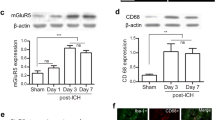
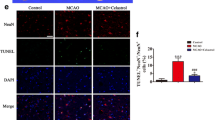
Abstract
Stroke is the major cause of death and the most frequent cause of disability in the adult population worldwide. Guanosine plays an important neuroprotective role in several cerebral ischemic models and is involved in the modulation of oxidative responses and glutamatergic parameters. Because the excessive reactive oxygen species produced during an ischemic event can trigger an inflammatory response, the purpose of this study was to evaluate the hypothesis that guanosine is neuroprotective against focal cerebral ischemia, inhibits microglia/macrophages activation, and mediates an inflammatory response ameliorating the neural damage. Permanent focal cerebral ischemia was induced in adult rats, and guanosine was administered immediately, 1, 3, and 6 h after surgery. Twenty-four hours after ischemia, the asymmetry scores were evaluated by the cylinder test; neuronal damage was evaluated by Fluoro-Jade C (FJC) staining and propidium iodide (PI) incorporation; microglia and immune cells were evaluated by anti-Iba-1 antibody; and inflammatory parameters such as interleukins (IL): IL-1, IL-6, IL-10; tumor necrosis factors alpha (TNF-α); and interferon-gamma (INF-γ) were evaluated in the brain tissue and cerebrospinal fluid. The ischemic event increased the levels of Iba-1-positive cells and pro-inflammatory cytokines and decreased IL-10 levels (an anti-inflammatory cytokine) in the lesion periphery. The guanosine treatment attenuated the changes in these inflammatory parameters and also reduced the infarct volume, PI incorporation, and number of FJC-positive cells, improving the functional recovery. Thus, guanosine may have been a promising therapeutic agent for the treatment of ischemic brain injury by reduction of inflammatory process triggered in an ischemic event.








Similar content being viewed by others
References
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB (2014) Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 129(3):e28–e292. doi:10.1161/01.cir.0000441139.02102.80
Doyle KP, Simon RP, Stenzel-Poore MP (2008) Mechanisms of ischemic brain damage. Neuropharmacology 55(3):310–318. doi:10.1016/j.neuropharm.2008.01.005
Brouns R, De Deyn PP (2009) The complexity of neurobiological processes in acute ischemic stroke. Clin Neurol Neurosurg 111(6):483–495. doi:10.1016/j.clineuro.2009.04.001
Donnan GA, Fisher M, Macleod M, Davis SM (2008) Stroke. Lancet 371(9624):1612–1623. doi:10.1016/S0140-6736(08)60694-7
Lipton P (1999) Ischemic cell death in brain neurons. Physiol Rev 79(4):1431–1568
Durukan A, Tatlisumak T (2007) Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol, Biochem Behav 87(1):179–197. doi:10.1016/j.pbb.2007.04.015
Jin R, Yang G, Li G (2010) Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol 87(5):779–789. doi:10.1189/jlb.1109766
Kettenmann H, Hanisch UK, Noda M, Verkhratsky A (2011) Physiology of microglia. Physiol Rev 91(2):461–553. doi:10.1152/physrev.00011.2010
Lambertsen KL, Biber K, Finsen B (2012) Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab 32(9):1677–1698. doi:10.1038/jcbfm.2012.88
Shichita T, Ago T, Kamouchi M, Kitazono T, Yoshimura A, Ooboshi H (2012) Novel therapeutic strategies targeting innate immune responses and early inflammation after stroke. J Neurochem 123(Suppl 2):29–38. doi:10.1111/j.1471-4159.2012.07941.x
Ooboshi H, Ibayashi S, Iida M (2006) Gene therapy for ischemic stroke. Fukuoka Igaku Zasshi 97(4):117–122
Tarozzi A, Merlicco A, Morroni F, Bolondi C, Di Iorio P, Ciccarelli R, Romano S, Giuliani P, Hrelia P (2010) Guanosine protects human neuroblastoma cells from oxidative stress and toxicity induced by Amyloid-beta peptide oligomers. J Biol Regul Homeost Agents 24(3):297–306
