Abstract
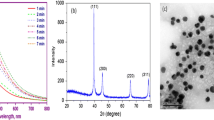
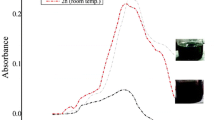
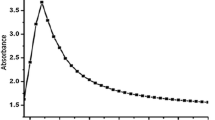
Green evolutionary products such as biologically fabricated nanoparticles (NPs) pose a hazard to aquatic creatures. Herein, biogenic silver nanoparticles (AgNPs) were synthesized by the reaction between ionic silver (AgNO3) and aqueous onion peel extract (Allium cepa L). The synthesized biogenic AgNPs were characterized with UV–Visible spectrophotometer, XRD, FT-IR, and TEM with EDS analysis; then, their toxicity was assessed on common carp fish (Cyprinus carpio) using biomarkers of haematological alterations, oxidative stress, histological changes, differential gene expression patterns, and bioaccumulation. The 96 h lethal toxicity was analysed with various concentrations (2, 4, 6, 8, and 10 mg/l) of biogenic AgNPs. Based on 96 h LC50, sublethal concentrations (1/15th, 1/10th, and 1/5th) were given to C. carpio for 28 days. At the end of experiment, the bioaccumulations of Ag content were accumulated mainly in the gills, followed by the liver and muscle. At an interval of 7 days, the haematological alterations showed significance (p < 0.05) and elevation of antioxidant defence mechanism reveals the toxicity of biogenic synthesized AgNPs. Adverse effects on oxidative stress were probably related to the histopathological damage of its vital organs like gill, liver, and muscle. Finally, the fish treated with biogenic synthesized AgNPs were significantly (p < 0.05) downregulates the oxidative stress genes such as Cu–Zn SOD, CAT, GPx1a, GST-α, CYP1A, and Nrf-2 expression patterns. The present study provides evidence of biogenic synthesized AgNPs influence on the aquatic life through induction of oxidative stress.







Similar content being viewed by others
Data Availability
Data and materials are available for all authors and included in this published article.
Change history
19 March 2022
A Correction to this paper has been published: https://doi.org/10.1007/s12011-022-03194-7
References
Fabrega J, Luoma SN, Tyler CR et al (2011) Silver nanoparticles: behaviour and effects in the aquatic environment. Environ Int 37:517–531. https://doi.org/10.1016/j.envint.2010.10.012
Jeevanandam J, Barhoum A, Chan YS et al (2018) Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J Nanotechnol 9:1050–1074. https://doi.org/10.3762/bjnano.9.98
Shen Z, Wu A, Chen X (2017) Iron oxide nanoparticle based contrast agents for magnetic resonance imaging. Mol Pharm 14:1352–1364. https://doi.org/10.1021/acs.molpharmaceut.6b00839
Senthilkumar N, Sharma PK, Sood N, Bhalla N (2021) Designing magnetic nanoparticles for in vivo applications and understanding their fate inside human body. Coord Chem Rev 445:214082. https://doi.org/10.1016/j.ccr.2021.214082
Montes MO, Hanna SK, Lenihan HS, Keller AA (2012) Uptake, accumulation, and biotransformation of metal oxide nanoparticles by a marine suspension-feeder. J Hazard Mater 225–226:139–145. https://doi.org/10.1016/j.jhazmat.2012.05.009
Alavi M, Varma RS (2021) Phytosynthesis and modification of metal and metal oxide nanoparticles/nanocomposites for antibacterial and anticancer activities: recent advances. Sustain Chem Pharm 21:100412. https://doi.org/10.1016/j.scp.2021.100412
Giráldez-Pérez RM, Grueso E, Domínguez I et al (2021) Biocompatible DNA/5-fluorouracil-gemini surfactant-functionalized gold nanoparticles as promising vectors in lung cancer therapy. Pharmaceutics 13:423. https://doi.org/10.3390/pharmaceutics13030423
García-Fernández C, Fornaguera C, Borrós S (2020) Nanomedicine in non-small cell lung cancer: from conventional treatments to immunotherapy. Cancers (Basel) 12:1609. https://doi.org/10.3390/cancers12061609
Goudarzi F, Asadi A, Afsharpour M, Jamadi RH (2018) In vitro characterization and evaluation of the cytotoxicity effects of Nisin and Nisin-loaded PLA-PEG-PLA nanoparticles on gastrointestinal (AGS and KYSE-30), hepatic (HepG2) and blood (K562) cancer cell lines. AAPS PharmSciTech 19:1554–1566. https://doi.org/10.1208/s12249-018-0969-4
Chang K-B, Shen C-C, Hsu S et al (2021) Functionalized collagen-silver nanocomposites for evaluation of the biocompatibility and vascular differentiation capacity of mesenchymal stem cells. Colloids Surfaces A Physicochem Eng Asp 624:126814. https://doi.org/10.1016/j.colsurfa.2021.126814
Ko SW, Lee JY, Rezk AI et al (2021) In-situ cellulose-framework templates mediated monodispersed silver nanoparticles via facile UV-light photocatalytic activity for anti-microbial functionalization. Carbohydr Polym 269:118255. https://doi.org/10.1016/j.carbpol.2021.118255
Fung MC, Bowen DL (1996) Silver products for medical indications: risk-benefit assessment. J Toxicol - Clin Toxicol 34:119–126
Alexander JW (2009) History of the medical use of silver. Surg Infect (Larchmt) 10:289–292. https://doi.org/10.1089/sur.2008.9941
Barillo DJ, Marx DE (2014) Silver in medicine: a brief history BC 335 to present. Burns 40:S3–S8. https://doi.org/10.1016/j.burns.2014.09.009
Kahru A, Ivask A (2013) Mapping the dawn of nanoecotoxicological research. Acc Chem Res 46:823–833. https://doi.org/10.1021/ar3000212
Khan B, Ho KT, Burgess RM (2020) Application of biomarker tools using bivalve models toward the development of adverse outcome pathways for contaminants of emerging concern. Environ Toxicol Chem 39:1472–1484. https://doi.org/10.1002/etc.4757
Wang L, Wu WM, Bolan NS et al (2021) Environmental fate, toxicity and risk management strategies of nanoplastics in the environment: current status and future perspectives. J Hazard Mater 401:123415. https://doi.org/10.1016/j.jhazmat.2020.123415
Genchi G, Carocci A, Lauria G et al (2020) Nickel: human health and environmental toxicology. Int J Environ Res Public Health 17:679. https://doi.org/10.3390/ijerph17030679
Ağçeli GK, Hammachi H, Kodal SP et al (2020) A Novel approach to synthesize TiO2 nanoparticles: biosynthesis by using Streptomyces sp. HC1. J Inorg Organomet Polym Mater 30:3221–3229. https://doi.org/10.1007/s10904-020-01486-w
Ajmal N, Saraswat K, Bakht MA et al (2019) Cost-effective and eco-friendly synthesis of titanium dioxide (TiO2) nanoparticles using fruit’s peel agro-waste extracts: characterization, in vitro antibacterial, antioxidant activities. Green Chem Lett Rev 12:244–254. https://doi.org/10.1080/17518253.2019.1629641
