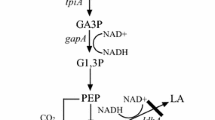
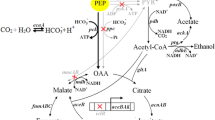
Abstract
α-Ketoglutarate is accumulated as the main byproduct during the aerobic succinate production from glycerol by Escherichia coli BL21(DE3) in minimal medium. To address this issue, here a strategy of directed pathway evolution was developed to enhance the alternative succinate production route—the glyoxylate shunt. Via the directed pathway evolution, the glyoxylate shunt was recruited as the primary anaplerotic pathway in a ppc mutant, which restored its viability in glycerol minimal medium. Subsequently, the operon sdhCDAB was deleted and the gene ppc was reverted in the evolved strain for succinate production. The resulting strain E2-Δsdh-ppc produced 30 % more succinate and 46 % less α-ketoglutarate than the control strain. A G583T mutation in gene icdA, which significantly decreased the activity of isocitrate dehydrogenase, was identified in the evolved strain as the main mutation responsible for the observed phenotype. Overexpression of α-ketoglutarate dehydrogenase complex in E2-Δsdh-ppc further reduced the amount of byproduct and improved succinate production. The final strain E2-Δsdh-ppc-sucAB produced 366 mM succinate from 1.3 M glycerol in minimal medium in fed-batch fermentation. The maximum and average succinate volumetric productivities were 19.2 and 6.55 mM h−1, respectively, exhibiting potential industrial production capacity from the low-priced substrate.






Similar content being viewed by others
References
Aoshima M, Ishii M, Yamagishi A, Oshima T, Igarashi Y (2003) Metabolic characteristics of an isocitrate dehydrogenase defective derivative of Escherichia coli BL21(DE3). Biotechnol Bioeng 84(6):732–737
Beauprez JJ, De Mey M, Soetaert WK (2010) Microbial succinic acid production: natural versus metabolic engineered producers. Process Biochem 45(7):1103–1114. doi:10.1016/j.procbio.2010.03.035
Blankschien MD, Clomburg JM, Gonzalez R (2010) Metabolic engineering of Escherichia coli for the production of succinate from glycerol. Metab Eng 12(5):409–419. doi:10.1016/j.ymben.2010.06.002
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chell RM, Sundaram TK, Wilkinson AE (1978) Isolation and characterization of isocitrate lyase from a thermophilic Bacillus sp. Biochem J 173(1):165–177
Cherepanov PP, Wackernagel W (1995) Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158(1):9–14. doi:10.1016/0378-1119(95)00193-A
Cortay JC, Nègre D, Scarabel M, Ramseier TM, Vartak NB, Reizer J, Saier MH, Cozzone AJ (1994) In vitro asymmetric binding of the pleiotropic regulatory protein, FruR, to the ace operator controlling glyoxylate shunt enzyme synthesis. J Biol Chem 269(21):14885–14891
da Silva GP, Mack M, Contiero J (2009) Glycerol: a promising and abundant carbon source for industrial microbiology. Biotechnol Adv 27(1):30–39. doi:10.1016/j.biotechadv.2008.07.006
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA 97(12):6640–6645. doi:10.1073/pnas.120163297
Dharmadi Y, Murarka A, Gonzalez R (2006) Anaerobic fermentation of glycerol by Escherichia coli: a new platform for metabolic engineering. Biotechnol Bioeng 94(5):821–829. doi:10.1002/bit.21025
Dobson R, Gray V, Rumbold K (2012) Microbial utilization of crude glycerol for the production of value-added products. J Ind Microbiol Biotechnol 39(2):217–226. doi:10.1007/s10295-011-1038-0
Fong SS (2006) Latent pathway activation and increased pathway capacity enable Escherichia coli adaptation to loss of key metabolic enzymes. J Biol Chem 281(12):8024–8033. doi:10.1074/jbc.M510016200
Gonzalez R, Murarka A, Dharmadi Y, Yazdani SS (2008) A new model for the anaerobic fermentation of glycerol in enteric bacteria: trunk and auxiliary pathways in Escherichia coli. Metab Eng 10(5):234–245. doi:10.1016/j.ymben.2008.05.001
Hoyt JC, Robertson EF, Berlyn KA, Reeves HC (1988) Escherichia coli isocitrate lyase: properties and comparisons. Biochim Biophys Acta 966(1):30–35. doi:10.1016/0304-4165(88)90125-0
