Abstract
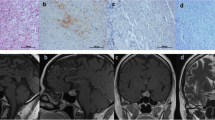
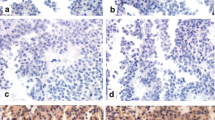
The spectrum of corticotroph cell adenomas is very wide. Though rarely, silent corticotroph cell adenomas (SCA) may transform into corticotroph cell adenomas associated with Cushing’s disease (CD). The aim of the study was to investigate the role of prohormone convertase 1/3 (PC1/3) in the transformation of SCA into CD. We reviewed the records of 1259 consecutive endoscopic endonasal procedures for pituitary adenomas from 1998 to 2013. Of these, 132 were CD and 44 were SCA. During the follow-up, three patients with SCA showed a clear transformation from SCA into CD and underwent surgery once again to remove the recurrent tumour. The PC1/3 expression was analysed by both immunohistochemistry and quantitative real time-polymerase chain reaction (qRT-PCR) in primary and recurrent tumours. The immunohistochemical PC1/3 expression was negative or weak in the three patients in the initial phase of SCA, while a strong expression was observed in the majority of neoplastic cells in tissue specimens obtained from the same three patients at the time of recurrence as CD. The immunohistochemical PC1/3 expression showed a strict correlation with the PC1/3 levels obtained by qRT-PCR. In 14 cases of SCA with no change of phenotype during the follow-up, the immunohistochemical PC1/3 expression was low and strictly associated with the level of PC1/3 obtained by qRT-PCR both in primary (14/14 cases) and in recurrent tumours (4/4 cases). Our study provides insight into the crucial role of the PC1/3 protein in the transformation of phenotype from SCA to CD.




Similar content being viewed by others
References
K. Kovacs, E. Horvath, T.A. Bayley, S.T. Hassaram, C. Ezrin, Silent corticotroph cell adenoma with lysosomal accumulation and crinophagy. A distinct clinicopathologic entity. Am. J. Med. 64(3), 492–499 (1978). doi:10.0002/93437890236-X
E. Horvath, K. Kovacs, D.W. Killinger, H.S. Smyth, M.E. Platts, W. Singer, Silent corticotropic adenomas of the human pituitary gland: a histologic, immunocytologic, and ultrastructural study. Am. J. Pathol. 98(3), 617–638 (1980)
B.W. Scheithauer, A.J. Jaap, E. Horvath, K. Kovacs, R.V. Lloyd, F.B. Meyer, E.R. Laws Jr., W.F. Young Jr., Clinically silent corticotroph tumors of the pituitary gland. Neurosurgery 47(3), 723–729 (2000). discussion 729–730
G. Raverot, A. Wierinckx, E. Jouanneau, C. Auger, F. Borson-Chazot, J. Lachuer, M. Pugeat, J. Trouillas, Clinical, hormonal and molecular characterization of pituitary ACTH adenomas without (silent corticotroph adenomas) and with Cushing’s disease. Eur. J. Endocrinol. 163(1), 35–43 (2010). doi:10.1530/EJE-10-0076
E. Melcescu, A.W. Gannon, A.D. Parent, J.F. Fratkin, W.C. Nicholas, C.A. Koch, A. Galhom, Silent or subclinical corticotroph pituitary macroadenoma transforming into cushing disease: 11-year follow-up. Neurosurgery 72(1), E144–E146 (2013). doi:10.1227/NEU.0b013e3182750850
E. Jouanneau, A. Wierinckx, F. Ducray, V. Favrel, F. Borson-Chazot, J. Honnorat, J. Trouillas, G. Raverot, New targeted therapies in pituitary carcinoma resistant to temozolomide. Pituitary 15(1), 37–43 (2012). doi:10.1007/s11102-011-0341-0
T. Psaras, J. Honegger, R. Buslei, W. Saeger, D. Klein, D. Capper, R. Meyermann, M. Mittelbronn, Atypical type II silent corticotrophic adenoma developing into Cushing’s disease upon second recurrence. Exp. Clin. Endocrinol. Diabetes 115(9), 610–615 (2007). doi:10.1055/s-2007-984437