Alavi M, Karimi N (2019) Ultrasound assisted-phytofabricated Fe3O4 NPs with antioxidant properties and antibacterial effects on growth, biofilm formation, and spreading ability of multidrug resistant bacteria. Artif Cells, Nanomedicine, Biotechnol 47:2405–2423. https://doi.org/10.1080/21691401.2019.1624560
Alavi M, Kennedy JF (2021) Recent advances of fabricated and modified Ag, Cu, CuO and ZnO nanoparticles by herbal secondary metabolites, cellulose and pectin polymers for antimicrobial applications. Cellulose 28:3297–3310. https://doi.org/10.1007/s10570-021-03746-5
Gaiser BK, Biswas A, Rosenkranz P et al (2011) Effects of silver and cerium dioxide micro- and nano-sized particles on Daphnia magna. J Environ Monit 13:1227–1235. https://doi.org/10.1039/c1em10060b
García-Alonso J, Khan FR, Misra SK et al (2011) Cellular internalization of silver nanoparticles in gut epithelia of the estuarine polychaete nereis diversicolor. Environ Sci Technol 45:4630–4636. https://doi.org/10.1021/es2005122
Walters CR, Cheng P, Pool E, Somerset V (2016) Effect of temperature on oxidative stress parameters and enzyme activity in tissues of Cape River crab (Potamanautes perlatus) following exposure to silver nanoparticles (AgNP). J Toxicol Environ Heal - Part A Curr Issues 79:61–70. https://doi.org/10.1080/15287394.2015.1106357
Heinlaan M, Kahru A, Kasemets K et al (2011) Changes in the Daphnia magna midgut upon ingestion of copper oxide nanoparticles: a transmission electron microscopy study. Water Res 45:179–190. https://doi.org/10.1016/j.watres.2010.08.026
Ale A, Liberatori G, Vannuccini ML et al (2019) Exposure to a nanosilver-enabled consumer product results in similar accumulation and toxicity of silver nanoparticles in the marine mussel Mytilus galloprovincialis. Aquat Toxicol 211:46–56. https://doi.org/10.1016/j.aquatox.2019.03.018
Al-Asgah NA, Abdel-Warith AWA, Younis ESM, Allam HY (2015) Haematological and biochemical parameters and tissue accumulations of cadmium in Oreochromis niloticus exposed to various concentrations of cadmium chloride. Saudi J Biol Sci 22:543–550. https://doi.org/10.1016/j.sjbs.2015.01.002
Al-Bairuty GA, Shaw BJ, Handy RD, Henry TB (2013) Histopathological effects of waterborne copper nanoparticles and copper sulphate on the organs of rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 126:104–115. https://doi.org/10.1016/j.aquatox.2012.10.005
Duran S, Tuncsoy M, Ay O et al (2017) Accumulation of copper oxide nanoparticles in gill, liver and muscle tissues of Clarias gariepinus. Toxicol Lett 280:S186. https://doi.org/10.1016/j.toxlet.2017.07.522
Tunçsoy M, Duran S, Ay Ö et al (2017) Effects of copper oxide nanoparticles on antioxidant enzyme activities and on tissue accumulation of Oreochromis niloticus. Bull Environ Contam Toxicol 99:360–364. https://doi.org/10.1007/s00128-017-2129-z
Griffitt RJ, Weil R, Hyndman KA et al (2007) Exposure to copper nanoparticles causes gill injury and acute lethality in zebrafish (Danio rerio). Environ Sci Technol 41:8178–8186. https://doi.org/10.1021/es071235e
Ates M, Demir V, Adiguzel R, Arslan Z (2013) Bioaccumulation, subacute toxicity, and tissue distribution of engineered titanium dioxide nanoparticles in goldfish (Carassius auratus). J Nanomater 2013:1–6. https://doi.org/10.1155/2013/460518
Jurgelėnė Ž, Stankevičius M, Stankevičiūtė M et al (2021) Imaging of the internal chorion structure of rainbow trout Oncorhynchus mykiss live embryos and the distribution of quantum dots therein: towards a deeper understanding of potential nanotoxicity. Sci Total Environ 785:147302. https://doi.org/10.1016/j.scitotenv.2021.147302
Pérez S, la Farré M, Barceló D (2009) Analysis, behavior and ecotoxicity of carbon-based nanomaterials in the aquatic environment. TrAC - Trends Anal Chem 28:820–832. https://doi.org/10.1016/j.trac.2009.04.001
Bat L, Sezgin M, Üstün F, Şahin F (2012) Heavy metal concentrations in ten species of fishes caught in Sinop coastal waters of the Black Sea, Turkey. Turkish J Fish Aquat Sci 12:371–376. https://doi.org/10.4194/1303-2712-v12_2_24
Krishna D, Sachan HK (2021) Nano-toxicity and aquatic food chain. In: Singh P, Singh R, Verma P, Bhadouria R, Kumar A, Kaushik M. (ed) Plant-Microbes-Engineered Nano-particles (PM-ENPs) Nexus in Agro-Ecosystems. Advances in science, technology and innovation. (IEREK Interdisciplinary Series for Sustainable Development). Springer, Cham.189–198. https://doi.org/10.1007/978-3-030-66956-0_13
Mallik A, Xavier KAM, Naidu BC, Nayak BB (2021) Ecotoxicological and physiological risks of microplastics on fish and their possible mitigation measures. Sci Total Environ 779:146433. https://doi.org/10.1016/j.scitotenv.2021.146433
Zhu X, Hondroulis E, Liu W, Li CZ (2013) Biosensing approaches for rapid genotoxicity and cytotoxicity assays upon nanomaterial exposure. Small 9:1821–1830. https://doi.org/10.1002/smll.201201593
Gomes HIO, Martins CSM, Prior JAV (2021) Silver nanoparticles as carriers of anticancer drugs for efficient target treatment of cancer cells. Nanomaterials 11:964. https://doi.org/10.3390/nano11040964
Matsuda Y, Torimoto T, Kameya T et al (2013) ZnS-AgInS2 nanoparticles as a temperature sensor. Sensors Actuators, B Chem 176:505–508. https://doi.org/10.1016/j.snb.2012.09.005
Alavi M, Dehestaniathar S, Mohammadi S et al (2020) Antibacterial activities of phytofabricated ZnO and CuO NPs by Mentha pulegium leaf/flower mixture extract against antibiotic resistant bacteria. Adv Pharm Bull 11:497–504. https://doi.org/10.34172/apb.2021.057
Aljelehawy Q, Karimi N, Alavi M (2021) Comparison of antibacterial and cytotoxic activities of phytosynthesized ZnONPs by leaves extract of Daphne mucronata at different salt sources. Mater Technol 36:747–759. https://doi.org/10.1080/10667857.2020.1794280
Shankar PD, Shobana S, Karuppusamy I et al (2016) A review on the biosynthesis of metallic nanoparticles (gold and silver) using bio-components of microalgae: formation mechanism and applications. Enzyme Microb Technol 95:28–44. https://doi.org/10.1016/j.enzmictec.2016.10.015
