Abstract
Purpose
Assess cyclin A in nonfunctioning pituitary adenomas (NFPA) and compare its expression in non-invasive and non-proliferative tumors with invasive and proliferative tumors (12× higher risk of recurrence).
Methods
Quantitative real time polymerase chain reaction to analyze cyclin A using normal pituitary gland as reference. Fold change (FC) > 1 was considered as increased. Tumor invasion was based on Knosp criteria (grades 3–4 considered invasive) and proliferation on the presence of at least two of three criteria: Ki-67 ≥ 3%; mitoses > 2/10; positive p53. Both groups were compared with Mann–Whitney test considering p value < 0.05 as statistically significant.
Results
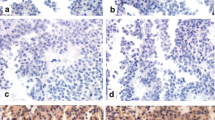
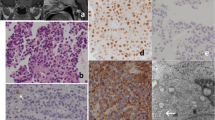
Thirty-one patients with NFPA were included. Tumors were mainly of gonadotrophic origin (74.2%), followed by corticotrophic (19.4%) and lactotrophic (3.2%) origins and null-cell adenomas (3.2%). Median tumor diameter was 3.5 cm (1.8–8.0) and Ki-67 was 3.0% (0.3–11%). Sixteen patients had tumors classified as non-invasive and non-proliferative and 15 as invasive and proliferative. Median FC was 0.31 in all tumors (0.13–1.94). Cyclin A was not related to invasion or proliferation (FC 0.41 in non-invasive and non-proliferative tumors and FC 0.30 in invasive and proliferative tumors; p = 0.968). Four (12.9%) patients had tumors that exhibited increased cyclin A [median FC of 1.04 (1.02–1.94)]—three of gonadotrophic origin and one null-cell adenoma, with two tumors classified as non-invasive and non-proliferative and two tumors classified as invasive and proliferative. Median tumor diameter in these samples was 3.4 cm (2.4–3.6) and Ki-67 was 5.1% (2–11%).
Conclusions
Cyclin A was increased in a minority of NFPA and does not seem to be related to invasion or proliferation.


Similar content being viewed by others
References
M.B.S. Lopes, The 2017 World Health Organization classification of tumors of the pituitary gland: a summary. Acta Neuropathol. 134(4), 521–535 (2017). https://doi.org/10.1007/s00401-017-1769-8
S. Melmed, Pituitary tumors. Endocrinol. Metab. Clin. N. Am. 44(1), 1–9 (2015). https://doi.org/10.1016/j.ecl.2014.11.004
Y. Greenman, N. Stern, Non-functioning pituitary adenomas. Best Pract. Res. Clin. Endocrinol. Metab. 23(5), 625–638 (2009). https://doi.org/10.1016/j.beem.2009.05.005
H. Buurman, W. Saeger, Subclinical adenomas in postmortem pituitaries: classification and correlations to clinical data. Eur. J. Endocrinol. 154(5), 753–758 (2006). https://doi.org/10.1530/eje.1.02107
W. Saeger, D.K. Ludecke, M. Buchfelder, R. Fahlbusch, H.J. Quabbe, S. Petersenn, Pathohistological classification of pituitary tumors: 10 years of experience with the German Pituitary Tumor Registry. Eur. J. Endocrinol. 156(2), 203–216 (2007). https://doi.org/10.1530/eje.1.02326
R.V. Lloyd, O.R., G. Klöppel, J. Rosai. WHO Classification of Tumours of Endocrine Organs, 4th edn. (IARC Press, Lyon, 2017).
