Abstract
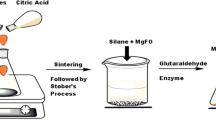
In this study, we synthesized super magnetic Fe(OH)3@Fe3O4 nanoparticles (SPIONs) by the co-precipitation method and introduction of amine groups via chemisorption of l-aspartic acid (LAA) on the surface of SPIONs. Penaeus vannamei protease (PVP) was immobilized onto amine-functionalized supermagnetic nanoparticles (ASPIONs), and conditions affecting PVP immobilization were investigated. PVP immobilized onto ASPIONs exhibited shifts in both working optimum pH and temperature with an increase from pH 7 to pH 8, and increased optimum temperature by 10 °C compared to free enzyme. Similarly, the thermal, pH, and storage stabilities of the immobilized PVP were superior to those of free form of the enzyme. In comparison to the free enzyme, the immobilized enzyme was reusable for 15 cycles while retaining 73% of its initial activity. The Michaelis–Menten kinetic constant (Km) and maximum reaction velocity (Vmax) for free PVP were 2.3 µM and 88 µM min−1, respectively, whereas Km and Vmax values of immobilized enzyme were 2.5 µM and 85 µM min−1, respectively. These results indicated that immobilized PVP was efficient in terms of catalytic activity and can be applied to continuous casein processing applications in the different industries.







Similar content being viewed by others
References
Sharifian S, Homaei A, Kim SK, Satari M (2018) Production of newfound alkaline phosphatases from marine organisms with potential functions and industrial applications. Process Biochem 64:103–115. https://doi.org/10.1016/j.procbio.2017.10.005
Sharifian S, Homaei A, Hemmati R, Khajeh K (2017) Light emission miracle in the sea and preeminent applications of bioluminescence in recent new biotechnology. J Photochem Photobiol B Biol 172:115–128. https://doi.org/10.1016/j.jphotobiol.2017.05.021
Ding C, Sun H, Ren J, Qu X (2017) Immobilization of enzyme on chiral polyelectrolyte surface. Anal Chim Acta 952:88–95. https://doi.org/10.1016/j.aca.2016.11.047
Homaei A, Saberi D (2015) Immobilization of α-amylase on gold nanorods: an ideal system for starch processing. Process Biochem 50:1394–1399. https://doi.org/10.1016/j.procbio.2015.06.002
Homaei A, Etemadipour R (2015) Improving the activity and stability of actinidin by immobilization on gold nanorods. Int J Biol Macromol 72:1176–1181. https://doi.org/10.1016/j.ijbiomac.2014.10.029
Homaei AA, Sajedi RH, Sariri R et al (2010) Cysteine enhances activity and stability of immobilized papain. Amino Acids 38:937–942. https://doi.org/10.1007/s00726-009-0302-3
Homaei A (2015) Enhanced activity and stability of papain immobilized on CNBr-activated sepharose. Int J Biol Macromol 75:373–377. https://doi.org/10.1016/j.ijbiomac.2015.01.055
Homaei A (2015) Enzyme immobilization and its application in the food industry. In: Ravishankar, Rai (eds) Advances in food biotechnology, first. Wiley, Oxford, pp 145–164
Mohamed SA, Al-Harbi MH, Almulaiky YQ et al (2017) Immobilization of horseradish peroxidase on Fe3O4 magnetic nanoparticles. Electron J Biotechnol. https://doi.org/10.1016/j.ejbt.2017.03.010
Liu W, Wang L, Jiang R (2012) Specific enzyme immobilization approaches and their application with nanomaterials. Top Catal 55:1146–1156. https://doi.org/10.1007/s11244-012-9893-0
Shi L, Ma F, Han Y et al (2014) Removal of sulfonamide antibiotics by oriented immobilized laccase on Fe3O4 nanoparticles with natural mediators. J Hazard Mater 279:203–211. https://doi.org/10.1016/j.jhazmat.2014.06.070
Homaei A, Lavajoo F, Sariri R (2016) Development of marine biotechnology as a resource for novel proteases and their role in modern biotechnology. Int J Biol Macromol 88:542–552. https://doi.org/10.1016/j.ijbiomac.2016.04.023
Shojaei F, Homaei A, Taherizadeh MR, Kamrani E (2017) Characterization of biosynthesized chitosan nanoparticles from Penaeus vannamei for the immobilization of P. vannamei protease: an eco-friendly nanobiocatalyst. Int J Food Prop 20:S1413–S1423
Dadshahi Z, Homaei A, Zeinali F et al (2016) Extraction and purification of a highly thermostable alkaline caseinolytic protease from wastes Litopenaeus vannamei suitable for food and detergent industries. Food Chem 202:110–115. https://doi.org/10.1016/j.foodchem.2016.01.104
Arefi M, Saberi D, Karimi M, Heydari A (2015) Superparamagnetic Fe(OH)3@Fe3O4 nanoparticles: an efficient and recoverable catalyst for tandem oxidative amidation of alcohols with amine hydrochloride salts. ACS Comb Sci 17:341–347. https://doi.org/10.1021/co5001844
