Abstract
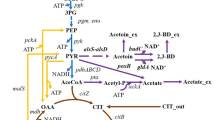
Poly-γ-glutamic acid (γ-PGA) is an anionic polymer with wide-ranging applications in the areas of medicine, light chemical industry, wastewater treatment, and agriculture. However, the production cost of γ-PGA is high for the requirement of adding the expensive precursor L-glutamic acid during fermentation, which hinders its widespread application. In this study, in order to improve γ-PGA yield, central carbon metabolism was engineered to enhance the carbon flux of tricarboxylic acid (TCA) cycle and glutamic acid synthesis in a γ-PGA production strain Bacillus licheniformis WX-02. Firstly, pyruvate dehydrogenase (PdhABCD) and citrate synthase (CitA) were overexpressed to strengthen the flux of pyruvate into TCA cycle, resulting in 34.93% and 11.14% increase of γ-PGA yield in B. licheniformis WX-02, respectively. Secondly, the carbon flux to glyoxylate shunt was rewired via varying the expression of isocitrate lyase (AceA), and a 23.24% increase of γ-PGA yield was obtained in AceA down-regulated strain WXPbacAaceBA. Thirdly, deletion of pyruvate formate-lyase gene pflB led to a 30.70% increase of γ-PGA yield. Finally, combinatorial metabolic engineering was applied, and γ-PGA titer was enhanced to 12.02 g/L via overexpressing pdhABCD and citA, repressing aceA, and deleting pflB, with a 69.30% improvement compared to WX-02. Collectively, metabolic engineering of central carbon metabolism is an effective strategy for enhanced γ-PGA production in B. licheniformis, and this research provided a promising strain for industrial production of γ-PGA.





Similar content being viewed by others
References
Sirisansaneeyakul, S., Cao, M., Kongklom, N., Chuensangjun, C., Shi, Z., & Chisti, Y. (2017). Microbial production of poly-gamma-glutamic acid. World Journal of Microbiology & Biotechnology, 33(9), 173.
Xu, G., Zha, J., Cheng, H., Ibrahim, M. H. A., Yang, F., Dalton, H., Cao, R., Zhu, Y., Fang, J., Chi, K., Zheng, P., Zhang, X., Shi, J., Xu, Z., Gross, R. A., & Koffas, M. A. G. (2019). Engineering Corynebacterium glutamicum for the de novo biosynthesis of tailored poly-gamma-glutamic acid. Metabolic Engineering, 56, 39–49.
Zhan, Y., Sheng, B., Wang, H., Shi, J., Cai, D., Yi, L., Yang, S., Wen, Z., Ma, X., & Chen, S. (2018). Rewiring glycerol metabolism for enhanced production of poly-gamma-glutamic acid in Bacillus licheniformis. Biotechnology for Biofuels, 11, 306.
Cai, D., He, P., Lu, X., Zhu, C., Zhu, J., Zhan, Y., Wang, Q., Wen, Z., & Chen, S. (2017). A novel approach to improve poly-gamma-glutamic acid production by NADPH regeneration in Bacillus licheniformis WX-02. Scientific Reports, 7, 43404.
Zhang, W., He, Y., Gao, W., Feng, J., Cao, M., Yang, C., Song, C., & Wang, S. (2015). Deletion of genes involved in glutamate metabolism to improve poly-gamma-glutamic acid production in B. amyloliquefaciens LL3. Journal of Industrial Microbiology & Biotechnology, 42(2), 297–305.
Feng, J., Quan, Y., Gu, Y., Liu, F., Huang, X., Shen, H., Dang, Y., Cao, M., Gao, W., Lu, X., Wang, Y., Song, C., & Wang, S. (2017). Enhancing poly-gamma-glutamic acid production in Bacillus amyloliquefaciens by introducing the glutamate synthesis features from Corynebacterium glutamicum. Microbial Cell Factories, 16(1), 88.
Qin, N., Li, L., Ji, X., Li, X., Zhang, Y., Larsson, C., Chen, Y., Nielsen, J., & Liu, Z. (2020). Rewiring central carbon metabolism ensures increased provision of Acetyl-CoA and NADPH required for 3-OH-propionic acid production. Acs Synthetic Biology, 9(12), 3236–3244.
Kopp, D., & Sunna, A. (2020). Alternative carbohydrate pathways - enzymes, functions and engineering. Critical Reviews in Biotechnology, 40(7), 895–912.