J.W. Tomlinson, N. Holden, R.K. Hills, K. Wheatley, R.N. Clayton, A.S. Bates, M.C. Sheppard, P.M. Stewart, Association between premature mortality and hypopituitarism. West Midlands Prospective Hypopituitary Study Group. Lancet 357(9254), 425–431 (2001)
D.S. Olsson, B.A. Bengtsson, Hypopituitarism-needs modern individualized treatment. Endocrine 56(1), 1–3 (2017). https://doi.org/10.1007/s12020-016-1211-3
J.R. Anderson, N. Antoun, N. Burnet, K. Chatterjee, O. Edwards, J.D. Pickard, N. Sarkies, Neurology of the pituitary gland. J. Neurol. Neurosurg. Psychiatry 66(6), 703–721 (1999)
L.E. Wildemberg, A. Glezer, M.D. Bronstein, M.R. Gadelha, Apoplexy in nonfunctioning pituitary adenomas. Pituitary 21(2), 138–144 (2018). https://doi.org/10.1007/s11102-018-0870-x
L.N. Vieira, C.L. Boguszewski, L.A. Araujo, M.D. Bronstein, P.A. Miranda, N.R. Musolino, L.A. Naves, L. Vilar, A.J. Ribeiro-Oliveira, M.R. Gadelha, A review on the diagnosis and treatment of patients with clinically nonfunctioning pituitary adenoma by the Neuroendocrinology Department of the Brazilian Society of Endocrinology and Metabolism. Arch. Endocrinol. Metab. 60(4), 374–390 (2016). https://doi.org/10.1590/2359-3997000000179
K. Boelaert, N.J. Gittoes, Radiotherapy for non-functioning pituitary adenomas. Eur. J. Endocrinol. 144(6), 569–575 (2001)
Y. Chen, C.D. Wang, Z.P. Su, Y.X. Chen, L. Cai, Q.C. Zhuge, Z.B. Wu, Natural history of postoperative nonfunctioning pituitary adenomas: a systematic review and meta-analysis. Neuroendocrinology 96(4), 333–342 (2012). https://doi.org/10.1159/000339823
B.W. Scheithauer, K.T. Kovacs, E.R. Laws Jr, R.V. Randall, Pathology of invasive pituitary tumors with special reference to functional classification. J. Neurosurg. 65(6), 733–744 (1986). https://doi.org/10.3171/jns.1986.65.6.0733
L. Kasuki, G. Raverot. Definition and diagnosis of aggressive pituitary tumors. Rev. Endocr. Metab. Disord. (2019). https://doi.org/10.1007/s11154-019-09531-x
M.R. Gadelha, G. Trivellin, L.C. Hernandez Ramirez, M. Korbonits, Genetics of pituitary adenomas. Front. Horm. Res. 41, 111–140 (2013). https://doi.org/10.1159/000345673
R. Yu, S. Melmed, Pathogenesis of pituitary tumors. Prog. Brain Res. 182, 207–227 (2010). https://doi.org/10.1016/S0079-6123(10)82009-6
S. Melmed, Pathogenesis of pituitary tumors. Nat. Rev. Endocrinol. 7(5), 257–266 (2011). https://doi.org/10.1038/nrendo.2011.40
V. Quereda, M. Malumbres, Cell cycle control of pituitary development and disease. J. Mol. Endocrinol. 42(2), 75–86 (2009). https://doi.org/10.1677/JME-08-0146
C. Desdouets, G. Matesic, C.A. Molina, N.S. Foulkes, P. Sassone-Corsi, C. Brechot, J. Sobczak-Thepot, Cell cycle regulation of cyclin A gene expression by the cyclic AMP-responsive transcription factors CREB and CREM. Mol. Cell. Biol. 15(6), 3301–3309 (1995). https://doi.org/10.1128/mcb.15.6.3301
A. Loukil, C.T. Cheung, N. Bendris, B. Lemmers, M. Peter, J.M. Blanchard, Cyclin A2: at the crossroads of cell cycle and cell invasion. World J. Biol. Chem. 6(4), 346–350 (2015). https://doi.org/10.4331/wjbc.v6.i4.346
C. Miao, Z. Wang, J. Yang, J. Li, X. Gao, Expression and mutation analysis of Cyclin A and Ki-67 in glioma and their correlation with tumor progression. Oncol. Lett. 10(3), 1716–1720 (2015). https://doi.org/10.3892/ol.2015.3474
C.H. Yam, T.K. Fung, R.Y. Poon, Cyclin A in cell cycle control and cancer. Cell. Mol. Life Sci. 59(8), 1317–1326 (2002). https://doi.org/10.1007/s00018-002-8510-y
S. Diederichs, N. Baumer, P. Ji, S.K. Metzelder, G.E. Idos, T. Cauvet, W. Wang, M. Moller, S. Pierschalski, J. Gromoll, M.G. Schrader, H.P. Koeffler, W.E. Berdel, H. Serve, C. Muller-Tidow, Identification of interaction partners and substrates of the cyclin A1-CDK2 complex. J. Biol. Chem. 279(32), 33727–33741 (2004). https://doi.org/10.1074/jbc.M401708200
