Abstract
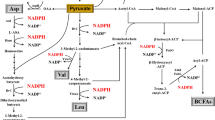
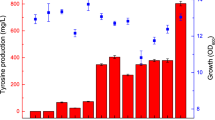
Acetolactate synthase catalyzes two molecules of pyruvates to form α-acetolactate, which is further converted to acetoin and 2,3-butanediol. In this study, by heterologous expression in Escherichia coli, the enzymatic properties of acetolactate synthase (AlsS) from Bacillus licheniformis WX-02 were characterized. Its K m and k cat for pyruvate were 3.96 mM and 514/s, respectively. It has the optimal activity at pH 6.5, 37 °C and was feedback inhibited by l-valine, l-leucine and l-isoleucine. Furthermore, the alsS-deficient strain could not produce acetoin, 2,3-butanediol, and l-valine, while the complementary strain was able to restore these capacities. The alsS overexpressing strain produced higher amounts of acetoin/2,3-butanediol (57.06 g/L) and l-valine (2.68 mM), which were 10.90 and 92.80% higher than those of the control strain, respectively. This is the first report regarding the in-depth understanding of AlsS enzymatic properties and its functions in B. licheniformis, and overexpression of AlsS can effectively improve acetoin/2,3-butanediol and l-valine production in B. licheniformis. We envision that this AlsS can also be applied in the improvement of acetoin/2,3-butanediol and l-valine production in other microbes.





Similar content being viewed by others
References
Xiao Z, Lu JR (2014) Strategies for enhancing fermentative production of acetoin: a review. Biotechnol Adv 32:492–503
Zhang L, Liu Q, Ge Y, Li L, Gao C, Xu P, Ma C (2016) Biotechnological production of acetoin, a bio-based platform chemical, from a lignocellulosic resource by metabolically engineered Enterobacter cloacae. Green Chem 18:1560–1570
Ge Y, Li K, Li L, Gao C, Zhang L, Ma C, Xu P (2016) Contracted but effective: production of enantiopure 2,3-butanediol by thermophilic and GRAS Bacillus licheniformis. Green Chem 18:4693–4703
Kim SJ, Seo SO, Jin YS, Seo JH (2013) Production of 2,3-butanediol by engineered Saccharomyces cerevisiae. Bioresour Technol 146:274–281
Lu M, Park C, Lee S, Kim B, Oh M-K, Um Y, Kim J, Lee J (2014) The regulation of 2,3-butanediol synthesis in Klebsiella pneumoniae as revealed by gene over-expressions and metabolic flux analysis. Bioprocess Biosyst Eng 37:343–353
Blombach B, Schreiner ME, Bartek T, Oldiges M, Eikmanns BJ (2008) Corynebacterium glutamicum tailored for high-yield l-valine production. Appl Microbiol Biotechnol 79:471–479
Liang C, Huo Y, Qi G, Wei X, Wang Q, Chen S (2015) Enhancement of l-valine production in Bacillus licheniformis by blocking three branched pathways. Biotechnol Lett 37:1243–1248
Bartek T, Rudolf C, Kerßen U, Klein B, Blombach B, Lang S, Eikmanns BJ, Oldiges M (2010) Studies on substrate utilisation in l-valine-producing Corynebacterium glutamicum strains deficient in pyruvate dehydrogenase complex. Bioprocess Biosyst Eng 33:873–883
Lee S-C, Kim J, La I-J, Kim S-K, Yoon M-Y (2013) Characterization of recombinant FAD-independent catabolic acetolactate synthase from Enterococcus faecalis V583. Enzyme Microb Technol 52:54–59
Renna MC, Najimudin N, Winik LR, Zahler SA (1993) Regulation of the Bacillus subtilisalsS, alsD, and alsR genes involved in post-exponential-phase production of acetoin. J Bacteriol 175:3863–3875
Lee S-C, Jung I-P, Baig I-A, Chien P-N, La I-J, Yoon M-Y (2015) Mutational analysis of critical residues of FAD-independent catabolic acetolactate synthase from Enterococcus faecalis V583. Int J Biol Macromol 72:104–109
Pang SS, Duggleby RG, Guddat LW (2002) Crystal structure of yeast acetohydroxyacid synthase: a target for herbicidal inhibitors. J Mol Biol 317:249–262
Park JH, Jang YS, Lee JW, Lee SY (2011) Escherichia coli W as a new platform strain for the enhanced production of l-valine by systems metabolic engineering. Biotechnol Bioeng 108:1140–1147
Chang AK, Duggleby RG (1997) Expression, purification and characterization of Arabidopsis thaliana acetohydroxyacid synthase. Biochem J 327:161–169
Duggleby RG, Pang SS (2000) Acetohydroxyacid synthase. BMB Rep 33:1–36
Holtzclaw WD, Chapman LF (1975) Degradative acetolactate synthase of Bacillus subtilis: purification and properties. J Bacteriol 121:917–922
Kisrieva IuS, Serebrennikov VM, Zagustina NA, Bezborodov AM (2000) Isolation and purification of acetolactate synthase and acetolactate decarboxylase from a Lactococcus lactis culture. Prikl Biokhim Mikrobiol 36:131–137
Snoep JL, De Mattos MT, Starrenburg MJ, Hugenholtz J (1992) Isolation, characterization, and physiological role of the pyruvate dehydrogenase complex and alpha-acetolactate synthase of Lactococcus lactis subsp. lactis bv. diacetylactis. J Bacteriol 174:4838–4841