Mani R, Vijayakumar P, Dhas S, et al (2022) Biogenic nanoscale silver particles synthesis using butter fruit pulp extract and study of antibacterial efficacy against Providencia vermicola in rohu fish. J King Saud Univ - Sci 34:101814. https://doi.org/10.1016/j.jksus.2021.101814
Li S, Shen Y, Xie A et al (2007) Green synthesis of silver nanoparticles using Capsicum annuum L extract. Green Chem 9:852. https://doi.org/10.1039/b615357g
Song JY, Kim BS (2009) Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst Eng 32:79. https://doi.org/10.1007/s00449-008-0224-6
Singh J, Dutta T, Kim KH et al (2018) “Green” synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J Nanobiotechnology 16:84. https://doi.org/10.1186/s12951-018-0408-4
Kumar B, Smita K, Cumbal L, Debut A (2017) Green synthesis of silver nanoparticles using Andean blackberry fruit extract. Saudi J Biol Sci 24:45–50. https://doi.org/10.1016/j.sjbs.2015.09.006
Antony JJ, Nivedheetha M, Siva D et al (2013) Antimicrobial activity of Leucas aspera engineered silver nanoparticles against Aeromonas hydrophila in infected Catla catla. Colloids Surfaces B Biointerfaces 109:20–24. https://doi.org/10.1016/j.colsurfb.2013.03.020
Baldissera MD, Souza CF, Zeppenfeld CC et al (2020) Dietary supplementation with nerolidol nanospheres improves growth, antioxidant status and fillet fatty acid profiles in Nile tilapia: benefits of nanotechnology for fish health and meat quality. Aquaculture 516:734635. https://doi.org/10.1016/j.aquaculture.2019.734635
Rather MA, Bhat IA, Sharma N et al (2017) Synthesis and characterization of Azadirachta indica constructed silver nanoparticles and their immunomodulatory activity in fish. Aquac Res 48:3742–3754. https://doi.org/10.1111/are.13199
Shah BR, Mraz J (2020) Advances in nanotechnology for sustainable aquaculture and fisheries. Rev Aquac 12:925–942. https://doi.org/10.1111/raq.12356
Vignesh V, Felix Anbarasi K, Karthikeyeni S et al (2013) A superficial phyto-assisted synthesis of silver nanoparticles and their assessment on hematological and biochemical parameters in Labeo rohita (Hamilton, 1822). Colloids Surfaces A Physicochem Eng Asp 439:184–192. https://doi.org/10.1016/j.colsurfa.2013.04.011
Choi JE, Kim S, Ahn JH et al (2010) Induction of oxidative stress and apoptosis by silver nanoparticles in the liver of adult zebrafish. Aquat Toxicol 100:151–159. https://doi.org/10.1016/j.aquatox.2009.12.012
Lubick N (2008) Nanosilver toxicity: ions, nanoparticles — or both? Environ Sci Technol 42:8617. https://doi.org/10.1021/es8026314
Mulenos MR, Liu J, Lujan H et al (2020) Copper, silver, and titania nanoparticles do not release ions under anoxic conditions and release only minute ion levels under oxic conditions in water: evidence for the low toxicity of nanoparticles. Environ Chem Lett 18:1319–1328. https://doi.org/10.1007/s10311-020-00985-z
Sakka Y, Skjolding LM, Mackevica A et al (2016) Behavior and chronic toxicity of two differently stabilized silver nanoparticles to Daphnia magna. Aquat Toxicol 177:526–535. https://doi.org/10.1016/j.aquatox.2016.06.025
Shen MH, Zhou XX, Yang XY et al (2015) Exposure medium: key in identifying free Ag+ as the exclusive species of silver nanoparticles with acute toxicity to Daphnia magna. Sci Rep 5:9674. https://doi.org/10.1038/srep09674
Khoshnamvand M, Hao Z, Fadare OO et al (2020) Toxicity of biosynthesized silver nanoparticles to aquatic organisms of different trophic levels. Chemosphere 258:127346. https://doi.org/10.1016/j.chemosphere.2020.127346
Nie X, Zhu K, Zhao S et al (2020) Interaction of Ag+ with soil organic matter: elucidating the formation of silver nanoparticles. Chemosphere 243:125413. https://doi.org/10.1016/j.chemosphere.2019.125413
Oya-Silva LF, Vicari T, Rodrigo Disner G et al (2021) Tissue-specific genotoxicity and antioxidant imbalance of titanium dioxide nanoparticles (NPTiO2) and inorganic lead (PbII) in a neotropical fish species. Environ Toxicol Pharmacol 82:103551. https://doi.org/10.1016/j.etap.2020.103551
Bathi JR, Moazeni F, Upadhyayula VKK et al (2021) Behavior of engineered nanoparticles in aquatic environmental samples: current status and challenges. Sci Total Environ 793:148560. https://doi.org/10.1016/j.scitotenv.2021.148560
He X, Aker WG, Fu PP, Hwang HM (2015) Toxicity of engineered metal oxide nanomaterials mediated by nano-bio-eco-interactions: a review and perspective. Environ Sci Nano 2:564–582. https://doi.org/10.1039/C5EN00094G
Krishnasamy Sekar R, Sridhar A, Perumalsamy B et al (2020) In vitro antioxidant, antipathogenicity and cytotoxicity effect of silver nanoparticles fabricated by onion (Allium cepa L.) peel extract. Bionanoscience 10:235–248. https://doi.org/10.1007/s12668-019-00691-3
OECD (2019) Test No. 203: fish, acute toxicity test, OECD Guidelines for the testing of chemicals, Section 2, OECD Publishing, Paris. https://doi.org/10.1787/9789264069961-en
Finney DJ (1952) Probit analysis Cambridge University Press, New York. J Am Pharm Assoc (Scientific ed) 41:627. https://doi.org/10.1002/jps.3030411125
Zhang Y, Lu X, Wang N et al (2016) Heavy metals in aquatic organisms of different trophic levels and their potential human health risk in Bohai Bay, China. Environ Sci Pollut Res 23:17801–17810. https://doi.org/10.1007/s11356-016-6948-y
Dacie JV, Lewis SM (1975) Practical haematology, 5th edn. Churchill Livingstone, London
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275. https://doi.org/10.1016/0922-338X(96)89160-4
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474. https://doi.org/10.1111/j.1432-1033.1974.tb03714.x
Claiborne A (1985) Catalase activity. In: Greenwald RA (ed) CRC Handbook of Methods for Oxygen Radical Research. CRC Press, Boca Raton, Florida pp 283–284
Rotruck JT, Pope AL, Ganther HE et al (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588–590. https://doi.org/10.1126/science.179.4073.588
Habig WH, Pabst M, Jakoby W (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139. https://doi.org/10.2307/j.ctv18b5cjk.40
Moron MS, Depierre JW, Mannervik B (1979) Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. BBA - Gen Subj 582:67–78. https://doi.org/10.1016/0304-4165(79)90289-7