L.R. Salgado, M.C. Machado, A. Cukiert, B. Liberman, C.T. Kanamura, V.A. Alves, Cushing’s disease arising from a clinically nonfunctioning pituitary adenoma. Endocr. Pathol. 17(2), 191–199 (2006). doi:10.1385/EP:17:2:191
S.E. Baldeweg, J.R. Pollock, M. Powell, J. Ahlquist, A spectrum of behaviour in silent corticotroph pituitary adenomas. Br. J. Neurosurg. 19(1), 38–42 (2005). doi:10.1080/02688690500081230
T. Sano, K. Kovacs, S.L. Asa, S. Yamada, N. Sanno, S. Yokoyama, H. Takami, Pituitary adenoma with ‘honeycomb Golgi’ appearance showing a phenotypic change at recurrence from clinically nonfunctioning to typical Cushing disease. Endocr. Pathol. 13(2), 125–130 (2002). doi:10.1385/EP:13:2:125
R.G. Gheri, W. Boddi, F. Ammannati, J. Olivotto, C. Nozzoli, A. Franchi, L. Bordi, M.L. Luisi, P. Mennonna, Two-step development of a pituitary adenoma: from hyperprolactinemic syndrome to Cushing’s disease. J. Endocrinol. Invest. 20(4), 240–244 (1997)
T. Mindermann, K. Kovacs, C.B. Wilson, Changes in the immunophenotype of recurrent pituitary adenomas. Neurosurgery 35(1), 39–44 (1994)
R.A. Bonner, K. Mukai, J.H. Oppenheimer, Two unusual variants of Nelson’s syndrome. J. Clin. Endocrinol. Metab. 49(1), 23–29 (1979). doi:10.1210/jcem-49-1-23
S. Ohta, S. Nishizawa, Y. Oki, T. Yokoyama, H. Namba, Significance of absent prohormone convertase 1/3 in inducing clinically silent corticotroph pituitary adenoma of subtype I—immunohistochemical study. Pituitary 5(4), 221–223 (2002)
V. Hook, L. Funkelstein, T. Toneff, C. Mosier, S.R. Hwang, Human pituitary contains dual cathepsin L and prohormone convertase processing pathway components involved in converting POMC into the peptide hormones ACTH, alpha-MSH, and beta-endorphin. Endocrine 35(3), 429–437 (2009). doi:10.1007/s12020-009-9163-5
A.W. Artenstein, S.M. Opal, Proprotein convertases in health and disease. N. Engl. J. Med. 365(26), 2507–2518 (2011). doi:10.1056/NEJMra1106700
T. Tateno, H. Izumiyama, M. Doi, T. Yoshimoto, M. Shichiri, N. Inoshita, K. Oyama, S. Yamada, Y. Hirata, Differential gene expression in ACTH-secreting and non-functioning pituitary tumors. Eur. J. Endocrinol. 157(6), 717–724 (2007). doi:10.1530/EJE-07-0428
I. Takumi, D.F. Steiner, N. Sanno, A. Teramoto, R.Y. Osamura, Localization of prohormone convertases 1/3 and 2 in the human pituitary gland and pituitary adenomas: analysis by immunohistochemistry, immunoelectron microscopy, and laser scanning microscopy. Mod. Pathol. 11(3), 232–238 (1998)
R.V. Lloyd, L. Jin, X. Qian, B.W. Scheithauer, W.F. Young Jr., D.H. Davis, Analysis of the chromogranin A post-translational cleavage product pancreastatin and the prohormone convertases PC2 and PC3 in normal and neoplastic human pituitaries. Am. J. Pathol. 146(5), 1188–1198 (1995)
L. Scopsi, M. Gullo, F. Rilke, S. Martin, D.F. Steiner, Proprotein convertases (PC1/PC3 and PC2) in normal and neoplastic human tissues: their use as markers of neuroendocrine differentiation. J. Clin. Endocrinol. Metab 80(1), 294–301 (1995). doi:10.1210/jcem.80.1.7829629
A. Righi, L. Morandi, E. Leonardi, A. Farnedi, G. Marucci, A. Sisto, G. Frank, M. Faustini-Fustini, M. Zoli, D. Mazzatenta, R. Agati, M.P. Foschini, Galectin-3 expression in pituitary adenomas as a marker of aggressive behavior. Hum. Pathol. 44(11), 2400–2409 (2013). doi:10.1016/j.humpath.2013.05.020
