Abstract
Clinically nonfunctioning pituitary adenomas (NFAs) may be hormonally inactive tumors of differentiated cells, mainly not only gonadotroph adenomas (GAs) but also silent corticotroph adenomas (SCAs) and other differentiated silent adenomas. Recently, the use of transcription factors has been recommended to confirm cytodiffererentiation of these neoplasms. Our objective was to assess the clinical significance of the new classification system using transcription factors. Five hundred sixteen consecutive NFAs were studied retrospectively. They were initially classified based on hormone immunohistochemistry as follows: 119 hormone-negative adenomas (23.1 %), 300 GAs (58.1 %), 51 SCAs (9.9 %), and 46 other silent adenomas. The 119 hormone-negative adenomas were further evaluated for expression of transcription factors including steroidogenic factor-1 (SF-1), estrogen receptor-α (ERα), pituitary-specific transcription factor 1 (Pit-1), and t-box transcription factor (Tpit). One hundred thirteen of 119 (95 %) hormone-negative adenomas showed mutually exclusive lineage-specific differentiation as gonadotrophs (SF-1 positive), corticotrophs (Tpit positive), or somatotrophs/mammosomatotrophs/lactotrophs/thyrotrophs (Pit-1 positive) in 79 cases (66.4 %), 32 cases (26.9 %), and 2 cases, respectively. The 32 ACTH-negative and Tpit-positive adenomas had higher pro-opiomelanocortin mRNA expression levels compared with GAs (P = 0.0001) on quantitative real-time PCR. They showed a female preponderance (P < 0.0001) and were more frequently giant adenomas (P = 0.0028) associated with marked cavernous sinus invasion (P < 0.0001) compared with GAs. These clinical features were identical to those of the 51 ACTH-positive SCAs. Our results justify the complementary role of transcription factors in the precise classification of NFAs that can more accurately characterize biological behavior. Our data suggest that more than one quarter of hormone-negative adenomas are SCAs that share distinct clinicopathological features with ACTH-expressing SCAs.



Similar content being viewed by others
References
Asa SL (2011) Tumors of the Pituitary Gland. Fascicle 15, 4th series. The Atlas of Tumor Pathology. Armed Forces Institute of Pathology, Washington DC
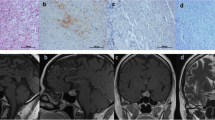
Nishioka H, Inoshita N, Sano T, Fukuhara N, Yamada S (2012) Correlation between histological subtypes and MRI findings in clinically nonfunctioning pituitary adenomas. Endocr Pathol 23:151-6
Cooper O, Ben-Shlomo A, Bonert V, Bannykh S, Mirocha J, Melmed S (2010) Silent corticotroph adenomas: clinical and cellular characteristics and long-term outcomes. Horm Canc 1: 80-92
Horvath E, Kovacs K (1992) Ultrastructural diagnosis of human pituitary adenomas. Microsc Res Tech 20: 107-135
Jameson JL, Klibanski A, Black PM, Zervas NT, Lindell CM, Hsu DW, Ridgway EC, Habener JF (1987) Glycoprotein hormone genes are expressed in clinically nonfunctioning pituitary adenomas. J Clin Invest 80: 1472-78
Kovacs K, Asa SL, Horvath E, Ryan N, Singer W, Killinger DW, Smyth HS, Scheithauer BW, Ebersold MJ (1990) Null cell adenomas of the pituitary: Attempts to resolve their cytogenesis. In: J Lechago, T Kameya (eds) Endocrine Pathology Update. Field and Wood, Philadelphia, pp 17-31
Yamada S, Asa SL, Kovacs K, Muller P, Smyth HS (1989) Analysis of hormone secretion by clinically nonfunctioning human pituitary adenomas using the reverse hemolytic plaque assay. J Clin Endocrinol Metab 68: 73-80
Al-Brahim NYY, Asa SL (2006) My approach to pathology of the pituitary gland. J Clin Pathol 59: 1245-53
Asa SL (2008) Practical pituitary pathology. What does the pathologists need to know? Arch Pathol Lab Med 132:1231-49