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Benítez V, Mollá E, Martín-Cabrejas MA et al (2011) Characterization of industrial onion wastes (Allium cepa L.): dietary fibre and bioactive compounds. Plant Foods Hum Nutr 66:48–57. https://doi.org/10.1007/s11130-011-0212-x
Ahmed MJ, Murtaza G, Mehmood A, Bhatti TM (2015) Green synthesis of silver nanoparticles using leaves extract of Skimmia laureola: characterization and antibacterial activity. Mater Lett 153:10–13. https://doi.org/10.1016/j.matlet.2015.03.143
He Y, Du Z, Ma S et al (2016) Biosynthesis, antibacterial activity and anticancer effects against prostate cancer (PC-3) cells of silver nanoparticles using Dimocarpus Longan Lour peel extract. Nanoscale Res Lett 11:300. https://doi.org/10.1186/s11671-016-1511-9
Li S, Shen Y, Xie A et al (2007) Green synthesis of silver nanoparticles using Capsicum annuum L. extract. Green Chem 9:852–885. https://doi.org/10.1039/b615357g
Tripathy A, Raichur AM, Chandrasekaran N et al (2010) Process variables in biomimetic synthesis of silver nanoparticles by aqueous extract of Azadirachta indica (Neem) leaves. J Nanoparticle Res 12:237–246. https://doi.org/10.1007/s11051-009-9602-5
Whiteman SC, Yang Y, Jones JM, Spiteri MA (2008) FTIR spectroscopic analysis of sputum: preliminary findings on a potential novel diagnostic marker for COPD. Ther Adv Respir Dis 2:23–31. https://doi.org/10.1177/1753465807087972
Jain N, Bhargava A, Majumdar S et al (2011) Extracellular biosynthesis and characterization of silver nanoparticles using Aspergillus flavus NJP08: a mechanism perspective. Nanoscale 3:635–641. https://doi.org/10.1039/c0nr00656d
Ghaseminezhad SM, Hamedi S, Shojaosadati SA (2012) Green synthesis of silver nanoparticles by a novel method: comparative study of their properties. Carbohydr Polym 89:467–472. https://doi.org/10.1016/j.carbpol.2012.03.030
Abdel-Aziz MS, Shaheen MS, El-Nekeety AA, Abdel-Wahhab MA (2014) Antioxidant and antibacterial activity of silver nanoparticles biosynthesized using Chenopodium murale leaf extract. J Saudi Chem Soc 18:356–363. https://doi.org/10.1016/j.jscs.2013.09.011
Kumar KG, Avinash BS, Rahimi-Gorji M, Alarifi IM (2019) Optical and electrical properties of Ti1-XSnXO2 nanoparticles. J Mol Liq 293:111556. https://doi.org/10.1016/j.molliq.2019.111556
Kanipandian N, Kannan S, Ramesh R et al (2014) Characterization, antioxidant and cytotoxicity evaluation of green synthesized silver nanoparticles using Cleistanthus collinus extract as surface modifier. Mater Res Bull 49:494–502. https://doi.org/10.1016/j.materresbull.2013.09.016
Judy JD, Kirby JK, Creamer C et al (2015) Effects of silver sulfide nanomaterials on mycorrhizal colonization of tomato plants and soil microbial communities in biosolid-amended soil. Environ Pollut 206:256–263. https://doi.org/10.1016/j.envpol.2015.07.002
Xia T, Kovochich M, Brant J et al (2006) Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett 6:1794–1807
Kleiven M, Macken A, Oughton DH (2019) Growth inhibition in Raphidocelis subcapita – Evidence of nanospecific toxicity of silver nanoparticles. Chemosphere 221:785–792. https://doi.org/10.1016/j.chemosphere.2019.01.055
Kakakhel MA, Wu F, Feng H et al (2021) Biological synthesis of silver nanoparticles using animal blood, their preventive efficiency of bacterial species, and ecotoxicity in common carp fish. Microsc Res Tech 84:1765–1774. https://doi.org/10.1002/jemt.23733
Liaqat F, Hanif U, Bahadur S et al (2021) Comparative evaluation of the toxicological effect of silver salt (AgNO3) and silver nanoparticles on Cyprinus carpio synthesized by chemicals and marine algae using scanning electron microscopy. Microsc Res Tech 84:1531–1541. https://doi.org/10.1002/jemt.23710
Khosravi-Katuli K, Shabani A, Paknejad H, Imanpoor MR (2018) Comparative toxicity of silver nanoparticle and ionic silver in juvenile common carp (Cyprinus carpio): accumulation, physiology and histopathology. J Hazard Mater 359:373–381. https://doi.org/10.1016/j.jhazmat.2018.07.064
Ramachandran R, Krishnaraj C, Kumar VKA et al (2018) In vivo toxicity evaluation of biologically synthesized silver nanoparticles and gold nanoparticles on adult zebrafish: a comparative study. 3 Biotech 8:441. https://doi.org/10.1007/s13205-018-1457-y
Krishnaraj C, Harper SL, Il YS (2016) In vivo toxicological assessment of biologically synthesized silver nanoparticles in adult Zebrafish (Danio rerio). J Hazard Mater 301:480–491. https://doi.org/10.1016/j.jhazmat.2015.09.022
Sarkar B, Netam SP, Mahanty A et al (2014) Toxicity evaluation of chemically and plant derived silver nanoparticles on zebrafish (Danio rerio). Proc Natl Acad Sci India Sect B - Biol Sci 84:885–892. https://doi.org/10.1007/s40011-013-0298-z
Bilberg K, Hovgaard MB, Besenbacher F, Baatrup E (2012) In vivo toxicity of silver nanoparticles and silver ions in zebrafish (Danio rerio). J Toxicol 2012:1–9. https://doi.org/10.1155/2012/293784
Girilal M, Krishnakumar V, Poornima P et al (2015) A comparative study on biologically and chemically synthesized silver nanoparticles induced heat shock proteins on fresh water fish Oreochromis niloticus. Chemosphere 139:461–468. https://doi.org/10.1016/j.chemosphere.2015.08.005
Massarsky A, Dupuis L, Taylor J et al (2013) Assessment of nanosilver toxicity during zebrafish (Danio rerio) development. Chemosphere 92:59–66. https://doi.org/10.1016/j.chemosphere.2013.02.060
Olasagasti M, Gatti AM, Capitani F et al (2014) Toxic effects of colloidal nanosilver in zebrafish embryos. J Appl Toxicol 34:562–575. https://doi.org/10.1002/jat.2975
Il KJ, Cui R, Nam S-H et al (2016) Multispecies toxicity test for silver nanoparticles to derive hazardous concentration based on species sensitivity distribution for the protection of aquatic ecosystems. Nanotoxicology 10:521–530. https://doi.org/10.3109/17435390.2015.1090028
Croteau MN, Misra SK, Luoma SN, Valsami-Jones E (2014) Bioaccumulation and toxicity of CuO nanoparticles by a freshwater invertebrate after waterborne and dietborne exposures. Environ Sci Technol 48:10929–10937. https://doi.org/10.1021/es5018703
Utembe W, Wepener V, Yu IJ, Gulumian M (2018) An assessment of applicability of existing approaches to predicting the bioaccumulation of conventional substances in nanomaterials. Environ Toxicol Chem 37:2972–2988. https://doi.org/10.1002/etc.4253
