochondrial chain complexes and their gene expression in rats by boswellic acid, Metab. Brain Dis., 36, 255-264, https://doi.org/10.1007/s11011-020-00639-7.
Ji, D., Wu, X., Li, D., Liu, P., Zhang, S., et al. (2020) Protective effects of chondroitin sulphate nano-selenium on a mouse model of Alzheimer’s disease, Int. J. Biol. Macromol., 154, 233-245, https://doi.org/10.1016/j.ijbiomac.2020.03.079.
Saretzki, G., and Wan, T. (2021) Telomerase in brain: the new kid on the block and its role in neurodegenerative diseases, Biomedicines, 9, 490, https://doi.org/10.3390/biomedicines9050490.
Funding
This research was financially supported in part by the Russian Foundation for Basic Research (grant no. 19-34-90165).
Author information
Authors and Affiliations
Contributions
K. K. Epremyan, R. A. Zvyagilskaya – concept and management; K. K. Epremyan – writing the text; R. A. Zvyagilskaya, T. N. Goleva – editing the text of the article.
Corresponding author
Ethics declarations
The authors declare no conflicts of interest in financial or any other sphere. This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Epremyan, K.K., Goleva, T.N. & Zvyagilskaya, R.A. Effect of Tau Protein on Mitochondrial Functions. Biochemistry Moscow 87, 689–701 (2022). https://doi.org/10.1134/S0006297922080028
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297922080028