H.E. Turner, Z. Nagy, N. Sullivan, M.M. Esiri, J.A. Wass, Expression analysis of cyclins in pituitary adenomas and the normal pituitary gland. Clin. Endocrinol. 53(3), 337–344 (2000)
B. Henglein, X. Chenivesse, J. Wang, D. Eick, C. Brechot, Structure and cell cycle-regulated transcription of the human cyclin A gene. Proc. Natl Acad. Sci. USA 91(12), 5490–5494 (1994)
A.R. Rosenberg, F. Zindy, F. Le Deist, H. Mouly, P. Metezeau, C. Brechot, E. Lamas, Overexpression of human cyclin A advances entry into S phase. Oncogene 10(8), 1501–1509 (1995)
K. Allan, R.C. Jordan, L.C. Ang, M. Taylor, B. Young, Overexpression of cyclin A and cyclin B1 proteins in astrocytomas. Arch. Pathol. Lab. Med. 124(2), 216–220 (2000). https://doi.org/10.1043/0003-9985(2000)1242.0.CO;2
M. Furihata, T. Ishikawa, A. Inoue, C. Yoshikawa, H. Sonobe, Y. Ohtsuki, K. Araki, S. Ogoshi, Determination of the prognostic significance of unscheduled cyclin A overexpression in patients with esophageal squamous cell carcinoma. Clin. Cancer Res. 2(10), 1781–1785 (1996)
R.D. Mashal, S. Lester, C. Corless, J.P. Richie, R. Chandra, K.J. Propert, A. Dutta, Expression of cell cycle-regulated proteins in prostate cancer. Cancer Res. 56(18), 4159–4163 (1996)
J. Kushner, G. Bradley, B. Young, R.C. Jordan, Aberrant expression of cyclin A and cyclin B1 proteins in oral carcinoma. J. Oral Pathol. Med. 28(2), 77–81 (1999). https://doi.org/10.1111/j.1600-0714.1999.tb02000.x
J. Trouillas, P. Roy, N. Sturm, E. Dantony, C. Cortet-Rudelli, G. Viennet, J.F. Bonneville, R. Assaker, C. Auger, T. Brue, A. Cornelius, H. Dufour, E. Jouanneau, P. Francois, F. Galland, F. Mougel, F. Chapuis, L. Villeneuve, C.A. Maurage, D. Figarella-Branger, G. Raverot, A. Barlier, M. Bernier, F. Bonnet, F. Borson-Chazot, G. Brassier, S. Caulet-Maugendre, O. Chabre, P. Chanson, J.F. Cottier, B. Delemer, E. Delgrange, L. Di Tommaso, S. Eimer, S. Gaillard, M. Jan, J.J. Girard, V. Lapras, H. Loiseau, J.G. Passagia, M. Patey, A. Penfornis, J.Y. Poirier, G. Perrin, A. Tabarin,Members of HYPOPRONOS, A new prognostic clinicopathological classification of pituitary adenomas: a multicentric case-control study of 410 patients with 8 years post-operative follow-up. Acta Neuropathol. 126(1), 123–135 (2013). https://doi.org/10.1007/s00401-013-1084-y
R. Zahr, M. Fleseriu, Updates in diagnosis and treatment of acromegaly. Eur. Endocrinol. 14(2), 57–61 (2018). https://doi.org/10.17925/EE.2018.14.2.57
L.K. Nieman, B.M. Biller, J.W. Findling, J. Newell-Price, M.O. Savage, P.M. Stewart, V.M. Montori, The diagnosis of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 93(5), 1526–1540 (2008). https://doi.org/10.1210/jc.2008-0125
S. Melmed, F.F. Casanueva, A.R. Hoffman, D.L. Kleinberg, V.M. Montori, J.A. Schlechte, J.A. Wass, S. Endocrine, Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 96(2), 273–288 (2011). https://doi.org/10.1210/jc.2010-1692
E. Knosp, E. Steiner, K. Kitz, C. Matula, Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery 33(4), 610–617 (1993). https://doi.org/10.1227/00006123-199310000-00008. discussion 617-618
P. Lundin, F. Pedersen, Volume of pituitary macroadenomas: assessment by MRI. J. Comput. Assist. Tomogr. 16(4), 519–528 (1992). https://doi.org/10.1097/00004728-199207000-00004
H. Nishioka, N. Inoshita, O. Mete, S.L. Asa, K. Hayashi, A. Takeshita, N. Fukuhara, M. Yamaguchi-Okada, Y. Takeuchi, S. Yamada, The complementary role of transcription factors in the accurate diagnosis of clinically nonfunctioning pituitary adenomas. Endocr. Pathol. 26(4), 349–355 (2015). https://doi.org/10.1007/s12022-015-9398-z