Velicogna JR, Schwertfeger DM, Jesmer AH et al (2017) The bioaccumulation of silver in Eisenia andrei exposed to silver nanoparticles and silver nitrate in soil. NanoImpact 6:11–18. https://doi.org/10.1016/j.impact.2017.03.001
Iversen TG, Skotland T, Sandvig K (2011) Endocytosis and intracellular transport of nanoparticles: present knowledge and need for future studies. Nano Today 6:176–185. https://doi.org/10.1016/j.nantod.2011.02.003
Mansouri B, Maleki A, Johari SA et al (2016) Copper bioaccumulation and depuration in common carp (Cyprinus carpio) following co-exposure to TiO2 and CuO nanoparticles. Arch Environ Contam Toxicol 71:541–552. https://doi.org/10.1007/s00244-016-0313-5
Sayadi MH, Pavlaki MD, Martins R et al (2021) Bioaccumulation and toxicokinetics of zinc oxide nanoparticles (ZnO NPs) co-exposed with graphene nanosheets (GNs) in the blackfish (Capoeta fusca). Chemosphere 269:128689. https://doi.org/10.1016/j.chemosphere.2020.128689
Mansouri B, Johari SA, Azadi NA, Sarkheil M (2018) Effects of waterborne ZnO nanoparticles and Zn2+ ions on the gills of rainbow trout (Oncorhynchus mykiss): bioaccumulation, histopathological and ultrastructural changes. Turkish J Fish Aquat Sci 18:739–746. https://doi.org/10.4194/1303-2712-v18_5_09
Ali I, Khan S, Shah K et al (2021) Microscopic analysis of plant-mediated silver nanoparticle toxicity in rainbow trout fish (Oncorhynchus mykiss). Microsc Res Tech 84:2302–2310. https://doi.org/10.1002/jemt.23785
Scown TM, Santos EM, Johnston BD et al (2010) Effects of aqueous exposure to silver nanoparticles of different sizes in rainbow trout. Toxicol Sci 115:521–534. https://doi.org/10.1093/toxsci/kfq076
Kaya H, Aydin F, Gürkan M et al (2015) Effects of zinc oxide nanoparticles on bioaccumulation and oxidative stress in different organs of tilapia (Oreochromis niloticus). Environ Toxicol Pharmacol 40:936–947. https://doi.org/10.1016/j.etap.2015.10.001
Yoo-iam M, Chaichana R, Satapanajaru T (2014) Toxicity, bioaccumulation and biomagnification of silver nanoparticles in green algae (Chlorella sp.), water flea (Moina macrocopa), blood worm (Chironomus spp.) and silver barb (Barbonymus gonionotus). Chem Speciat Bioavailab 26:257–265. https://doi.org/10.3184/095422914X14144332205573
Johari SA, Kalbassi MR, Yu IJ, Lee JH (2015) Chronic effect of waterborne silver nanoparticles on rainbow trout (Oncorhynchus mykiss): histopathology and bioaccumulation. Comp Clin Path 24:995–1007. https://doi.org/10.1007/s00580-014-2019-2
Xiao B, Wang X, Yang J et al (2020) Bioaccumulation kinetics and tissue distribution of silver nanoparticles in zebrafish: the mechanisms and influence of natural organic matter. Ecotoxicol Environ Saf 194:110454. https://doi.org/10.1016/j.ecoenv.2020.110454
Thummabancha K, Onparn N, Srisapoome P (2016) Analysis of hematologic alterations, immune responses and metallothionein gene expression in Nile tilapia (Oreochromis niloticus) exposed to silver nanoparticles. J Immunotoxicol 13:909–917. https://doi.org/10.1080/1547691X.2016.1242673
Dhanapakiam P, Ramasamy VK (2001) Toxic effects of copper and zinc mixtures on some haematological and biochemical parameters in common carp, Cyprinus carpio (Linn). J Environ Biol 22:105–111
Alkaladi A, El-Deen NAMN, Afifi M, Zinadah OAA (2015) Hematological and biochemical investigations on the effect of vitamin E and C on Oreochromis niloticus exposed to zinc oxide nanoparticles. Saudi J Biol Sci 22:556–563. https://doi.org/10.1016/j.sjbs.2015.02.012
Remyla SR, Ramesh M, Sajwan KS, Senthil Kumar K (2008) Influence of zinc on cadmium induced haematological and biochemical responses in a freshwater teleost fish Catla catla. Fish Physiol Biochem 34:169–174. https://doi.org/10.1007/s10695-007-9157-2
Lavanya S, Ramesh M, Kavitha C, Malarvizhi A (2011) Hematological, biochemical and ionoregulatory responses of Indian major carp Catla catla during chronic sublethal exposure to inorganic arsenic. Chemosphere 82:977–985. https://doi.org/10.1016/j.chemosphere.2010.10.071
Kuhn V, Diederich L, Keller TCS et al (2017) Red blood cell function and dysfunction: redox regulation, nitric oxide metabolism, anemia. antioxid redox signal 26:718–742
Levine EA, Rosen AL, Sehgal LR et al (1990) Physiologic effects of acute anemia: implications for a reduced transfusion trigger. Transfusion 30:11–14. https://doi.org/10.1046/j.1537-2995.1990.30190117621.x
Tsai AG, Acero C, Nance PR et al (2005) Elevated plasma viscosity in extreme hemodilution increases perivascular nitric oxide concentration and microvascular perfusion. Am J Physiol - Hear Circ Physiol 288:H1730–H1739. https://doi.org/10.1152/ajpheart.00998.2004
Das S, Patra A, Mandal A et al (2021) Study on impacts of direct supplementation of choline into semi-intensive fish culture system based on haematopoietic alterations. Environ Sustain Indic 9:100089. https://doi.org/10.1016/j.indic.2020.100089
Fazio F, Piccione G, Arfuso F, Faggio C (2015) Peripheral blood and head kidney haematopoietic tissue response to experimental blood loss in mullet (Mugil cephalus). Mar Biol Res 11:197–202. https://doi.org/10.1080/17451000.2014.898850
Khabbazi M, Harsij M, Hedayati SAA et al (2014) Effect of CuO nanoparticles on some hematological indices of rainbow trout Oncorhynchus mykiss and their potential toxicity. Nanomedicine J 2:67–73. https://doi.org/10.7508/nmj.2015.01.008
Farmen E, Mikkelsen HN, Evensen, et al (2012) Acute and sub-lethal effects in juvenile Atlantic salmon exposed to low μg/L concentrations of Ag nanoparticles. Aquat Toxicol 108:78–84. https://doi.org/10.1016/j.aquatox.2011.07.007
Nussey G, Van Vuren JHJ, du Preez HH (1995) Effect of copper on the haematology and osmoregulation of the Mozambique tilapia, Oreochromis mossambicus (Cichlidae). Comp Biochem Physiol Part C Comp 111:369–380. https://doi.org/10.1016/0742-8413(95)00063-1
Shaw BJ, Al-Bairuty G, Handy RD (2012) Effects of waterborne copper nanoparticles and copper sulphate on rainbow trout, (Oncorhynchus mykiss): physiology and accumulation. Aquat Toxicol 116–117:90–101. https://doi.org/10.1016/j.aquatox.2012.02.032
