Abstract
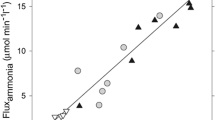
The nucleoside guanosine (GUO) increases glutamate uptake by astrocytes and acts as antioxidant, thereby providing neuroprotection against glutamatergic excitotoxicity, as we have recently demonstrated in an animal model of chronic hepatic encephalopathy. Here, we investigated the neuroprotective effect of GUO in an acute ammonia intoxication model. Adult male Wistar rats received an intraperitoneal (i.p.) injection of vehicle or GUO 60 mg/kg, followed 20 min later by an i.p. injection of vehicle or 550 mg/kg of ammonium acetate. Afterwards, animals were observed for 45 min, being evaluated as normal, coma (i.e., absence of corneal reflex), or death status. In a second cohort of rats, video-electroencephalogram (EEG) recordings were performed. In a third cohort of rats, the following were measured: (i) plasma levels of glucose, transaminases, and urea; (ii) cerebrospinal fluid (CSF) levels of ammonia, glutamine, glutamate, and alanine; (iii) glutamate uptake in brain slices; and (iv) brain redox status and glutamine synthetase activity in cerebral cortex. GUO drastically reduced the lethality rate and the duration of coma. Animals treated with GUO had improved EEG traces, decreased CSF levels of glutamate and alanine, lowered oxidative stress in the cerebral cortex, and increased glutamate uptake by astrocytes in brain slices compared with animals that received vehicle prior to ammonium acetate administration. This study provides new evidence on mechanisms of guanine-derived purines in their potential modulation of glutamatergic system, contributing to GUO neuroprotective effects in a rodent model of by acute ammonia intoxication.







Similar content being viewed by others
References
Daroff RB, Bradley WG (2012) Bradley’s neurology in clinical practice, 6th edn. Elsevier/Saunders, Philadelphia
Jackson P, Khan A (2015) Delirium in critically ill patients. Crit Care Clin 31(3):589–603. doi:10.1016/j.ccc.2015.03.011
Liu A, Perumpail RB, Kumari R, Younossi ZM, Wong RJ, Ahmed A (2015) Advances in cirrhosis: optimizing the management of hepatic encephalopathy. World J Hepatol 7(29):2871–2879. doi:10.4254/wjh.v7.i29.2871
Poordad FF (2007) Review article: the burden of hepatic encephalopathy. Aliment Pharmacol Ther 25(Suppl 1):3–9. doi:10.1111/j.1746-6342.2006.03215.x
Felipo V (2013) Hepatic encephalopathy: effects of liver failure on brain function. Nat Rev Neurosci 14(12):851–858. doi:10.1038/nrn3587
Ciecko-Michalska I, Szczepanek M, Slowik A, Mach T (2012) Pathogenesis of hepatic encephalopathy. Gastroenterol Res Pract 2012:642108. doi:10.1155/2012/642108
Amodio P, Del Piccolo F, Petteno E, Mapelli D, Angeli P, Iemmolo R, Muraca M, Musto C et al (2001) Prevalence and prognostic value of quantified electroencephalogram (EEG) alterations in cirrhotic patients. J Hepatol 35(1):37–45
Agrawal A, Sharma BC, Sharma P, Sarin SK (2012) Secondary prophylaxis of hepatic encephalopathy in cirrhosis: an open-label, randomized controlled trial of lactulose, probiotics, and no therapy. Am J Gastroenterol 107(7):1043–1050. doi:10.1038/ajg.2012.113
Stepanova M, Mishra A, Venkatesan C, Younossi ZM (2012) In-hospital mortality and economic burden associated with hepatic encephalopathy in the United States from 2005 to 2009. Clin Gastroenterol Hepatol 10(9):1034–1041. doi:10.1016/j.cgh.2012.05.016, e1031