Tavares RG, Schmidt AP, Tasca CI, Souza DO (2008) Quinolinic acid-induced seizures stimulate glutamate uptake into synaptic vesicles from rat brain: effects prevented by guanine-based purines. Neurochem Res 33(1):97–102. doi:10.1007/s11064-007-9421-y
Petronilho F, Perico SR, Vuolo F, Mina F, Constantino L, Comim CM, Quevedo J, Souza DO, Dal-Pizzol F (2012) Protective effects of guanosine against sepsis-induced damage in rat brain and cognitive impairment. Brain Behav Immun 26(6):904–910. doi:10.1016/j.bbi.2012.03.007
Pettifer KM, Jiang S, Bau C, Ballerini P, D'Alimonte I, Werstiuk ES, Rathbone MP (2007) MPP(+)-induced cytotoxicity in neuroblastoma cells: antagonism and reversal by guanosine. Purinergic Signal 3(4):399–409. doi:10.1007/s11302-007-9073-z
Giuliani P, Romano S, Ballerini P, Ciccarelli R, Petragnani N, Cicchitti S, Zuccarini M, Jiang S, Rathbone MP, Caciagli F, Di Iorio P (2012) Protective activity of guanosine in an in vitro model of Parkinson's disease. Panminerva Med 54(1 Suppl 4):43–51
Quincozes-Santos A, Bobermin LD, de Souza DG, Bellaver B, Goncalves CA, Souza DO (2013) Gliopreventive effects of guanosine against glucose deprivation in vitro. Purinergic Signal. doi:10.1007/s11302-013-9377-0
Schmidt AP, Bohmer AE, Schallenberger C, Antunes C, Tavares RG, Wofchuk ST, Elisabetsky E, Souza DO (2010) Mechanisms involved in the antinociception induced by systemic administration of guanosine in mice. Br J Pharmacol 159(6):1247–1263. doi:10.1111/j.1476-5381.2009.00597.x
Rathbone M, Pilutti L, Caciagli F, Jiang S (2008) Neurotrophic effects of extracellular guanosine. Nucleosides, Nucleotides Nucleic Acids 27(6):666–672. doi:10.1080/15257770802143913
Schmidt AP, Lara DR, Souza DO (2007) Proposal of a guanine-based purinergic system in the mammalian central nervous system. Pharmacol Ther 116(3):401–416. doi:10.1016/j.pharmthera.2007.07.004
Schmidt AP, Souza DO (2010) The role of the guanosine-based purinergic system in seizures and epilepsy. Open Neurosci J 4:102–113
Dal-Cim T, Martins WC, Santos AR, Tasca CI (2011) Guanosine is neuroprotective against oxygen/glucose deprivation in hippocampal slices via large conductance Ca(2)+-activated K+ channels, phosphatidilinositol-3 kinase/protein kinase B pathway activation and glutamate uptake. Neuroscience 183:212–220. doi:10.1016/j.neuroscience.2011.03.022
Thomazi AP, Boff B, Pires TD, Godinho G, Battu CE, Gottfried C, Souza DO, Salbego C, Wofchuk ST (2008) Profile of glutamate uptake and cellular viability in hippocampal slices exposed to oxygen and glucose deprivation: developmental aspects and protection by guanosine. Brain Res 1188:233–240. doi:10.1016/j.brainres.2007.10.037
Chang R, Algird A, Bau C, Rathbone MP, Jiang S (2008) Neuroprotective effects of guanosine on stroke models in vitro and in vivo. Neurosci Lett 431(2):101–105. doi:10.1016/j.neulet.2007.11.072
Oleskovicz SP, Martins WC, Leal RB, Tasca CI (2008) Mechanism of guanosine-induced neuroprotection in rat hippocampal slices submitted to oxygen-glucose deprivation. Neurochem Int 52(3):411–418. doi:10.1016/j.neuint.2007.07.017
Dal-Cim T, Ludka FK, Martins WC, Reginato C, Parada E, Egea J, Lopez MG, Tasca CI (2013) Guanosine controls inflammatory pathways to afford neuroprotection of hippocampal slices under oxygen and glucose deprivation conditions. J Neurochem 126(4):437–450. doi:10.1111/jnc.12324
Moretto MB, Boff B, Lavinsky D, Netto CA, Rocha JB, Souza DO, Wofchuk ST (2009) Importance of schedule of administration in the therapeutic efficacy of guanosine: early intervention after injury enhances glutamate uptake in model of hypoxia-ischemia. J Mol Neurosci 38(2):216–219. doi:10.1007/s12031-008-9154-7
Connell BJ, Di Iorio P, Sayeed I, Ballerini P, Saleh MC, Giuliani P, Saleh TM, Rathbone MP, Su C, Jiang S (2013) Guanosine protects against reperfusion injury in rat brains after ischemic stroke. J Neurosci Res 91(2):262–272. doi:10.1002/jnr.23156