Remya AS, Ramesh M, Saravanan M et al (2015) Iron oxide nanoparticles to an Indian major carp, Labeo rohita: impacts on hematology, iono regulation and gill Na+/K+ ATPase activity. J King Saud Univ - Sci 27:151–160. https://doi.org/10.1016/j.jksus.2014.11.002
Ramsden CS, Smith TJ, Shaw BJ, Handy RD (2009) Dietary exposure to titanium dioxide nanoparticles in rainbow trout, (Oncorhynchus mykiss): no effect on growth, but subtle biochemical disturbances in the brain. Ecotoxicology 18:939–951. https://doi.org/10.1007/s10646-009-0357-7
Smith SD, Bolwell BJ, Rybicki LA et al (2007) Autologous hematopoietic stem cell transplantation in peripheral T-cell lymphoma using a uniform high-dose regimen. Bone Marrow Transplant 40:239–243. https://doi.org/10.1038/sj.bmt.1705712
Cogun HY, Firat Ö, Firat Ö et al (2012) Protective effect of selenium against mercury-induced toxicity on hematological and biochemical parameters of Oreochromis niloticus. J Biochem Mol Toxicol 26:117–122. https://doi.org/10.1002/jbt.20417
Pirsaheb M, Azadi NA, Miglietta ML et al (2019) Toxicological effects of transition metal-doped titanium dioxide nanoparticles on goldfish (Carassius auratus) and common carp (Cyprinus carpio). Chemosphere 215:904–915. https://doi.org/10.1016/j.chemosphere.2018.10.111
Kappus H (1985) Lipid peroxidation: mechanisms, analysis, enzymology and biological relevance. Oxidative Stress 273:273–310. https://doi.org/10.1016/b978-0-12-642760-8.50016-8
Govindasamy R, Rahuman AA (2012) Histopathological studies and oxidative stress of synthesized silver nanoparticles in Mozambique tilapia (Oreochromis mossambicus). J Environ Sci (China) 24:1091–1098. https://doi.org/10.1016/S1001-0742(11)60845-0
Zhu X, Zhu L, Lang Y, Chen Y (2008) Oxidative stress and growth inhibition in the freshwater fish Carassius auratus induced by chronic exposure to sublethal fullerene aggregates. Environ Toxicol Chem 27:1979–1985. https://doi.org/10.1897/07-573.1
Atli G, Alptekin Ö, Tükel S, Canli M (2006) Response of catalase activity to Ag+, Cd2+, Cr6+, Cu2+ and Zn2+ in five tissues of freshwater fish Oreochromis niloticus. Comp Biochem Physiol - C Toxicol Pharmacol 143:218–224. https://doi.org/10.1016/j.cbpc.2006.02.003
Hansen BH, Rømma S, Garmo A et al (2006) Antioxidative stress proteins and their gene expression in brown trout (Salmo trutta) from three rivers with different heavy metal levels. Comp Biochem Physiol - C Toxicol Pharmacol 143:263–274. https://doi.org/10.1016/j.cbpc.2006.02.010
Husak VV, Mosiichuk NM, Kubrak OI et al (2018) Acute exposure to copper induces variable intensity of oxidative stress in goldfish tissues. Fish Physiol Biochem 44:841–852. https://doi.org/10.1007/s10695-018-0473-5
Vieira MC, Torronteras R, Córdoba F, Canalejo A (2012) Acute toxicity of manganese in goldfish Carassius auratus is associated with oxidative stress and organ specific antioxidant responses. Ecotoxicol Environ Saf 78:212–217. https://doi.org/10.1016/j.ecoenv.2011.11.015
Fırat Ö, Bozat RC (2019) Assessment of biochemical and toxic responses induced by titanium dioxide nanoparticles in Nile tilapia Oreochromis niloticus. Hum Ecol Risk Assess 25:1438–1447. https://doi.org/10.1080/10807039.2018.1465338
Hao L, Wang Z, Xing B (2009) Effect of sub-acute exposure to TiO2 nanoparticles on oxidative stress and histopathological changes in Juvenile Carp (Cyprinus carpio). J Environ Sci (China) 21:1459–1466. https://doi.org/10.1016/s1001-0742(08)62440-7
Afifi M, Saddick S, Abu Zinada OA (2016) Toxicity of silver nanoparticles on the brain of Oreochromis niloticus and Tilapia zillii. Saudi J Biol Sci 23:754–760. https://doi.org/10.1016/j.sjbs.2016.06.008
Wu Y, Zhou Q (2013) Silver nanoparticles cause oxidative damage and histological changes in medaka (Oryzias latipes) after 14 days of exposure. Environ Toxicol Chem 32:165–173. https://doi.org/10.1002/etc.2038
Massarsky A, Abraham R, Nguyen KC et al (2014) Nanosilver cytotoxicity in rainbow trout (Oncorhynchus mykiss) erythrocytes and hepatocytes. Comp Biochem Physiol - C Toxicol Pharmacol 159:10–21. https://doi.org/10.1016/j.cbpc.2013.09.008
Lee B, Duong CN, Cho J, et al (2012) Toxicity of citrate-capped silver nanoparticles in common carp (Cyprinus carpio). J Biomed Biotechnol. 2012:262670. https://doi.org/10.1155/2012/262670
Monteiro DA, Rantin FT, Kalinin AL (2010) Inorganic mercury exposure: toxicological effects, oxidative stress biomarkers and bioaccumulation in the tropical freshwater fish matrinxã, Brycon amazonicus (Spix and Agassiz, 1829). Ecotoxicology 19:105–123. https://doi.org/10.1007/s10646-009-0395-1
Chae YJ, Pham CH, Lee J et al (2009) Evaluation of the toxic impact of silver nanoparticles on Japanese medaka (Oryzias latipes). Aquat Toxicol 94:320–327. https://doi.org/10.1016/j.aquatox.2009.07.019
Al-Abdan MA, Bin-Jumah MN, Alarifi S (2020) Exploration of cadmium dioxide nanoparticles on bioaccumulation, oxidative stress, and carcinogenic potential in Oreochromis mossambicus L. Oxid Med Cell Longev 2020:1–11. https://doi.org/10.1155/2020/5407159
Alak G, Ucar A, Parlak V et al (2020) Antioxidant potential of ulexite in zebrafish brain: assessment of oxidative DNA damage, apoptosis, and response of antioxidant defense system. Biol Trace Elem Res 199:1092–1099. https://doi.org/10.1007/s12011-020-02231-7
Hoseinifar SH, Yousefi S, Van Doan H et al (2021) Oxidative stress and antioxidant defense in fish: the implications of probiotic, prebiotic, and synbiotics. Rev Fish Sci Aquac 29:198–217. https://doi.org/10.1080/23308249.2020.1795616
Reddy UA, Prabhakar PV, Mahboob M (2017) Biomarkers of oxidative stress for in vivo assessment of toxicological effects of iron oxide nanoparticles. Saudi J Biol Sci 24:1172–1180. https://doi.org/10.1016/j.sjbs.2015.09.029
Jifa W, Yu Z, Xiuxian S, You W (2006) Response of integrated biomarkers of fish (Lateolabrax japonicus) exposed to benzo[a]pyrene and sodium dodecylbenzene sulfonate. Ecotoxicol Environ Saf 65:230–236. https://doi.org/10.1016/j.ecoenv.2005.08.002
Arora S, Jain J, Rajwade JM, Paknikar KM (2008) Cellular responses induced by silver nanoparticles: In vitro studies. Toxicol Lett 179:93–100. https://doi.org/10.1016/j.toxlet.2008.04.009
Hajirezaee S, Mohammadi G, Naserabad SS (2020) The protective effects of vitamin C on common carp (Cyprinus carpio) exposed to titanium oxide nanoparticles (TiO2-NPs). Aquaculture 518:734734. https://doi.org/10.1016/j.aquaculture.2019.734734