Desjardins P, Du T, Jiang W, Peng L, Butterworth RF (2012) Pathogenesis of hepatic encephalopathy and brain edema in acute liver failure: role of glutamine redefined. Neurochem Int 60(7):690–696. doi:10.1016/j.neuint.2012.02.001
Felipo V, Butterworth RF (2002) Neurobiology of ammonia. Prog Neurobiol 67(4):259–279
Ott P, Vilstrup H (2014) Cerebral effects of ammonia in liver disease: current hypotheses. Metab Brain Dis 29(4):901–911. doi:10.1007/s11011-014-9494-7
Hermenegildo C, Monfort P, Felipo V (2000) Activation of N-methyl-D-aspartate receptors in rat brain in vivo following acute ammonia intoxication: characterization by in vivo brain microdialysis. Hepatology 31(3):709–715. doi:10.1002/hep.510310322
Bruck J, Gorg B, Bidmon HJ, Zemtsova I, Qvartskhava N, Keitel V, Kircheis G, Haussinger D (2011) Locomotor impairment and cerebrocortical oxidative stress in portal vein ligated rats in vivo. J Hepatol 54(2):251–257. doi:10.1016/j.jhep.2010.06.035
Rose C (2002) Increased extracellular brain glutamate in acute liver failure: decreased uptake or increased release? Metab Brain Dis 17(4):251–261
Chan H, Butterworth RF (2003) Cell-selective effects of ammonia on glutamate transporter and receptor function in the mammalian brain. Neurochem Int 43(4-5):525–532
Paniz LG, Calcagnotto ME, Pandolfo P, Machado DG, Santos GF, Hansel G, Almeida RF, Bruch RS et al (2014) Neuroprotective effects of guanosine administration on behavioral, brain activity, neurochemical and redox parameters in a rat model of chronic hepatic encephalopathy. Metab Brain Dis 29(3):645–654. doi:10.1007/s11011-014-9548-x
Cauli O, Gonzalez-Usano A, Cabrera-Pastor A, Gimenez-Garzo C, Lopez-Larrubia P, Ruiz-Sauri A, Hernandez-Rabaza V, Duszczyk M et al (2014) Blocking NMDA receptors delays death in rats with acute liver failure by dual protective mechanisms in kidney and brain. Neruomol Med 16(2):360–375. doi:10.1007/s12017-013-8283-5
Rodrigo R, Cauli O, Boix J, ElMlili N, Agusti A, Felipo V (2009) Role of NMDA receptors in acute liver failure and ammonia toxicity: therapeutical implications. Neurochem Int 55(1-3):113–118. doi:10.1016/j.neuint.2009.01.007
Schmidt AP, Lara DR, Souza DO (2007) Proposal of a guanine-based purinergic system in the mammalian central nervous system. Pharmacol Ther 116(3):401–416. doi:10.1016/j.pharmthera.2007.07.004
Connell BJ, Di Iorio P, Sayeed I, Ballerini P, Saleh MC, Giuliani P, Saleh TM, Rathbone MP et al (2013) Guanosine protects against reperfusion injury in rat brains after ischemic stroke. J Neurosci Res 91(2):262–272. doi:10.1002/jnr.23156
Hansel G, Tonon AC, Guella FL, Pettenuzzo LF, Duarte T, Duarte MM, Oses JP, Achaval M et al (2015) Guanosine protects against cortical focal ischemia. Involvement of inflammatory response. Mol Neurobiol 52(3):1791–1803. doi:10.1007/s12035-014-8978-0
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates, 6th edn. Elsevier, Amsterdam; Boston
Vogels BA, Maas MA, Daalhuisen J, Quack G, Chamuleau RA (1997) Memantine, a noncompetitive NMDA receptor antagonist improves hyperammonemia-induced encephalopathy and acute hepatic encephalopathy in rats. Hepatology 25(4):820–827. doi:10.1002/hep.510250406