Doi, S., Komatsu, M., & Ikeda, H. (2020). Modifications to central carbon metabolism in an engineered Streptomyces host to enhance secondary metabolite production. Journal of Bioscience and Bioengineering, 130(6), 563–570.
Liu, H., Song, R., Liang, Y., Zhang, T., Deng, L., Wang, F., & Tan, T. (2018). Genetic manipulation of Escherichia coli central carbon metabolism for efficient production of fumaric acid. Bioresource Technology, 270, 96–102.
Choi, S., Kim, H. U., Kim, T. Y., & Lee, S. Y. (2016). Systematic engineering of TCA cycle for optimal production of a four-carbon platform chemical 4-hydroxybutyric acid in Escherichia coli. Metabolic Engineering, 38, 264–273.
Gu, Y., Lv, X., Liu, Y., Li, J., Du, G., Chen, J., Rodrigo, L. A., & Liu, L. (2019). Synthetic redesign of central carbon and redox metabolism for high yield production of N-acetylglucosamine in Bacillus subtilis. Metabolic Engineering, 51, 59–69.
Liu, Z., Yu, W., Nomura, C. T., Li, J., Chen, S., Yang, Y., & Wang, Q. (2018). Increased flux through the TCA cycle enhances bacitracin production by Bacillus licheniformis DW2. Applied Microbiology and Biotechnology, 102(16), 6935–6946.
Gao, W., He, Y., Zhang, F., Zhao, F., Huang, C., Zhang, Y., Zhao, Q., Wang, S., & Yang, C. (2019). Metabolic engineering of Bacillus amyloliquefaciens LL3 for enhanced poly-gamma-glutamic acid synthesis. Microbial Biotechnology, 12(5), 932–945.
Zhang, H., Zhu, J., Zhu, X., Cai, J., Zhang, A., Hong, Y., Huang, J., Huang, L., & Xu, Z. (2012). High-level exogenous glutamic acid-independent production of poly-(gamma-glutamic acid) with organic acid addition in a new isolated Bacillus subtilis C10. Bioresource Technology, 116, 241–246.
Li, B. C., Cai, D. B., Hu, S. Y., Zhu, A. T., He, Z. L., & Chen, S. W. (2018). Enhanced synthesis of poly gamma glutamic acid by increasing the intracellular reactive oxygen species in the Bacillus licheniformis 1-pyrroline-5-carboxylate dehydrogenase gene ycgN-deficient strain. Applied Microbiology and Biotechnology, 102(23), 10127–10137.
Cai, D., Chen, Y., He, P., Wang, S., Mo, F., Li, X., Wang, Q., Nomura, C. T., Wen, Z., Ma, X., & Chen, S. (2018). Enhanced production of poly-gamma-glutamic acid by improving ATP supply in metabolically engineered Bacillus licheniformis. Biotechnology and Bioengineering, 115(10), 2541–2553.
Wang, J., Yuan, H., Wei, X., Chen, J., & Chen, S. (2016). Enhancement of poly-γ-glutamic acid production by alkaline pH stress treatment in Bacillus licheniformis WX-02. Journal of Chemical Technology & Biotechnology, 91(9), 2399–2403.
Wei, X., Ji, Z., & Chen, S. (2010). Isolation of halotolerant Bacillus licheniformis WX-02 and regulatory effects of sodium chloride on yield and molecular sizes of poly-gamma-glutamic acid. Applied Biochemistry and Biotechnology, 160(5), 1332–1340.
Cai, D., Hu, S., Chen, Y., Liu, L., Yang, S., Ma, X., & Chen, S. (2018). Enhanced production of poly-gamma-glutamic acid by overexpression of the global anaerobic regulator Fnr in Bacillus licheniformis WX-02. Applied Biochemistry and Biotechnology, 185(4), 958–970.
Nemeria, N., Yan, Y., Zhang, Z., Brown, A. M., Arjunan, P., Furey, W., Guest, J. R., & Jordan, F. (2001). Inhibition of the Escherichia coli pyruvate dehydrogenase complex E1 subunit and its tyrosine 177 variants by thiamin 2-thiazolone and thiamin 2-thiothiazolone diphosphates. Evidence for reversible tight-binding inhibition. The Journal of Biological Chemistry, 276(49), 45969–45978.
Schendel, F. J., August, P. R., Anderson, C. R., Hanson, R. S., & Flickinger, M. C. (1992). Cloning and nucleotide sequence of the gene coding for citrate synthase from a thermotolerant Bacillus sp. Applied and Environmental Microbiology, 58(1), 335–345.