Mikhaylova M, Kim DK, Berry CC et al (2004) BSA immobilization on amine functionalized superparamagnetic iron oxide nanoparticles. Chem Mater 16:2344–2354
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Homaei A, Barkheh H, Sariri R, Stevanato R (2014) Immobilized papain on gold nanorods as heterogeneous biocatalysts. Amino Acids 46:1649–1657. https://doi.org/10.1007/s00726-014-1724-0
Homaei A, Samari F (2017) Investigation of activity and stability of papain by adsorption on multi-wall carbon nanotubes. Int J Biol Macromol 105:1630–1635. https://doi.org/10.1016/j.ijbiomac.2017.02.038
Homaei A (2017) Immobilization of Penaeus merguiensis alkaline phosphatase on gold nanorods for heavy metal detection. Ecotoxicol Environ Saf 136:1–7. https://doi.org/10.1016/j.ecoenv.2016.10.023
Fortes CCS, Daniel-da-Silva AL, Xavier AMRB., Tavares APM (2017) Optimization of enzyme immobilization on functionalized magnetic nanoparticles for laccase biocatalytic reactions. Chem Eng Process Process Intensif. https://doi.org/10.1016/j.cep.2017.03.009
Al-Dhrub AHA, Sahin S, Ozmen I et al (2017) Immobilization and characterization of human carbonic anhydrase I on amine functionalized magnetic nanoparticles. Process Biochem. https://doi.org/10.1016/j.procbio.2017.03.025
Das A, Singh J, Yogalakshmi KN (2017) International biodeterioration and biodegradation laccase immobilized magnetic iron nanoparticles: fabrication and its performance evaluation in chlorpyrifos degradation. Int Biodeterior Biodegrad 117:183–189. https://doi.org/10.1016/j.ibiod.2017.01.007
Wanjari S, Prabhu C, Yadav R et al (2011) Immobilization of carbonic anhydrase on chitosan beads for enhanced carbonation reaction. Process Biochem 46:1010–1018. https://doi.org/10.1016/j.procbio.2011.01.023
Arrhenius S (1889) Uber die Reaktionsgeschwindigkeit bei der Inversion von Rohrzucker durch Säuren. Z Phys Chem 4:226
Homaei AA, Sariri R, Vianello F, Stevanato R (2013) Enzyme immobilization: an update. J Chem Biol 6:185–205. https://doi.org/10.1007/s12154-013-0102-9
Brena B, González-Pombo P, Batista-Viera F (2013) Immobilization of enzymes: a literature survey. Immobil Enzym Cells 1051:15–31. https://doi.org/10.1007/978-1-62703-550-7_2
Kim BJ, Kang BK, Bahk YY et al (2009) Immobilization of horseradish peroxidase on multi-walled carbon nanotubes and its enzymatic stability. Curr Appl Phys 9:e263–e265. https://doi.org/10.1016/j.cap.2009.06.050
Chen C, Xie Q, Yang D et al (2013) Recent advances in electrochemical glucose biosensors: a review. RSC Adv 3:4473. https://doi.org/10.1039/c2ra22351a
Tischer W, Kasche V (1999) Immobilized enzymes: crystals or carriers? 17:326–335
Copeland RA (2000) Enzymes: a practical introduction to structure, mechanism, and data analysis. Wiley, Oxford
Palmer T (1995) Understanding enzymes. Prentice Hall/Ellis Horwood, Upper Saddle River
Jafary F, Panjehpour M, Varshosaz J, Yaghmaei P (2016) Stability improvement of immobilized alkaline phosphate using chitosan nanoparticles. Braz J Chem Eng 33:243–250. https://doi.org/10.1590/0104-6632.20160332s20140074
Samani NB, Nayeri H, Amiri G (2016) Effects of cadmium chloride as inhibitor on stability and kinetics of immobilized Lactoperoxidase (LPO) on silica-coated magnetite nanoparticles versus free LPO. 3:pp 230–239
Patel SKS, Otari SV, Chan Kang Y, Lee J-K (2017) Protein–inorganic hybrid system for efficient his-tagged enzymes immobilization and its application in l-xylulose production. RSC Adv 7:3488–3494. https://doi.org/10.1039/C6RA24404A
Contesini FJ, Figueira J, de A, Kawaguti HY, et al (2013) Potential applications of carbohydrases immobilization in the food industry. Int J Mol Sci 14:1335–1369. https://doi.org/10.3390/ijms14011335
Acknowledgements
Authors are grateful to the University of Hormozgan for the financial support to this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This work is free from any conflict of interest.
Rights and permissions
About this article
Cite this article
Moslemi, M., Homaei, A. & Toiserkani, H. Aspartic acid introduce the functional amine groups on the surface of superparamagnetic Fe(OH)3@Fe3O4 nanoparticles for efficient immobilization of Penaeus vannamei protease. Bioprocess Biosyst Eng 41, 749–756 (2018). https://doi.org/10.1007/s00449-018-1908-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-018-1908-1