Joyce AR, Reed JL, White A, Edwards R, Osterman A, Baba T, Mori H, Lesely SA, Palsson BO, Agarwalla S (2006) Experimental and computational assessment of conditionally essential genes in Escherichia coli. J Bacteriol 188(23):8259–8271. doi:10.1128/Jb.00740-06
LaPorte DC (1993) The isocitrate dehydrogenase phosphorylation cycle: regulation and enzymology. J Cell Biochem 51(1):14–18
Lee EC, Yu D, Martinez de Velasco J, Tessarollo L, Swing DA, Court DL, Jenkins NA, Copeland NG (2001) A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics 73(1):56–65
Lee PC, Lee SY, Chang HN (2009) Kinetic study on succinic acid and acetic acid formation during continuous cultures of Anaerobiospirillum succiniciproducens grown on glycerol. Bioprocess Biosyst Eng 33(4):465–471. doi:10.1007/s00449-009-0355-4
Lee PC, Lee WG, Lee SY, Chang HN (2001) Succinic acid production with reduced by-product formation in the fermentation of Anaerobiospirillum succiniciproducens using glycerol as a carbon source. Biotechnol Bioeng 72(1):41–48. doi:10.1002/1097-0290(20010105)72:1<41:aid-bit6>3.0.co;2-n
Lin H, Bennett GN, San K-Y (2005) Chemostat culture characterization of Escherichia coli mutant strains metabolically engineered for aerobic succinate production: a study of the modified metabolic network based on metabolite profile, enzyme activity, and gene expression profile. Metab Eng 7(5–6):337–352. doi:10.1016/j.ymben.2005.06.002
Lin H, Bennett GN, San K-Y (2005) Fed-batch culture of a metabolically engineered Escherichia coli strain designed for high-level succinate production and yield under aerobic conditions. Biotechnol Bioeng 90(6):775–779. doi:10.1002/bit.20458
Lin H, Bennett GN, San K-Y (2005) Genetic reconstruction of the aerobic central metabolism in Escherichia coli for the absolute aerobic production of succinate. Biotechnol Bioeng 89(2):148–156. doi:10.1002/bit.20298
Lin H, Bennett GN, San K-Y (2005) Metabolic engineering of aerobic succinate production systems in Escherichia coli to improve process productivity and achieve the maximum theoretical succinate yield. Metab Eng 7(2):116–127. doi:10.1016/j.ymben.2004.10.003
Litsanov B, Kabus A, Brocker M, Bott M (2012) Efficient aerobic succinate production from glucose in minimal medium with Corynebacterium glutamicum. Microb Biotechnol 5(1):116–128. doi:10.1111/j.1751-7915.2011.00310.x
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2 −ΔΔCT method. Methods 25(4):402–408
Martínez I, Bennett GN, San K-Y (2010) Metabolic impact of the level of aeration during cell growth on anaerobic succinate production by an engineered Escherichia coli strain. Metab Eng 12(6):499–509. doi:10.1016/j.ymben.2010.09.002
Morgunov I, Srere PA (1998) Interaction between citrate synthase and malate dehydrogenase: substrate channeling of oxaloacetate. J Biol Chem 273(45):29540–29544. doi:10.1074/jbc.273.45.29540
Murarka A, Dharmadi Y, Yazdani SS, Gonzalez R (2008) Fermentative utilization of glycerol by Escherichia coli and its implications for the production of fuels and chemicals. Appl Environ Microbiol 74(4):1124–1135. doi:10.1128/AEM.02192-07
Noronha SB, Yeh HJC, Spande TF, Shiloach J (2000) Investigation of the TCA cycle and the glyoxylate shunt in Escherichia coli BL21 and JM109 using 13C-NMR/MS. Biotechnol Bioeng 68(3):316–327. doi:10.1002/(sici)1097-0290(20000505)68:3<316:aid-bit10>3.0.co;2-2
Ogawa T, Murakami K, Mori H, Ishii N, Tomita M, Yoshin M (2007) Role of phosphoenolpyruvate in the NADP-isocitrate dehydrogenase and isocitrate lyase reaction in Escherichia coli. J Bacteriol 189(3):1176–1178. doi:10.1128/jb.01628-06
Park SJ, McCabe J, Turna J, Gunsalus RP (1994) Regulation of the citrate synthase (gltA) gene of Escherichia coli in response to anaerobiosis and carbon supply: role of the arcA gene product. J Bacteriol 176(16):5086–5092
Peng L (2004) Metabolic flux analysis for a ppc mutant Escherichia coli based on 13C-labelling experiments together with enzyme activity assays and intracellular metabolite measurements. FEMS Microbiol Lett 235(1):17–23. doi:10.1016/j.femsle.2004.04.003
Peng L, Shimizu K (2003) Global metabolic regulation analysis for Escherichia coli K12 based on protein expression by 2-dimensional electrophoresis and enzyme activity measurement. Appl Microbiol Biotechnol 61(2):163–178