Phalip V, Schmitt P, Diviès C (1995) Purification and characterization of the catabolic α-acetolactate synthase from Leuconostoc mesenteroides subsp. cremoris. Curr Microbiol 31:316–321
Peng HL, Wang PY, Wu CM, Hwang DC, Chang HY (1992) Cloning, sequencing and heterologous expression of a Klebsiella pneumoniae gene encoding an FAD-independent acetolactate synthase. Gene 117:125–130
Ng CY, Jung MY, Lee J, Oh MK (2012) Production of 2,3-butanediol in Saccharomyces cerevisiae by in silico aided metabolic engineering. Microb Cell Fact 11:68
Nguyen DM, Lipscomb GL, Schut GJ, Vaccaro BJ, Basen M, Kelly RM, Adams MW (2016) Temperature-dependent acetoin production by Pyrococcus furiosus is catalyzed by a biosynthetic acetolactate synthase and its deletion improves ethanol production. Metab Eng 34:71–79
Qi G, Kang Y, Li L, Xiao A, Zhang S, Wen Z, Xu D, Chen S (2014) Deletion of meso-2,3-butanediol dehydrogenase gene budC for enhanced D-2,3-butanediol production in Bacillus licheniformis. Biotechnol Biofuels 7:16
Wei X, Ji Z, Chen S (2010) Isolation of halotolerant Bacillus licheniformis WX-02 and regulatory effects of sodium chloride on yield and molecular sizes of poly-gamma-glutamic acid. Appl Biochem Biotechnol 160:1332–1340
Liu Y, Zhang S, Yong Y-C, Ji Z, Ma X, Xu Z, Chen S (2011) Efficient production of acetoin by the newly isolated Bacillus licheniformis strain MEL09. Process Biochem 46:390–394
Tian G, Fu J, Wei X, Ji Z, Ma X, Qi G, Chen S (2014) Enhanced expression of pgdS gene for high production of poly-γ-glutamic aicd with lower molecular weight in Bacillus licheniformis WX-02. J Chem Technol Biotechnol 89:1825–1832
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Singh BK, Stidham MA, Shaner DL (1988) Assay of acetohydroxyacid synthase. Anal Biochem 171:173–179
Qiu Y, Zhang J, Li L, Wen Z, Nomura CT, Wu S, Chen S (2016) Engineering Bacillus licheniformis for the production of meso-2,3-butanediol. Biotechnol Biofuels 9:117
Xue GP, Johnson JS, Dalrymple BP (1999) High osmolarity improves the electro-transformation efficiency of the gram-positive bacteria Bacillus subtilis and Bacillus licheniformis. J Microbiol Methods 34:183–191
Fradrich C, March A, Fiege K, Hartmann A, Jahn D, Hartig E (2012) The transcription factor AlsR binds and regulates the promoter of the alsSD operon responsible for acetoin formation in Bacillus subtilis. J Bacteriol 194:1100–1112
Tsau JL, Guffanti AA, Montville TJ (1992) Conversion of pyruvate to acetoin helps to maintain pH homeostasis in Lactobacillus plantarum. Appl Environ Microbiol 58:891–894
Guo Y, Han M, Xu J, Zhang W (2015) Analysis of acetohydroxyacid synthase variants from branched-chain amino acids-producing strains and their effects on the synthesis of branched-chain amino acids in Corynebacterium glutamicum. Protein Expr Purif 109:106–112
Radmacher E, Vaitsikova A, Burger U, Krumbach K, Sahm H, Eggeling L (2002) Linking central metabolism with increased pathway flux: l-valine accumulation by Corynebacterium glutamicum. Appl Environ Microbiol 68:2246–2250
Park JH, Lee KH, Kim TY, Lee SY (2007) Metabolic engineering of Escherichia coli for the production of l-valine based on transcriptome analysis and in silico gene knockout simulation. Proc Natl Acad Sci USA 104:7797–7802
Gao X, Xu N, Li S, Liu L (2014) Metabolic engineering of Candida glabrata for diacetyl production. PLoS One 9:e89854
Atsumi S, Li Z, Liao JC (2009) Acetolactate synthase from Bacillus subtilis serves as a 2-ketoisovalerate decarboxylase for isobutanol biosynthesis in Escherichia coli. Appl Environ Microbiol 75:6306–6311
Kaushal A, Pabbi S, Sharma P (2003) Characterization of 2,3-butanediol-forming and valine-sensitive α-acetolactate synthase of Enterobacter cloacae. World J Microbiol Biotechnol 19:487–493
Zhu Y, Chen X, Chen T, Zhao X (2007) Enhancement of riboflavin production by overexpression of acetolactate synthase in a pta mutant of Bacillus subtilis. FEMS Microbiol Lett 266:224–230
Acknowledgements
This work was supported by National Program on Key Basic Research Project (973 Program, No. 2015CB150505), the Science and Technology Program of Wuhan (20160201010086), rural areas of the national science and technology plan in the 12th five-year plan of China (No. 2013AA102801-52).
Author information
Authors and Affiliations
Contributions
SC designed and supervised the study. YH and YZ performed the experiments. YH, YZ, QW, SL, SY, CN, CW and SC analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Huo, Y., Zhan, Y., Wang, Q. et al. Acetolactate synthase (AlsS) in Bacillus licheniformis WX-02: enzymatic properties and efficient functions for acetoin/butanediol and l-valine biosynthesis. Bioprocess Biosyst Eng 41, 87–96 (2018). https://doi.org/10.1007/s00449-017-1847-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-017-1847-2