Almeida RF, Comasseto DD, Ramos DB, Hansel G, Zimmer ER, Loureiro SO, Ganzella M, Souza DO (2016) Guanosine anxiolytic-like effect involves adenosinergic and glutamatergic neurotransmitter systems. Mol Neurobiol. doi:10.1007/s12035-015-9660-x
Schmidt AP, Tort AB, Silveira PP, Bohmer AE, Hansel G, Knorr L, Schallenberger C, Dalmaz C et al (2009) The NMDA antagonist MK-801 induces hyperalgesia and increases CSF excitatory amino acids in rats: reversal by guanosine. Pharmacol Biochem Behav 91(4):549–553. doi:10.1016/j.pbb.2008.09.009
Thomazi AP, Godinho GF, Rodrigues JM, Schwalm FD, Frizzo ME, Moriguchi E, Souza DO, Wofchuk ST (2004) Ontogenetic profile of glutamate uptake in brain structures slices from rats: sensitivity to guanosine. Mech Ageing Dev 125(7):475–481. doi:10.1016/j.mad.2004.04.005
Quincozes-Santos A, Bobermin LD, Souza DG, Bellaver B, Goncalves CA, Souza DO (2014) Guanosine protects C6 astroglial cells against azide-induced oxidative damage: a putative role of heme oxygenase 1. J Neurochem 130(1):61–74. doi:10.1111/jnc.12694
Boveris A (1984) Determination of the production of superoxide radicals and hydrogen peroxide in mitochondria. Methods Enzymol 105:429–435
Wendel A (1981) Glutathione peroxidase. Methods Enzymol 77:325–333
Bellaver B, Souza DG, Bobermin LD, Goncalves CA, Souza DO, Quincozes-Santos A (2015) Guanosine inhibits LPS-induced pro-inflammatory response and oxidative stress in hippocampal astrocytes through the heme oxygenase-1 pathway. Purinergic signalling 11(4):571–580. doi:10.1007/s11302-015-9475-2
Clay AS, Hainline BE (2007) Hyperammonemia in the ICU. Chest 132(4):1368–1378. doi:10.1378/chest.06-2940
Rama Rao KV, Jayakumar AR, Norenberg MD (2014) Brain edema in acute liver failure: mechanisms and concepts. Metab Brain Dis 29(4):927–936. doi:10.1007/s11011-014-9502-y
Palomero-Gallagher N, Zilles K (2013) Neurotransmitter receptor alterations in hepatic encephalopathy: a review. Arch Biochem Biophys 536(2):109–121. doi:10.1016/j.abb.2013.02.010
Schmidt AP, Bohmer AE, Schallenberger C, Antunes C, Pereira MS, Leke R, Wofchuk ST, Elisabetsky E et al (2009) Spinal mechanisms of antinociceptive action caused by guanosine in mice. Eur J Pharmacol 613(1-3):46–53. doi:10.1016/j.ejphar.2009.04.018
Ramos DB, Muller GC, Rocha GB, Dellavia GH, Almeida RF, Pettenuzzo LF, Loureiro SO, Hansel G, Horn AC, Souza DO, Ganzella M (2015) Intranasal guanosine administration presents a wide therapeutic time window to reduce brain damage induced by permanent ischemia in rats. Purinergic signalling. doi:10.1007/s11302-015-9489-9
Dal-Cim T, Ludka FK, Martins WC, Reginato C, Parada E, Egea J, Lopez MG, Tasca CI (2013) Guanosine controls inflammatory pathways to afford neuroprotection of hippocampal slices under oxygen and glucose deprivation conditions. J Neurochem 126(4):437–450. doi:10.1111/jnc.12324
Palsson E, Jakobsson J, Sodersten K, Fujita Y, Sellgren C, Ekman CJ, Agren H, Hashimoto K et al (2015) Markers of glutamate signaling in cerebrospinal fluid and serum from patients with bipolar disorder and healthy controls. Eur Neuropsychopharmacol 25(1):133–140. doi:10.1016/j.euroneuro.2014.11.001
Rodan LH, Gibson KM, Pearl PL (2015) Clinical use of CSF neurotransmitters. Pediatr Neurol 53(4):277–286. doi:10.1016/j.pediatrneurol.2015.04.016