M.E. Torregrosa-Quesada, A. Garcia-Martinez, S. Silva-Ortega, S. Martinez-Lopez, R. Camara, C. Fajardo, C. Lamas, I. Aranda, A. Pico. How valuable is the RT-qPCR of pituitary-specific transcription factors for identifying pituitary neuroendocrine tumor subtypes according to the new WHO 2017 criteria? Cancers 11(12), (2019). https://doi.org/10.3390/cancers11121990
A. de Almeida Verdolin, E.B. Lamback, N. Ventura, A. Guasti, P.J. da Mata Pereira, M.R. Gadelha, L. Chimelli. Collision sellar lesions: coexistence of pituitary adenoma and Rathke cleft cyst-a single-center experience. Endocrine (2019). https://doi.org/10.1007/s12020-019-02149-8
K.R. Normann, K.A.B. Oystese, J.P. Berg, T. Lekva, J. Berg-Johnsen, J. Bollerslev, N.C. Olarescu, Selection and validation of reliable reference genes for RT-qPCR analysis in a large cohort of pituitary adenomas. Mol. Cell. Endocrinol. 437, 183–189 (2016). https://doi.org/10.1016/j.mce.2016.08.030
K.J. Livak, T.D. Schmittgen, Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4), 402–408 (2001). https://doi.org/10.1006/meth.2001.1262
M. Musat, V.V. Vax, N. Borboli, M. Gueorguiev, S. Bonner, M. Korbonits, A.B. Grossman, Cell cycle dysregulation in pituitary oncogenesis. Front. Horm. Res. 32, 34–62 (2004). https://doi.org/10.1159/000079037
H. Nakabayashi, I. Sunada, M. Hara, Immunohistochemical analyses of cell cycle-related proteins, apoptosis, and proliferation in pituitary adenomas. J. Histochem. Cytochem. 49(9), 1193–1194 (2001). https://doi.org/10.1177/002215540104900916
G. O’Hurley, E. Sjostedt, A. Rahman, B. Li, C. Kampf, F. Ponten, W.M. Gallagher, C. Lindskog, Garbage in, garbage out: a critical evaluation of strategies used for validation of immunohistochemical biomarkers. Mol. Oncol. 8(4), 783–798 (2014). https://doi.org/10.1016/j.molonc.2014.03.008
H.P. Sinn, A. Schneeweiss, M. Keller, K. Schlombs, M. Laible, J. Seitz, S. Lakis, E. Veltrup, P. Altevogt, S. Eidt, R.M. Wirtz, F. Marme, Comparison of immunohistochemistry with PCR for assessment of ER, PR, and Ki-67 and prediction of pathological complete response in breast cancer. BMC Cancer 17(1), 124 (2017). https://doi.org/10.1186/s12885-017-3111-1
C. Ramirez, S. Cheng, G. Vargas, S.L. Asa, S. Ezzat, B. Gonzalez, L. Cabrera, G. Guinto, M. Mercado, Expression of Ki-67, PTTG1, FGFR4, and SSTR 2, 3, and 5 in nonfunctioning pituitary adenomas: a high throughput TMA, immunohistochemical study. J. Clin. Endocrinol. Metab. 97(5), 1745–1751 (2012). https://doi.org/10.1210/jc.2011-3163
I. Kalaszczynska, Y. Geng, T. Iino, S. Mizuno, Y. Choi, I. Kondratiuk, D.P. Silver, D.J. Wolgemuth, K. Akashi, P. Sicinski, Cyclin A is redundant in fibroblasts but essential in hematopoietic and embryonic stem cells. Cell 138(2), 352–365 (2009). https://doi.org/10.1016/j.cell.2009.04.062
F. Traganos, Cycling without cyclins. Cell Cycle 3(1), 32–34 (2004)
S. Jordan, K. Lidhar, M. Korbonits, D.G. Lowe, A.B. Grossman, D. Cyclin, and cyclin E expression in normal and adenomatous pituitary. Eur. J. Endocrinol. 143(1), R1–R6 (2000). https://doi.org/10.1530/eje.0.143r001
N.A. Hibberts, D.J. Simpson, J.E. Bicknell, J.C. Broome, P.R. Hoban, R.N. Clayton, W.E. Farrell, Analysis of cyclin D1 (CCND1) allelic imbalance and overexpression in sporadic human pituitary tumors. Clin. Cancer Res. 5(8), 2133–2139 (1999)
Acknowledgements
We would like to thank Diego de Araújo Santos and Heliomar Pereira Marcos for preparing the histopathological sections.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was approved by the Instituto Estadual do Cérebro Paulo Niemeyer Research and Ethics Committee (Certificate of Ethical Approval 24084719.0.0000.8110).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lamback, E.B., Guterres, A., Barbosa, M.A. et al. Cyclin A in nonfunctioning pituitary adenomas. Endocrine 70, 380–387 (2020). https://doi.org/10.1007/s12020-020-02402-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-020-02402-5