Li, N., Zhang, B., Chen, T., Wang, Z., Tang, Y. J., & Zhao, X. (2013). Directed pathway evolution of the glyoxylate shunt in Escherichia coli for improved aerobic succinate production from glycerol. Journal of Industrial Microbiology & Biotechnology, 40(12), 1461–1475.
Sonenshein, A. L. (2007). Control of key metabolic intersections in Bacillus subtilis. Nature Reviews Microbiology, 5(12), 917–927.
Guo, H. W., Madzak, C., Du, G. C., Zhou, J. W., & Chen, J. (2014). Effects of pyruvate dehydrogenase subunits overexpression on the alpha-ketoglutarate production in Yarrowia lipolytica WSH-Z06. Applied Microbiology and Biotechnology, 98(16), 7003–7012.
Sha, Y., Sun, T., Qiu, Y., Zhu, Y., Zhan, Y., Zhang, Y., Xu, Z., Li, S., Feng, X., & Xu, H. (2019). Investigation of glutamate dependence mechanism for poly-gamma-glutamic acid production in Bacillus subtilis on the basis of transcriptome analysis. Journal of Agricultural and Food Chemistry, 67(22), 6263–6274.
Yangtse, W., Zhou, Y., Lei, Y., Qiu, Y., Wei, X., Ji, Z., Qi, G., Yong, Y., Chen, L., & Chen, S. (2012). Genome sequence of Bacillus licheniformis WX-02. Journal of Bacteriology., 194(13), 3561–3562.
Stokell, D. J., Donald, L. J., Maurus, R., Nguyen, N. T., Sadler, G., Choudhary, K., Hultin, P. G., Brayer, G. D., & Duckworth, H. W. (2003). Probing the roles of key residues in the unique regulatory NADH binding site of type II citrate synthase of Escherichia coli. The Journal of Biological Chemistry, 278(37), 35435–35443.
Yu, B. Q., Shen, W., Wang, Z. X., & Zhuge, J. (2005). Glyoxylate cycle is required for the overproduction of glutamate but is not essential for Corynebacterium glutamicum growth on glucose. Sheng Wu Gong Cheng Xue Bao, 21(2), 270–274.
Deng, Y., Ma, N., Zhu, K., Mao, Y., Wei, X., & Zhao, Y. (2018). Balancing the carbon flux distributions between the TCA cycle and glyoxylate shunt to produce glycolate at high yield and titer in Escherichia coli. Metabolic Engineering, 46, 28–34.
Alexeeva, S., de Kort, B., Sawers, G., Hellingwerf, K. J., & de Mattos, M. J. (2000). Effects of limited aeration and of the ArcAB system on intermediary pyruvate catabolism in Escherichia coli. Journal of Bacteriology, 182(17), 4934–4940.
Qi, G., Kang, Y., Li, L., Xiao, A., Zhang, S., Wen, Z., Xu, D., & Chen, S. (2014). Deletion of meso-2,3-butanediol dehydrogenase gene budC for enhanced D-2,3-butanediol production in Bacillus licheniformis. Biotechnology for Biofuels, 7(1), 16.
Mitsunaga, H., Meissner, L., Palmen, T., Bamba, T., Buchs, J., & Fukusaki, E. (2016). Metabolome analysis reveals the effect of carbon catabolite control on the poly(gamma-glutamic acid) biosynthesis of Bacillus licheniformis ATCC 9945. Journal of Bioscience and Bioengineering, 121(4), 413–419.
Fu, J., Huo, G., Feng, L., Mao, Y., Wang, Z., Ma, H., Chen, T., & Zhao, X. (2016). Metabolic engineering of Bacillus subtilis for chiral pure meso-2,3-butanediol production. Biotechnology for Biofuels, 9, 90.
Fu, J., Wang, Z., Chen, T., Liu, W., Shi, T., Wang, G., Tang, Y. J., & Zhao, X. (2014). NADH plays the vital role for chiral pure D-(-)-2,3-butanediol production in Bacillus subtilis under limited oxygen conditions. Biotechnology and Bioengineering, 111(10), 2126–2131.
Rebecchi, S., Pinelli, D., Zanaroli, G., Fava, F., & Frascari, D. (2018). Effect of oxygen mass transfer rate on the production of 2,3-butanediol from glucose and agro-industrial byproducts by Bacillus licheniformis ATCC9789. Biotechnology for Biofuels, 11, 145.