A. Righi, L. Jin, S. Zhang, G. Stilling, B.W. Scheithauer, K. Kovacs, R.V. Lloyd, Identification and consequences of galectin-3 expression in pituitary tumors. Mol. Cell. Endocrinol. 326(1-2), 8–14 (2010). doi:10.1016/j.mce.2010.04.026
O. Mete, S.L. Asa, Clinicopathological correlations in pituitary adenomas. Brain Pathol. 22(4), 443–453 (2012). doi:10.1111/j.1750-3639.2012.00599.x
O. Mete, S.L. Asa, Therapeutic implications of accurate classification of pituitary adenomas. Semin. Diagn. Pathol. 30(3), 158–164 (2013). doi:10.1053/j.semdp.2013.06.002
H. Nishioka, N. Inoshita, O. Mete, S.L. Asa, K. Hayashi, A. Takeshita, N. Fukuhara, M. Yamaguchi-Okada, Y. Takeuchi, S. Yamada, The complementary role of transcription factors in the accurate diagnosis of clinically nonfunctioning pituitary adenomas. Endocr. Pathol. 26(4), 349–355 (2015). doi:10.1007/s12022-015-9398-z
H. Alahmadi, D. Lee, J.R. Wilson, C. Hayhurst, O. Mete, F. Gentili, S.L. Asa, G. Zadeh, Clinical features of silent corticotroph adenomas. Acta. Neurochir. (Wien.) 154(8), 1493–1498 (2012). doi:10.1007/s00701-012-1378-1
M. Zoli, M. Faustini-Fustini, D. Mazzatenta, G. Marucci, E. De Carlo, A. Bacci, E. Pasquini, G. Lanzino, G. Frank, ACTH adenomas transforming their clinical expression: report of 5 cases. Neurosurg. Focus 38(2), E15 (2015). doi:10.3171/2014.11.FOCUS14679
G. Mustacchi, M.P. Sormani, P. Bruzzi, A. Gennari, F. Zanconati, D. Bonifacio, A. Monzoni, L. Morandi, Identification and validation of a new set of five genes for prediction of risk in early breast cancer. Int. J. Mol. Sci. 14(5), 9686–9702 (2013). doi:10.3390/ijms14059686
K.J. Livak, T.D. Schmittgen, Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4), 402–408 (2001). doi:10.1006/meth.2001.1262
S. Gibson, D.W. Ray, S.R. Crosby, T.L. Dornan, A.M. Jennings, J.S. Bevan, J.R. Davis, A. White, Impaired processing of proopiomelanocortin in corticotroph macroadenomas. J. Clin. Endocrinol. Metab. 81(2), 497–502 (1996). doi:10.1210/jcem.81.2.8636257
M. Reincke, B. Allolio, W. Saeger, D. Kaulen, W. Winkelmann, A pituitary adenoma secreting high molecular weight adrenocorticotropin without evidence of Cushing’s disease. J. Clin. Endocrinol. Metab. 65(6), 1296–1300 (1987). doi:10.1210/jcem-65-6-1296
Lila, A.R., Sarathi, V., Bandgar, T.R., Shah, N.S.: Paradoxical response to dexamethasone and spontaneous hypocortisolism in Cushing’s disease. BMJ Case Rep. 2013 (2013). doi:10.1136/bcr-2012-008035
R.D. Brown, G.R. Van Loon, D.N. Orth, G.W. Liddle, Cushing’s disease with periodic hormonogenesis: one explanation for paradoxical response to dexamethasone. J. Clin. Endocrinol. Metab. 36(3), 445–451 (1973). doi:10.1210/jcem-36-3-445
V. Popovic, D. Micic, M. Nesovic, T. Howlett, I. Doniach, A. Kendereski, P. Djordjevic, D. Manojlovic, J. Micic, M. Besser, Cushing’s disease cycling over ten years. Exp. Clin. Endocrinol. 96(2), 143–148 (1990). doi:10.1055/s-0029-1211003
T. Miyoshi, F. Otsuka, J. Suzuki, K. Inagaki, M. Takeda, Y. Kano, T. Yamashita, T. Ogura, I. Date, Y. Tanaka, K. Hashimoto, H. Makino, Periodic secretion of adrenocorticotropin in a patient with Cushing’s disease manifested during pregnancy. Endocr. J. 52(3), 287–292 (2005). doi:10.1507/endocrj.52.287
S. Asano, H. Ooka, R. Okazaki, T. Ishikawa, H. Ochiai, M. Nakashima, F. Ide, I. Hasegawa, S. Miyawaki, H. Nakaguchi, M. Murakami, Y. Ogino, K. Takano, A. Matsuno, Long-term remission of cyclic Cushing’s disease that was diagnosed and treated surgically in non-active phase. Endocr. J. 54(3), 407–412 (2007). doi:JST.JSTAGE/endocrj/K06-218