Mete O, Asa SL (2012) Clinicopathological correlations in pituitary adenomas. Brain Pathol 22: 443-453
Kovacs K, Horvath E, Bayley TA, Hassaram ST, Ezrin C (1978) Silent corticotroph cell adenoma with lysosomal accumulation and crinophagy. A distinct clinicopathologic entity. Am J Med 64: 492-9
Baldeweg SE, Pollock JR, Powell M, Ahlquist J (2005) A spectrum of silent corticotroph pituitary adenomas. Br J Neurosurg 19: 38-42
Raverot G, Wierinckx A, Jouanneau E, Auger C, Borson-Chazot F, Lachuer J, Pugeat M, Trouillas J (2010) Clinical, hormonal, and molecular characterization of pituitary adenomas without (silent corticotroph adenomas) and with Cushing’s disease. Eur J Endocrinol 163: 35-43
Sahli R, Christ ER, Seiler R, Kappeler A, Vajtai I (2006) Clinicopathologic correlations of silent corticotroph adenomas of the pituitary: report of four cases and literature review. Pathol Res Pract 202: 457-464
Scheithauer BW, Jaap AJ, Horvath E, Kovacs K, Lloyd RV, Meyer FB, Laws Jr ER, Young Jr WF (2000) Clinically silent corticotroph tumors of the pituitary gland. Neurosurgery 47: 723-730
Webb KM, Laurent JJ, Okonkwo DO, Lopes MB, Vance ML, Laws Jr ER (2003) Clinical characteristics of silent corticotroph adenomas and creation of an internet-accessible database to facilitate their multi-institutional study. Neurosurgery 53: 1076-85
Tateno T, Izumiyama H, Doi M, Yoshimoto T, Shichiri M, Inoshita N, Oyama K, Yamada S, Hirata Y (2007). Differential gene expression in ACTH-secreting and non-functioning pituitary tumors. Eur J Endocrinol 157: 717-24
Knosp E, Steiner E, Kitz K, Matla C (1993) Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery 33: 610-7
Du L, Bergsneider M, Mirsadraei L, Young SH, Jonker JW, Downes M, Yong WH, Evans RM, Heaney AP (2013) Evidence for orphan nuclear receptor TR4 in the etiology of Cushing disease. Proc Natl Acad Sci USA 110: 8555-60
DeLellis RA, Lloyd RV, Heitz PU, Eng C (2004) Pathology and genetics of tumours of the endocrine organs. IARC press, Lyon
Vallette-Kasic S, Figarella-Branger D, Grino M, Pulichino A-M, Dufour H, Grisoli F, Enjalbert A, Drouin J, Brue T (2003) Differential regulation of proopiomelanocortin and pituitary-restricted transcription factor (TPIT), a new marker of normal and adenomatous human corticotrophs. J Clin Endocrinol Metab 88: 3050-56
Yamada S, Ohyama K, Taguchi M, Takeshita A, Morita K, Takano K, Sano T (2007) A study of the correlation between morphological findings and biological activities in clinically nonfunctioning pituitary adenomas. Neurosurgery 61: 580-5
Young Jr WF, Scheithauer BW, Kovacs KT, Horvath E, Davis DH, Randall RV (1996) Gonadotroph adenoma of the pituitary gland: a clinicopathologic analysis of 100 cases. Mayo Clinic Proc 71: 649-56
Sano T, Mader R, Asa SL, Qian ZR, Hino A, Yamada S (2003) “Honeycomb Golgi” in pituitary adenomas: not a marker of gonadotroph adenomas. Endocr Pathol 14: 363-8
Nishioka H, Hara T, Usui M, Fukuhara N, Yamada S (2011) Simultaneous combined supra-infrasellar approach for giant/large multi-lobulated pituitary adenomas. World Neurosurg 77: 533-39
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Funding
This work was supported by the Okinaka Memorial Institute for Medical Research. This work was also supported in part by JSPS KAKENHI Grant Number 25460468.
Rights and permissions
About this article
Cite this article
Nishioka, H., Inoshita, N., Mete, O. et al. The Complementary Role of Transcription Factors in the Accurate Diagnosis of Clinically Nonfunctioning Pituitary Adenomas. Endocr Pathol 26, 349–355 (2015). https://doi.org/10.1007/s12022-015-9398-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-015-9398-z