Rathbone MP, Saleh TM, Connell BJ, Chang R, Su C, Worley B, Kim M, Jiang S (2011) Systemic administration of guanosine promotes functional and histological improvement following an ischemic stroke in rats. Brain Res 1407:79–89. doi:10.1016/j.brainres.2011.06.027
Hansel G, Ramos DB, Delgado CA, Souza DG, Almeida RF, Portela LV, Quincozes-Santos A, Souza DO (2014) The potential therapeutic effect of guanosine after cortical focal ischemia in rats. PLoS One 9(2):e90693. doi:10.1371/journal.pone.0090693
Gloire G, Legrand-Poels S, Piette J (2006) NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol 72(11):1493–1505. doi:10.1016/j.bcp.2006.04.011
Sethi G, Sung B, Aggarwal BB (2008) Nuclear factor-kappaB activation: from bench to bedside. Exp Biol Med 233(1):21–31. doi:10.3181/0707-MR-196
Szele FG, Alexander C, Chesselet MF (1995) Expression of molecules associated with neuronal plasticity in the striatum after aspiration and thermocoagulatory lesions of the cerebral cortex in adult rats. J Neurosci 15(6):4429–4448
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. Academic, Sydney
Macrae IM (2011) Preclinical stroke research—advantages and disadvantages of the most common rodent models of focal ischaemia. Br J Pharmacol 164(4):1062–1078. doi:10.1111/j.1476-5381.2011.01398.x
Schallert T (2006) Behavioral tests for preclinical intervention assessment. NeuroRx 3(4):497–504. doi:10.1016/j.nurx.2006.08.001
de Vasconcelos Dos Santos A, da Costa RJ, Diaz Paredes B, Moraes L, Jasmin G-GA, Mendez-Otero R (2010) Therapeutic window for treatment of cortical ischemia with bone marrow-derived cells in rats. Brain Res 1306:149–158. doi:10.1016/j.brainres.2009.09.094
Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR (1990) A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab 10(2):290–293. doi:10.1038/jcbfm.1990.47
Liu S, Zhen G, Meloni BP, Campbell K, Winn HR (2009) Rodent stroke model guidelines for preclinical stroke trials (1st edition). J Exp Stroke Transl Med 2(2):2–27
Gu Q, Schmued LC, Sarkar S, Paule MG, Raymick B (2012) One-step labeling of degenerative neurons in unfixed brain tissue samples using Fluoro-Jade C. J Neurosci Methods 208(1):40–43. doi:10.1016/j.jneumeth.2012.04.012
Giraldi-Guimaraes A, Rezende-Lima M, Bruno FP, Mendez-Otero R (2009) Treatment with bone marrow mononuclear cells induces functional recovery and decreases neurodegeneration after sensorimotor cortical ischemia in rats. Brain Res. doi:10.1016/j.brainres.2009.01.062
Heimfarth L, Loureiro SO, Dutra MF, Andrade C, Pettenuzzo L, Guma FT, Goncalves CA, da Rocha JB, Pessoa-Pureur R (2012) In vivo treatment with diphenyl ditelluride induces neurodegeneration in striatum of young rats: implications of MAPK and Akt pathways. Toxicol Appl Pharmacol 264(2):143–152. doi:10.1016/j.taap.2012.07.025
Moretto MB, Arteni NS, Lavinsky D, Netto CA, Rocha JB, Souza DO, Wofchuk S (2005) Hypoxic-ischemic insult decreases glutamate uptake by hippocampal slices from neonatal rats: prevention by guanosine. Exp Neurol 195(2):400–406. doi:10.1016/j.expneurol.2005.06.005
Oechmichen M, Meissner C (2006) Cerebral hypoxia and ischemia: the forensic point of view: a review. J Forensic Sci 51(4):880–887. doi:10.1111/j.1556-4029.2006.00174.x
Kaushal V, Schlichter LC (2008) Mechanisms of microglia-mediated neurotoxicity in a new model of the stroke penumbra. J Neurosci 28(9):2221–2230. doi:10.1523/JNEUROSCI. 5643-07.2008
Lakhan SE, Kirchgessner A, Hofer M (2009) Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med 7:97. doi:10.1186/1479-5876-7-97
Iadecola C, Anrather J (2011) The immunology of stroke: from mechanisms to translation. Nat Med 17(7):796–808. doi:10.1038/nm.2399
Woodruff TM, Thundyil J, Tang SC, Sobey CG, Taylor SM, Arumugam TV (2011) Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol Neurodegener 6(1):11. doi:10.1186/1750-1326-6-11