V. Bonert, N. Bose, J.D. Carmichael, Cyclic Cushing’s disease with misleading inferior petrosal sinus sampling results during a trough phase. Neurosurg. Focus 38(2), E7 (2015). doi:10.3171/2014.12.FOCUS14780
K.I. Alexandraki, G.A. Kaltsas, A.M. Isidori, S.A. Akker, W.M. Drake, S.L. Chew, J.P. Monson, G.M. Besser, A.B. Grossman, The prevalence and characteristic features of cyclicity and variability in Cushing’s disease. Eur. J. Endocrinol. 160(6), 1011–1018 (2009). doi:10.1530/EJE-09-0046
N.J. Vaughan, C.M. Laroche, I. Goodman, M.J. Davies, J.S. Jenkins, Pituitary Cushing’s disease arising from a previously non-functional corticotrophic chromophobe adenoma. Clin. Endocrinol. (Oxf.) 22(2), 147–153 (1985)
Y. Kojima, S. Suzuki, K. Yamamura, G. Ohhashi, I. Yamamoto, Comparison of ACTH secretion in Cushing’s adenoma and clinically silent corticotroph adenoma by cell immunoblot assay. Endocr. J. 49(3), 285–292 (2002)
A. Matsuno, R. Okazaki, Y. Oki, T. Nagashima, Secretion of high-molecular-weight adrenocorticotropic hormone from a pituitary adenoma in a patient without Cushing stigmata. J. Neurosurg. 101(5), 874–877 (2004). doi:10.3171/jns.2004.101.5.0874 Case report
M.E. Cooper, R.M. Murray, R. Kalnins, J. Woodward, G. Jerums, The development of Cushing’s syndrome from a previously silent pituitary tumour. Aust. N.Z. J. Med. 17(2), 249–251 (1987)
A.G. Lania, S. Ferrero, R. Pivonello, G. Mantovani, E. Peverelli, A. Di Sarno, P. Beck-Peccoz, A. Spada, A. Colao, Evolution of an aggressive prolactinoma into a growth hormone secreting pituitary tumor coincident with GNAS gene mutation. J. Clin. Endocrinol. Metab. 95(1), 13–17 (2010). doi:10.1210/jc.2009-1360
J. Hardy, M. Somma (eds.) Acromegaly. Surgical Treatment by Transsphenoidal Microsurgical Removal of the Pituitary Adenoma. (Raven Press, New York), 1979) 209–217
C.B. Wilson, A decade of pituitary microsurgery. The Herbert Olivecrona lecture. J. Neurosurg. 61(5), 814–833 (1984). doi:10.3171/jns.1984.61.5.0814
DeLellis R.A., Lloyd R.V., Heitz P.U., Eng C. (eds.) World Health Organization Classification of Tumors: Pathology and Genetics: Tumors of Endocrine Organs. (2004)Please provide name of publisher and place of publication in DeLellis et al. (2004).
Acknowledgments
The authors thank the following members of the team for their contributions to patient care and data collection: Dr. Giorgio Frank for his skilful contribution as project manager of the Centre of Pituitary and Endoscopic Skullbase Surgery, IRCCS Institute of Neurological Sciences of Bologna (ISNB); Dr. Carmelo Sturiale, chief of the Neurosurgery Unit, IRCCS Institute of Neurological Sciences of Bologna (ISNB).
Funding
This research was supported with grants from Department of Biomedical and Neuromotor Sciences (DIBINEM) of the University of Bologna (Fund for Fundamentally Oriented Research).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Alberto Righi and Marco Faustini-Fustini contributed equally to the study.
Rights and permissions
About this article
Cite this article
Righi, A., Faustini-Fustini, M., Morandi, L. et al. The changing faces of corticotroph cell adenomas: the role of prohormone convertase 1/3. Endocrine 56, 286–297 (2017). https://doi.org/10.1007/s12020-016-1028-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-016-1028-0