Abdelazim AM, Saadeldin IM, Swelum AAA et al (2018) Oxidative stress in the muscles of the fish Nile tilapia caused by zinc oxide nanoparticles and its modulation by vitamins C and E. Oxid Med Cell Longev 2018:6926712. https://doi.org/10.1155/2018/6926712
Cristina Soare L, Păunescu A, Cristina Maria P (2019) The morphophysiological, histological, and biochemical response of some nontarget organisms to the stress induced by the pesticides in the environment. In: Larramendy, Soloneski (ed) Pesticides — use and misuse and their impact in the environment. IntechOpen. https://doi.org/10.5772/intechopen.84332
Elgendy MY, Abumourad IK, Ali SEM et al (2017) Health status and genotoxic effects of metal pollution in Tilapia zillii and Solea vulgaris from polluted aquatic habitats. Int J Zool Res 13:54–63. https://doi.org/10.3923/ijzr.2017.54.63
Ossana NA, Baudou FG, Castañé PM et al (2019) Histological, genotoxic, and biochemical effects on Cnesterodon decemmaculatus (Jenyns 1842) (Cyprinodontiformes, Poeciliidae): early response bioassays to assess the impact of receiving waters. J Toxicol 2019:1–13. https://doi.org/10.1155/2019/4687685
Nero V, Farwell A, Lister A et al (2006) Gill and liver histopathological changes in yellow perch (Perca flavescens) and goldfish (Carassius auratus) exposed to oil sands process-affected water. Ecotoxicol Environ Saf 63:365–377. https://doi.org/10.1016/j.ecoenv.2005.04.014
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. EXS 101:133–164. https://doi.org/10.1007/2F978-3-7643-8340-4_6
Butchiram MS, Vijaya Kumar M, Tilak KS (2013) Studies on the histopathological changes in selected tissues of fish Labeo rohita exposed to phenol. J Environ Biol 34:247–251
Mansouri B, Maleki A, Johari SA et al (2017) Histopathological effects of copper oxide nanoparticles on the gill and intestine of common carp (Cyprinus carpio) in the presence of titanium dioxide nanoparticles. Chem Ecol 33:295–308. https://doi.org/10.1080/02757540.2017.1301436
Rajkumar KS, Kanipandian N, Thirumurugan R (2016) Toxicity assessment on haemotology, biochemical and histopathological alterations of silver nanoparticles-exposed freshwater fish Labeo rohita. Appl Nanosci 6:19–29. https://doi.org/10.1007/s13204-015-0417-7
Cassar S, Adatto I, Freeman JL et al (2020) Use of zebrafish in drug discovery toxicology. Chem Res Toxicol 33:95–118. https://doi.org/10.1021/acs.chemrestox.9b00335
Chen J, Dong X, Xin Y, Zhao M (2011) Effects of titanium dioxide nano-particles on growth and some histological parameters of zebrafish (Danio rerio) after a long-term exposure. Aquat Toxicol 101:493–499. https://doi.org/10.1016/j.aquatox.2010.12.004
Jang MH, Kim WK, Lee SK et al (2014) Uptake, tissue distribution, and depuration of total silver in common carp (Cyprinus carpio) after aqueous exposure to silver nanoparticles. Environ Sci Technol 48:11568–11574. https://doi.org/10.1021/es5022813
Shi H, Magaye R, Castranova V, Zhao J (2013) Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol 10:15. https://doi.org/10.1186/1743-8977-10-15
Vali S, Mohammadi G, Tavabe KR et al (2020) The effects of silver nanoparticles (Ag-NPs) sublethal concentrations on common carp (Cyprinus carpio): bioaccumulation, hematology, serum biochemistry and immunology, antioxidant enzymes, and skin mucosal responses. Ecotoxicol Environ Saf 194:110353. https://doi.org/10.1016/j.ecoenv.2020.110353
Hall AP, Elcombe CR, Foster JR et al (2012) Liver hypertrophy: a review of adaptive (adverse and non-adverse) changes-conclusions from the 3rd international ESTP expert workshop. Toxicol Pathol 40:971–994
Schramm M, Müller E, Triebskorn R (1998) Brown trout (Salmo trutta) liver ultrastructure as a biomarker for assessment of small stream pollution. Biomarkers 3:93–108. https://doi.org/10.1080/135475098231264
Bernet D, Schmidt H, Meier W et al (1999) Histopathology in fish: proposal for a protocol to assess aquatic pollution. J Fish Dis 22:25–34. https://doi.org/10.1046/j.1365-2761.1999.00134.x
Rodrigues S, Antunes SC, Nunes B, Correia AT (2019) Histopathological effects of the antibiotic erythromycin on the freshwater fish species Oncorhynchus mykiss. Ecotoxicol Environ Saf 181:1–10. https://doi.org/10.1016/j.ecoenv.2019.05.067
da Cunha RLD, de Brito-Gitirana L (2020) Effects of titanium dioxide nanoparticles on the intestine, liver, and kidney of Danio rerio. Ecotoxicol Environ Saf 203:111032. https://doi.org/10.1016/j.ecoenv.2020.111032
Huang CW, Li SW, Liao VHC (2019) Long-term sediment exposure to ZnO nanoparticles induces oxidative stress. Caenorhabditis elegans Environ Sci Nano 6:2602–2614. https://doi.org/10.1039/c9en00039a
Kim KS, Lee D, Song CG, Kang PM (2015) Reactive oxygen species-activated nanomaterials as theranostic agents. Nanomedicine 10:2709–2723.https://doi.org/10.2217/2Fnnm.15.108
Maharajan A, Kitto MR, Paruruckumani PS, Ganapiriya V (2016) Histopathology biomarker responses in Asian sea bass, Lates calcarifer (Bloch) exposed to copper. J Basic Appl Zool 77:21–30. https://doi.org/10.1016/j.jobaz.2016.02.001
Yao JW, Liu J, Kong XZ et al (2012) Induction of activation of the antioxidant response element and stabilization of Nrf2 by 3-(3-pyridylmethylidene)-2-indolinone (PMID) confers protection against oxidative stress-induced cell death. Toxicol Appl Pharmacol 259:227–235. https://doi.org/10.1016/j.taap.2011.12.027
Kobayashi M, Li L, Iwamoto N et al (2009) The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol Cell Biol 29:493–502. https://doi.org/10.1128/mcb.01080-08
Wu P, Liu Y, Jiang WD et al (2017) A comparative study on antioxidant system in fish hepatopancreas and intestine affected by choline deficiency: different change patterns of varied antioxidant enzyme genes and nrf2 signaling factors. PLoS ONE 12:e0169888. https://doi.org/10.1371/journal.pone.0169888
Imai H, Nakagawa Y (2003) Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic Biol Med 34:145–169. https://doi.org/10.1016/S0891-5849(02)01197-8
Neefjes VME, Evelo CTA, Baars LGM, Blanco CE (1999) Erythrocyte glutathione S transferase as a marker of oxidative stress at birth. Arch Dis Child Fetal Neonatal Ed 81:F130–F133. https://doi.org/10.1136/fn.81.2.F130