Portnoy VA, Bezdan D, Zengler K (2011) Adaptive laboratory evolution—harnessing the power of biology for metabolic engineering. Curr Opin Biotechnol 22(4):590–594. doi:10.1016/j.copbio.2011.03.007
Raab AM, Gebhardt G, Bolotina N, Weuster-Botz D, Lang C (2010) Metabolic engineering of Saccharomyces cerevisiae for the biotechnological production of succinic acid. Metab Eng 12(6):518–525. doi:10.1016/j.ymben.2010.08.005
Sauer U, Eikmanns BJ (2005) The PEP-pyruvate-oxaloacetate node as the switch point for carbon flux distribution in bacteria. FEMS Microbiol Rev 29(4):765–794. doi:10.1016/j.femsre.2004.11.002
Scholten E, Dägele D (2008) Succinic acid production by a newly isolated bacterium. Biotechnol Lett 30(12):2143–2146. doi:10.1007/s10529-008-9806-2
Scholten E, Renz T, Thomas J (2009) Continuous cultivation approach for fermentative succinic acid production from crude glycerol by Basfia succiniciproducens DD1. Biotechnol Lett 31(12):1947–1951. doi:10.1007/s10529-009-0104-4
Schweizer HP (1992) Allelic exchange in Pseudomonas aeruginosa using novel ColE1-type vectors and a family of cassettes containing a portable oriT and the counter-selectable Bacillus subtilis sacB marker. Mol Microbiol 6(9):1195–1204
Tsuruta H, Paddon CJ, Eng D, Lenihan JR, Horning T, Anthony LC, Regentin R, Keasling JD, Renninger NS, Newman JD (2009) High-level production of amorpha-4,11-diene, a precursor of the antimalarial agent artemisinin Escherichia coli. PLoS One 4(2):e4489. doi:10.1371/journal.pone.0004489
Vlysidis A, Binns M, Webb C, Theodoropoulos C (2011) Glycerol utilisation for the production of chemicals: conversion to succinic acid, a combined experimental and computational study. Biochem Eng J 58–59:1–11. doi:10.1016/j.bej.2011.07.004
Walsh K, Koshland DE (1984) Determination of flux through the branch point of two metabolic cycles. The tricarboxylic acid cycle and the glyoxylate shunt. J Biol Chem 259(15):9646–9654
Walsh K, Koshland DE Jr (1985) Characterization of rate-controlling steps in vivo by use of an adjustable expression vector. Proc Natl Acad Sci USA 82(11):3577–3581
Yamamoto K, Ishihama A (2003) Two different modes of transcription repression of the Escherichia coli acetate operon by IclR. Mol Microbiol 47(1):183–194. doi:10.1046/j.1365-2958.2003.03287.x
Yuzbashev TV, Yuzbasheva EY, Sobolevskaya TI, Laptev IA, Vybornaya TV, Larina AS, Matsui K, Fukui K, Sineoky SP (2010) Production of succinic acid at low pH by a recombinant strain of the aerobic yeast Yarrowia lipolytica. Biotechnol Bioeng 107(4):673–682. doi:10.1002/bit.22859
Zhang X, Jantama K, Moore JC, Jarboe LR, Shanmugam KT, Ingram LO (2009) Metabolic evolution of energy-conserving pathways for succinate production in Escherichia coli. Proc Natl Acad Sci USA 106(48):20180–20185. doi:10.1073/pnas.0905396106
Zhang X, Shanmugam KT, Ingram LO (2010) Fermentation of glycerol to succinate by metabolically engineered strains of Escherichia coli. Appl Environ Microbiol 76(8):2397–2401. doi:10.1128/aem.02902-09
Zhang Z, Gosset G, Barabote R, Gonzalez CS, Cuevas WA, Saier MH (2005) Functional interactions between the carbon and iron utilization regulators, Crp and Fur, in Escherichia coli. J Bacteriol 187(3):980–990. doi:10.1128/jb.187.3.980-990.2005
Zhu N, Xia H, Wang Z, Zhao X, Chen T (2013) Engineering of acetate recycling and citrate synthase to improve aerobic succinate production in Corynebacterium glutamicum. PLoS ONE 8(4):e60659. doi:10.1371/journal.pone.0060659
Acknowledgments
This work was supported by National 973 Project (2011CBA00804, 2012CB725203), National Natural Science Foundation of China (NSFC-21176182, NSFC-21206112) and National High-tech R&D Program of China (2012AA02A702, 2012AA022103).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, N., Zhang, B., Chen, T. et al. Directed pathway evolution of the glyoxylate shunt in Escherichia coli for improved aerobic succinate production from glycerol. J Ind Microbiol Biotechnol 40, 1461–1475 (2013). https://doi.org/10.1007/s10295-013-1342-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-013-1342-y