Madinier A, Bertrand N, Mossiat C, Prigent-Tessier A, Beley A, Marie C, Garnier P (2009) Microglial involvement in neuroplastic changes following focal brain ischemia in rats. PLoS One 4(12):e8101. doi:10.1371/journal.pone.0008101
Schilling M, Besselmann M, Leonhard C, Mueller M, Ringelstein EB, Kiefer R (2003) Microglial activation precedes and predominates over macrophage infiltration in transient focal cerebral ischemia: a study in green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol 183(1):25–33
Tanaka R, Komine-Kobayashi M, Mochizuki H, Yamada M, Furuya T, Migita M, Shimada T, Mizuno Y, Urabe T (2003) Migration of enhanced green fluorescent protein expressing bone marrow-derived microglia/macrophage into the mouse brain following permanent focal ischemia. Neuroscience 117(3):531–539
Schilling M, Strecker JK, Schabitz WR, Ringelstein EB, Kiefer R (2009) Effects of monocyte chemoattractant protein 1 on blood-borne cell recruitment after transient focal cerebral ischemia in mice. Neuroscience 161(3):806–812. doi:10.1016/j.neuroscience.2009.04.025
Breckwoldt MO, Chen JW, Stangenberg L, Aikawa E, Rodriguez E, Qiu S, Moskowitz MA, Weissleder R (2008) Tracking the inflammatory response in stroke in vivo by sensing the enzyme myeloperoxidase. Proc Natl Acad Sci U S A 105(47):18584–18589. doi:10.1073/pnas.0803945105
Jin R, Liu L, Zhang S, Nanda A, Li G (2013) Role of inflammation and its mediators in acute ischemic stroke. J Cardiovasc Transl Res 6(5):834–851. doi:10.1007/s12265-013-9508-6
Colton C, Wilcock DM (2010) Assessing activation states in microglia. CNS Neurol Disord: Drug Targets 9(2):174–191
Tuttolomondo A, Di Sciacca R, Di Raimondo D, Renda C, Pinto A, Licata G (2009) Inflammation as a therapeutic target in acute ischemic stroke treatment. Curr Top Med Chem 9(14):1240–1260
Nilupul Perera M, Ma HK, Arakawa S, Howells DW, Markus R, Rowe CC, Donnan GA (2006) Inflammation following stroke. J Clin Neurosci 13(1):1–8. doi:10.1016/j.jocn.2005.07.005
Rawdin BJ, Mellon SH, Dhabhar FS, Epel ES, Puterman E, Su Y, Burke HM, Reus VI, Rosser R, Hamilton SP, Nelson JC, Wolkowitz OM (2013) Dysregulated relationship of inflammation and oxidative stress in major depression. Brain Behav Immun 31:143–152. doi:10.1016/j.bbi.2012.11.011
Gomes da Silva S, Simoes PS, Mortara RA, Scorza FA, Cavalheiro EA, da Graca N-MM, Arida RM (2013) Exercise-induced hippocampal anti-inflammatory response in aged rats. J Neuroinflammation 10:61. doi:10.1186/1742-2094-10-61
Quincozes-Santos A, Bobermin LD, Souza DG, Bellaver B, Goncalves CA, Souza DO (2014) Guanosine protects C6 astroglial cells against azide-induced oxidative damage: a putative role of heme oxygenase 1. J Neurochem 130(1):61–74. doi:10.1111/jnc.12694
D'Alimonte I, Flati V, D'Auro M, Toniato E, Martinotti S, Rathbone MP, Jiang S, Ballerini P, Di Iorio P, Caciagli F, Ciccarelli R (2007) Guanosine inhibits CD40 receptor expression and function induced by cytokines and beta amyloid in mouse microglia cells. J Immunol 178(2):720–731
Madrigal JL, Garcia-Bueno B, Caso JR, Perez-Nievas BG, Leza JC (2006) Stress-induced oxidative changes in brain. CNS Neurol Disord: Drug Targets 5(5):561–568
Acknowledgments
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), Financiadora de Estudos e Projetos (FINEP), IBN.Net 01.06.0842-00, and Instituto Nacional de Ciência e Tecnologia para Excitotoxicidade e Neuroproteção (INCTEN).
Conflict of Interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hansel, G., Tonon, A.C., Guella, F.L. et al. Guanosine Protects Against Cortical Focal Ischemia. Involvement of Inflammatory Response. Mol Neurobiol 52, 1791–1803 (2015). https://doi.org/10.1007/s12035-014-8978-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8978-0