Cruz Ramos, H., Hoffmann, T., Marino, M., Nedjari, H., Presecan-Siedel, E., Dreesen, O., Glaser, P., & Jahn, D. (2000). Fermentative metabolism of Bacillus subtilis: Physiology and regulation of gene expression. Journal of Bacteriology, 182(11), 3072–3080.
Guo, J., Zhang, H., Wang, C., Chang, J. W., & Chen, L. L. (2016). Construction and analysis of a genome-scale metabolic network for Bacillus licheniformis WX-02. Research in Microbiology, 167(4), 282–289.
Guo, J., Cheng, G., Gou, X. Y., Xing, F., Li, S., Han, Y. C., Wang, L., Song, J. M., Shu, C. C., Chen, S. W., & Chen, L. L. (2015). Comprehensive transcriptome and improved genome annotation of Bacillus licheniformis WX-02. FEBS Letters, 589(18), 2372–2381.
Huo, Y., Zhan, Y., Wang, Q., Li, S., Yang, S., Nomura, C. T., Wang, C., & Chen, S. (2018). Acetolactate synthase (AlsS) in Bacillus licheniformis WX-02: Enzymatic properties and efficient functions for acetoin/butanediol and L-valine biosynthesis. Bioprocess and Biosystems Engineering, 41(1), 87–96.
Kozak, B. U., van Rossum, H. M., Luttik, M. A., Akeroyd, M., Benjamin, K. R., Wu, L., de Vries, S., Daran, J. M., Pronk, J. T., & van Maris, A. J. (2014). Engineering acetyl coenzyme A supply: Functional expression of a bacterial pyruvate dehydrogenase complex in the cytosol of Saccharomyces cerevisiae. MBio, 5(5), e01696-e1614.
Chiang, C. J., Ho, Y. J., Hu, M. C., & Chao, Y. P. (2020). Rewiring of glycerol metabolism in Escherichia coli for effective production of recombinant proteins. Biotechnology for Biofuels, 13(1), 205.
Li, Y., Huang, B., Wu, H., Li, Z., Ye, Q., & Zhang, Y. P. (2016). Production of succinate from acetate by metabolically engineered Escherichia coli. Acs Synthetic Biology., 5(11), 1299–1307.
Liu, M., Ding, Y., Chen, H., Zhao, Z., Liu, H., Xian, M., & Zhao, G. (2017). Improving the production of acetyl-CoA-derived chemicals in Escherichia coli BL21(DE3) through iclR and arcA deletion. Bmc Microbiology, 17(1), 10.
Zhao, H., Fang, Y., Wang, X., Zhao, L., Wang, J., & Li, Y. (2018). Increasing L-threonine production in Escherichia coli by engineering the glyoxylate shunt and the L-threonine biosynthesis pathway. Applied Microbiology and Biotechnology, 102(13), 5505–5518.
Zhu, L., Fang, Y., Ding, Z., Zhang, S., & Wang, X. (2019). Developing an l-threonine-producing strain from wild-type Escherichia coli by modifying the glucose uptake, glyoxylate shunt, and l-threonine biosynthetic pathway. Biotechnology and Applied Biochemistry, 66(6), 962–976.
Jo, M., Noh, M. H., Lim, H. G., Kang, C. W., Im, D. K., Oh, M. K., & Jung, G. Y. (2019). Precise tuning of the glyoxylate cycle in Escherichia coli for efficient tyrosine production from acetate. Microbial Cell Factories, 18(1), 57.
Noh, M. H., Lim, H. G., Park, S., Seo, S. W., & Jung, G. Y. (2017). Precise flux redistribution to glyoxylate cycle for 5-aminolevulinic acid production in Escherichia coli. Metabolic Engineering, 43(Pt A), 1–8.
Funding
This work was supported by the Educational Research Projects for Young and Middle-Aged Teachers in Fujian Province (No. JAT190788) and Fujian Provincial Key Laboratory of Eco-Industrial Green Technology (No. WYKF-GCT2020-3).
Author information
Authors and Affiliations
Contributions
B Li, D Cai, and S Chen designed the study. B Li carried out the molecular biology studies, construction of engineering strains, and the fermentation studies. B Li, D Cai, and S Chen analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, B., Cai, D. & Chen, S. Metabolic Engineering of Central Carbon Metabolism of Bacillus licheniformis for Enhanced Production of Poly-γ-glutamic Acid. Appl Biochem Biotechnol 193, 3540–3552 (2021). https://doi.org/10.1007/s12010-021-03619-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03619-4