Struyfs H, Niemantsverdriet E, Goossens J, Fransen E, Martin JJ, De Deyn PP, Engelborghs S (2015) Cerebrospinal fluid P-Tau181P: biomarker for improved differential dementia diagnosis. Front Neurol 6:138. doi:10.3389/fneur.2015.00138
Therrien G, Butterworth RF (1991) Cerebrospinal fluid amino acids in relation to neurological status in experimental portal-systemic encephalopathy. Metab Brain Dis 6(2):65–74
Rothman DL, De Feyter HM, Maciejewski PK, Behar KL (2012) Is there in vivo evidence for amino acid shuttles carrying ammonia from neurons to astrocytes? Neurochem Res 37(11):2597–2612. doi:10.1007/s11064-012-0898-7
Rama Rao KV, Norenberg MD (2014) Glutamine in the pathogenesis of hepatic encephalopathy: the trojan horse hypothesis revisited. Neurochem Res 39(3):593–598. doi:10.1007/s11064-012-0955-2
Cooper AJ, Kuhara T (2014) alpha-Ketoglutaramate: an overlooked metabolite of glutamine and a biomarker for hepatic encephalopathy and inborn errors of the urea cycle. Metab Brain Dis 29(4):991–1006. doi:10.1007/s11011-013-9444-9
Amodio P, Gatta A (2005) Neurophysiological investigation of hepatic encephalopathy. Metab Brain Dis 20(4):369–379. doi:10.1007/s11011-005-7921-5
Amodio P, Montagnese S (2015) Clinical neurophysiology of hepatic encephalopathy. J Clin Exp Hepatol 5(Suppl 1):S60–S68. doi:10.1016/j.jceh.2014.06.007
Petronilho F, Perico SR, Vuolo F, Mina F, Constantino L, Comim CM, Quevedo J, Souza DO et al (2012) Protective effects of guanosine against sepsis-induced damage in rat brain and cognitive impairment. Brain Behav Immun 26(6):904–910. doi:10.1016/j.bbi.2012.03.007
Giuliani P, Ballerini P, Buccella S, Ciccarelli R, Rathbone MP, Romano S, D'Alimonte I, Caciagli F et al (2015) Guanosine protects glial cells against 6-hydroxydopamine toxicity. Adv Exp Med Biol 837:23–33. doi:10.1007/5584_2014_73
Butterworth RF (2015) Pathogenesis of hepatic encephalopathy in cirrhosis: the concept of synergism revisited. Metab Brain Dis. doi:10.1007/s11011-015-9746-1
Bemeur C, Desjardins P, Butterworth RF (2010) Evidence for oxidative/nitrosative stress in the pathogenesis of hepatic encephalopathy. Metab Brain Dis 25(1):3–9. doi:10.1007/s11011-010-9177-y
Acknowledgments
This work was supported by Brazilian agencies and grants: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), and Brazilian Institute of Neuroscience—IBN Net FINEP, INCT—Excitotoxicity and Neuroprotection (573577/2008-5).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experiments were approved by the Ethics Commission (CEUA/UFRGS) under project number 29468 and followed the National Institutes of Health “Guide for the Care and Use of Laboratory Animals” (NIH publication No. 80-23, revised 1996).
Conflict of Interest
The authors declare that they have no competing financial interests.
Additional information
G. F. Cittolin-Santos and A. M. de Assis contributed equally to this work.
Rights and permissions
About this article
Cite this article
Cittolin-Santos, G.F., de Assis, A.M., Guazzelli, P.A. et al. Guanosine Exerts Neuroprotective Effect in an Experimental Model of Acute Ammonia Intoxication. Mol Neurobiol 54, 3137–3148 (2017). https://doi.org/10.1007/s12035-016-9892-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9892-4