Abstract
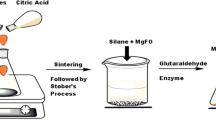
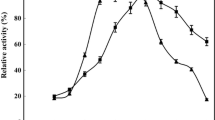
The potential of the modified magnetic nanoparticles for covalent immobilization of porcine pancreatic α-amylase has been investigated. The synthesis and immobilization processes were simple and fast. The co-precipitation method was used for synthesis of magnetic iron oxide (Fe3O4) nanoparticles (NPs) which were subsequently coated with silica through sol–gel reaction. The amino-functionalized NPs were prepared by treating silica-coated NPs with 3-aminopropyltriethoxysilane followed by covalent immobilization of α-amylase by glutaraldehyde. The optimum enzyme concentration and incubation time for immobilization reaction were 150 mg and 4 h, respectively. Upon this immobilization, the α-amylase retained more than 50 % of its initial specific activity. The optimum pH for maximal catalytic activity of the immobilized enzyme was 6.5 at 45 °C. The kinetic studies on the immobilized enzyme and its free counterpart revealed an acceptable change of Km and Vmax. The Km values were found as 4 and 2.5 mM for free and immobilized enzymes, respectively. The Vmax values for the free and immobilized enzymes were calculated as 1.75 and 1.03 μmol mg−1 min−1, in order, when starch was used as the substrate. A quick separation of immobilized amylase from reaction mixture was achieved when a magnetically active support was applied. In comparison to the free enzyme, the immobilized enzyme was thermally stable and was reusable for 9 cycles while retaining 68 % of its initial activity.













Similar content being viewed by others
References
Bornscheuer, U. T., & Angew. (2003). Chemie International Edition, 423, 336–337.
Sheldon, R. A. (1993). Chirotechnology: industrial synthesis of optically active compounds. New York: Marcel Dekker.
Mozhaev, V. V., Khmelnitsky, Y. L., Sergeeva, M. V., Belova, A. B., Klyachko, N. L., Levashov, A. V., & Martinek, K. (1989). Catalytic activity and denaturation of enzymes in water/organic cosolvent mixtures. European Journal of Biochemistry, 184, 597–602.
Ozyilmaz, G., & Yağız, E. (2012). Isoamylacetate production by entrapped and covalently bound Candida rugosa and porcine pancreatic lipases. Food Chemistry, 135, 2326–2332.
Berry, C. C., & Curtis, A. S. G. (2003). Functionalisation of magnetic nanoparticles for applications in biomedicine. Journal of Physics D: Applied Physics, 36, 198–206.
Konerack_a, M., Kopčanský, P., Antalík, M., Ramchand, C. N., Lobo, D., Mehta, R. V., & Upadhyay, R. V. (1999). Immobilization of proteins and enzymes to fine magnetic particles. Journal of Magnetism and Magnetic Materials, 201, 427–430.
Lee, J., Lee, Y., Youn, J., Na, H., Yu, T., Kim, H., Lee, S. M., Koo, Y. M., Kwak, J. H., Park, H. G., Chang, H. N., Hwang, M., Park, J. G., Kim, J., & Hyeon, T. (2008). Simple synthesis of functionalized superparamagnetic magnetite/silica core/shell nanoparticles and their application as magnetically separable high performance biocatalysts. Small, 4, 143–152.
Gupta, R., Gigras, P., Mohapatra, H., Goswami, V. K., & Chauhan, B. (2003). Microbial α-amylases: a biotechnological perspective. Process Biochemistry, 38, 1599–1616.
Kandra, L. (2003). a-Amylases of medical and industrial importance. Journal of Molecular Structure, 666, 487–498.
Pandey, A., Nigam, P., Soccol, C. R., Soccol, V. T., Singh, D., & Mohan, R. (2000). Advances in microbial amylases. Applied Biochemistry and Biotechnology, 31, 135–152.
Reddy, N. S., Nimmagadda, A., & Sambasiva Rao, K. R. S. (2003). An overview of the microbial a-amylase family. African Journal of Biotechnology, 2, 645–648.
Kahraman, M. V., Bayramoglu, G., Kayaman, N. A., & Gungor, A. (2007). Alpha-amylase immobilization on functionalized glass beads by covalent attachment. Food Chemistry, 104, 1385–1392.
Bellino, M. G., & Regazzoni, A. E. (2010). Amylase-functionalized mesoporous silica thin films as robust biocatalyst platforms. Applied Materials & Interfaces, 2, 360–365.
Tripathi, P., Kumari, A., Rath, P., & Kayastha, A. M. (2007). Immobilization of α-amylase from mung beans on amberlite MB 150 and chitosan beads: a comparative study. Journal of Molecular Catalysis B: Enzymatic, 49, 69–74.
Jaiswal, N., Prakash, O., Talat, M., Hasan, S. H., & Pandey, R. K. (2012). α-Amylase immobilizati on on gelatin: optimization of process variables. Journal Genetic Engineering and Biotechnology, 10, 161–167.
Tumturk, H., Aksoy, S., & Hasirci, N. (2000). Covalent immobilization of alpha-amylase onto poly(2-hydroxyethyl methacrylate) poly(styerene-2-hydroxyethyl methacrylate) microspheres and the effect of Ca2+ ions on the enzyme activity. Food Chemistry, 68, 259–266.
Pascoal, A. M., Mitidieri, S., & Fernandes, K. F. (2011). Immobilization of α-amylase from Aspergillus niger onto polyaniline. Food and Bioproducts Processing, 89, 300–306.