Yeo MK, Park HG (2012) Gene expression in zebrafish embryos following exposure to Cu-doped TiO2 and pure TiO2 nanometer-sized photocatalysts. Mol Cell Toxicol 8:127–137. https://doi.org/10.1007/s13273-012-0016-6
Van der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149. https://doi.org/10.1016/S1382-6689(02)00126-6
Oliva M, Gravato C, Guilhermino L et al (2014) EROD activity and cytochrome P4501A induction in liver and gills of Senegal sole Solea senegalensis from a polluted Huelva Estuary (SW Spain). Comp Biochem Physiol Part - C Toxicol Pharmacol 166:134–144. https://doi.org/10.1016/j.cbpc.2014.07.010
Valko M, Leibfritz D, Moncol J et al (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84. https://doi.org/10.1016/j.biocel.2006.07.001
Nakajima H, Nakajima-Takagi Y, Tsujita T et al (2011) Tissue-restricted expression of Nrf2 and its target genes in zebrafish with gene-specific variations in the induction profiles. PLoS ONE 6:e26884. https://doi.org/10.1371/journal.pone.0026884
Wu G, Fang YZ, Yang S et al (2004) Glutathione metabolism and its implications for health. J Nutr 134:489–492. https://doi.org/10.1093/jn/134.3.489
Ma Q (2013) Role of Nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 53:401–426. https://doi.org/10.1146/annurev-pharmtox-011112-140320
Nabavi SF, Barber AJ, Spagnuolo C et al (2016) Nrf2 as molecular target for polyphenols: a novel therapeutic strategy in diabetic retinopathy. Crit Rev Clin Lab Sci 53:293–312. https://doi.org/10.3109/10408363.2015.1129530
Zhang XD, Zhu YF, Cai LS, Wu TX (2008) Effects of fasting on the meat quality and antioxidant defenses of market-size farmed large yellow croaker (Pseudosciaena crocea). Aquaculture 280:136–139. https://doi.org/10.1016/j.aquaculture.2008.05.010
Zheng JL, Zhu QL, Wu CW et al (2016) Zinc acclimation mitigated high zinc induced oxidative stress by enhancing antioxidant defenses in large yellow croaker Pseudosciaena crocea. Aquat Toxicol 172:21–29. https://doi.org/10.1016/j.aquatox.2015.12.009
Pillet M, Castaldo G, De Weggheleire S et al (2019) Limited oxidative stress in common carp (Cyprinus carpio, L., 1758) exposed to a sublethal tertiary (Cu, Cd and Zn) metal mixture. Comp Biochem Physiol Part - C Toxicol Pharmacol 218:70–80. https://doi.org/10.1016/j.cbpc.2019.01.003
Wu P, Jiang WD, Liu Y et al (2014) Effect of choline on antioxidant defenses and gene expressions of Nrf2 signaling molecule in the spleen and head kidney of juvenile Jian carp (Cyprinus carpio var. Jian). Fish Shellfish Immunol 38:374–382. https://doi.org/10.1016/j.fsi.2014.03.032
Li Z-H, Li P, Shi Z-C (2015) Responses of the hepatic glutathione antioxidant defense system and related gene expression in juvenile common carp after chronic treatment with tributyltin. Ecotoxicology 24:700–705. https://doi.org/10.1007/s10646-014-1416-2
Hoseinifar SH, Ahmadi A, Khalili M et al (2017) The study of antioxidant enzymes and immune-related genes expression in common carp (Cyprinus carpio) fingerlings fed different prebiotics. Aquac Res 48:5447–5454. https://doi.org/10.1111/are.13359
Xing H, Zhang Z, Yao H et al (2014) Effects of atrazine and chlorpyrifos on cytochrome P450 in common carp liver. Chemosphere 104:244–250. https://doi.org/10.1016/j.chemosphere.2014.01.002
Zhang C, Zhang J, Fan W et al (2019) Effects of dietary Lactobacillus delbrueckii on growth performance, body composition, digestive and absorptive capacity, and gene expression of common carp (Cyprinus carpio Huanghe var). Aquac Nutr 25:166–175. https://doi.org/10.1111/anu.12840
Acknowledgements
The author Rajkumar Krishnasamy Sekar gratefully acknowledges the Department of Science and Technology (DST), Govt. of India, for providing fellowship under the DST-INSPIRE Fellowship (IF140546) scheme. The authors thank National Centre for Alternatives to Animal Experiments (NCAAE) under UGC-CPEPA scheme, Government of India (F.No.2-1/2013(NS/PE)), for RTqPCR studies in this work. The authors highly acknowledge the UGC-SAP-DRS-II (F.3-9/2013(SAP-II)), Department of Science and Technology Fund for Improvement of Science and Technology Infrastructure (DST-FIST)-Level-I (stage-II) (Ref. No. SR/FST/LSI-647/2015(C) Date.11.08.2016) and Department of Science and Technology Promotion of University Research and Scientific Excellence (DST PURSE Phase—II) (Ref. No. SR/PURSE PHASE 2/16(G)/&16(C) Date. 21.02.2017) for the instrumentation facility to the Department of Animal Science, Bharathidasan University, Tiruchirappalli 620 024, Tamil Nadu, India. The authors also thank “RUSA, 2.0—Biological Sciences, Bharathidasan University.”
Author information
Authors and Affiliations
Contributions
Investigation, methodology, data curation, writing — original draft: Rajkumar Krishnasamy Sekar; methodology, resources: Ramkumar Arunachalam, Murugadas Anbazhagan, and Sivagaami Palaniyappan; formal analysis, validation, writing — review and editing: Srinivasan Veeran and Arun Sridhar; conceptualization, project administration, supervision, validation, visualization, writing — review and editing: Thirumurugan Ramasamy. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Institutional Animal Ethical Committee (IAEC) of Bharathidasan University, Tiruchirappalli 620 024, Tamil Nadu, India, has approved this research work (Ref. No: BDU/IAEC/P28/2018).
Consent for Publication
All authors read and approved the final manuscript and have complete access to study data and agree with this manuscript publication.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised. equations 1 and 2 should be: 1. \(\mathrm{MCV }(\mathrm{cu}.\mathrm{ microns}) =\mathrm{ Hct}/\mathrm{RBC }(10^6/\mathrm{ml})\) 2. \(\mathrm{MCH }(\mathrm{pg}) = [\mathrm{Hb }(\mathrm{g}/\mathrm{l}) \times 10]/\mathrm{RBC }(10^6/\mathrm{ml})\)
Rights and permissions
About this article
Cite this article
Krishnasamy Sekar, R., Arunachalam, R., Anbazhagan, M. et al. Accumulation, Chronicity, and Induction of Oxidative Stress Regulating Genes Through Allium cepa L. Functionalized Silver Nanoparticles in Freshwater Common Carp (Cyprinus carpio). Biol Trace Elem Res 201, 904–925 (2023). https://doi.org/10.1007/s12011-022-03164-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03164-z