Singh, V., & Ahmed, S. (2012). Silver nanoparticles (AgNPs) doped gum acacia–gelatin–silica nanohybrid: an effective support for diastase immobilization. International Journal of Biological Macromolecules, 50, 353–361.
Eslamipour, F., & Hejazi, P. (2016). Evaluating effective factors on the activity and loading of immobilized a-amylase onto magnetic nanoparticles using a response surface-desirability approach. RSC Advances, 6, 20187–20197.
Guo, H., Tang, Y., Yu, Y., Xue, L., & Qian, J. Q. (2016). Covalent immobilization of α-amylase on magnetic particles ascatalyst for hydrolysis of high-amylose starch. International Journal of Biological Macromolecules, 87, 537–544.
Tuzmen, N., Kalburcu, T., & Denizli, A. (2012). α-Amylase immobilization onto dye attached magnetic beads: optimization and characterization. Journal of Molecular Catalysis B, 78, 16–23.
Baskar, G., Afrin Banu, N., Helan Leuca, G., Gayathri, V., & Jeyashree, N. (2015). Magnetic immobilization and characterization of α-amylase as nanobiocatalyst for hydrolysis of sweet potato starch. Chemical Engineering Journal, 102, 18–23.
Motevalizadeh, S. F., Khoobi, M., Sadighi, A., Khalilvand-Sedagheh, M., Pazhouhandeh, M., Ramazani, A., Faramarzi, M. A., & Abbas Shafiee, A. (2015). Lipase immobilization onto polyethylenimine coated magnetic nanoparticles assisted by divalent metal chelated ions. Journal of Molecular Catalysis B, 120, 75–83.
Chen, X., Lam, K. F., Zhang, Q., Pan, B., Arruebo, M., & Yeung, K. L. (2009). Synthesis of highly selective magnetic mesoporous adsorbent. Journal of Physical Chemistry, 113, 9804–9813.
Jang, J., & Lim, H. (2010). Characterization analytical application of surface modified magnetic nanoparticles. Microchemical Journal, 94, 148–158.
Abareshi, M., Goharshadi, E. K., Zebarjad, S. M., Fadafan, H. K., & Youssefi, A. (2010). Fabrication, characterization and measurement of thermal conductivity of Fe3O4 nanofluids. Journal of Magnetism and Magnetic Materials, 322, 3895–3901.
Stober, W., Fink, A., & Bohn, E. J. (1968). Controlled growth of monodisperse silica spheres in the micron size range. Journal of Colloid and Interface Science, 26, 62–69.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Bernfeld, P. (1955). α and β amylases. Methods in Enzymology, 1, 149–158.
Sohrabi, N., Rasouli, N., & Torkzadeh, M. (2014). Chemical Engineering Journal, 240, 426–433.
Ranjbakhsh, E., Bordbar, A. K., Abbasi, M., Khosropour, A. R., & Shams, E. (2012). Chemical Engineering Journal, 179, 272–276.
Swarnalatha, V., Esther, R. A., & Dhamodharan, R. (2013). Journal of Molecular Catalysis B: Enzymatic, 96, 6–13.
Singh, V., & Kumar, P. (2011). Carboxy methyl tamarind gum–silica nanohybrids for effective immobilization of amylase. Journal of Molecular Catalysis B: Enzymatic, 70, 67–73.
Jahir Khan, A., Husain, Q., & Azam, A. (2012). Biotechnology and Bioprocess Engineering, 17, 377–384.
Ramesh, M. V., & Lonsane, B. K. (1990). Effect of metal salts and protein modifying agents on activity of thermostable α-amylase produced by Bacillus licheniformis M27 under solid state fermentation. Chemie Mikrobiologie Technologie der Lebensmittel, 12, 129–136.
Lo, H., Lin, L., Chen, H., Hsu, W., & Chang, C. (2001). Enzymatic properties of a SDS-resistant Bacillus sp. TS-23 α-amylase produced by recombinant Escherichia coli. Process Biochemistry, 36, 743–750.
Ai, Z., Jiang, Z., Li, L., Deng, W., Kusakabe, I., & Li, H. (2005). Process Biochemistry, 40, 2707–2714.
Turunc, O., Kahraman, M. V., Akdemir, Z. S., Kayaman-Apohan, N., & Gungor, A. (2009). Immobilization of alpha-amylase onto cyclic carbonate bearing hybrid material. Food Chemistry, 112(4), 992–997.
Dey, G., Nagpal, V., & Banerjee, R. (2002). Immobilization of alpha-amylase from Bacillus circulans grs 313 on coconut fiber. Applied Biochemistry and Microbiology, 102, 303–313.
Mukherjee, A., Kumar, T., Rai, S., & Roy, J. (2010). Biotechnology and Bioprocess Engineering, 15, 984–992.
Sedaghat, M. E., Ghiaci, M., Aghaei, H., & Soleimanian-Zad, S. (2009). Immobilization of alpha-amylase on Na-bentonite and modified bentonite. Applied Clay Science, 46, 125–130.
Swarnalatha, V., Esther, R. A., & Dhamodharan, R. (2013). Immobilized of a-amylase on gum acacia stabilized magnetite nanoparticles, an easily recoverable and reusable support. Journal \of Molecular Catalysis B: Enzymatic, 96, 6–13.
Acknowledgments
The authors wish to acknowledge the support of this work by Shiraz University Research Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akhond, M., Pashangeh, K., Karbalaei-Heidari, H.R. et al. Efficient Immobilization of Porcine Pancreatic α-Amylase on Amino-Functionalized Magnetite Nanoparticles: Characterization and Stability Evaluation of the Immobilized Enzyme. Appl Biochem Biotechnol 180, 954–968 (2016). https://doi.org/10.1007/s12010-016-2145-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2145-1