Abstract
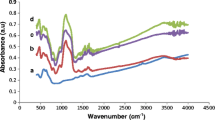
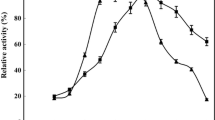
Ubiquitous nanomaterials have been extensively involved in multifarious biotransformation of diverse organic and synthetic techniques. This research study describes the robust immobilization of α-amylase on magnetic nanoparticles of magnesium ferrite (MgFO) functionalized with silane. The XRD patterns, FESEM and HRTEM images were analysed to study structural and morphological features. The crystallite size of MgFO is found to be 12 nm. The FTIR results confirmed the covalent attachment of the enzyme with the MgFO and VSM and Mössbauer spectrometric graphs were evaluated to study the magnetic behaviour of the prepared samples. The Kinetic parameters, reusability, storage capacity and catalytic activity of the enzyme at various reaction time, pH and temperature conditions before and after attachment with MgFO were examined to confirm the successful immobilization process. The reusability of the enzyme on modified MgFO is found to be good (> 50%) even after 12 consecutive usability cycles and also retain 50% catalytic efficiency over 30 days storage period.









Similar content being viewed by others
References
V. Atiroğlu, A. Atiroğlu, M. Özacar, Immobilization of α-amylase enzyme on a protein @metal–organic framework nanocomposite: a new strategy to develop the reusability and stability of the enzyme. Food Chem. 349(September), 2021 (2020). https://doi.org/10.1016/j.foodchem.2021.129127
A. Basso, S. Serban. Industrial applications of immobilized enzymes—a review. Mol. Catal. 479, 110607 (2019). https://doi.org/10.1016/j.mcat.2019.110607
U. Hanefeld, L. Gardossi, E. Magner, Understanding enzyme immobilisation. Chem. Soc. Rev. 38(2), 453–468 (2009). https://doi.org/10.1039/b711564b
S. Santos, J. Puna, J. Gomes. A review on bio-based catalysts (immobilized enzymes) used for biodiesel production. Energies 13(11). https://doi.org/10.3390/en13113013
R. A. Wahab, N. Elias, F. Abdullah, S. K. Ghoshal. On the taught new tricks of enzymes immobilization: an all-inclusive overview. React. Funct. Polym. 152, 104613 (2020). https://doi.org/10.1016/j.reactfunctpolym.2020.104613.
C. Bernal, K. Rodríguez, R. Martínez, Integrating enzyme immobilization and protein engineering: An alternative path for the development of novel and improved industrial biocatalysts. Biotechnol. Adv. 36(5), 1470–1480 (2018). https://doi.org/10.1016/j.biotechadv.2018.06.002
H.M. Salvi, G.D. Yadav, Process intensification using immobilized enzymes for the development of white biotechnology. Catal. Sci. Technol. 11(6), 1994–2020 (2021). https://doi.org/10.1039/d1cy00020a
M. Bilal, Y. Zhao, S. Noreen, S.Z.H. Shah, R.N. Bharagava, H.M.N. Iqbal, Modifying bio-catalytic properties of enzymes for efficient biocatalysis: A review from immobilization strategies viewpoint. Biocatal. Biotransf. 37(3), 159–182 (2019). https://doi.org/10.1080/10242422.2018.1564744
M. Razzaghi et al. Industrial applications of immobilized nano-biocatalysts. Bioprocess Biosyst. Eng. 0123456789 (2021). https://doi.org/10.1007/s00449-021-02647-y.
A. Sharma, A. Kumar, K.R. Meena, S. Rana, M. Singh, S.S. Kanwar, Fabrication and functionalization of magnesium nanoparticle for lipase immobilization in n-propyl gallate synthesis. J. King Saud Univ. Sci. 29(4), 536–546 (2017). https://doi.org/10.1016/j.jksus.2017.08.005
M. Bilal, Y. Zhao, T. Rasheed, H.M.N. Iqbal, Magnetic nanoparticles as versatile carriers for enzymes immobilization: a review. Int. J. Biol. Macromol. 120, 2530–2544 (2018). https://doi.org/10.1016/j.ijbiomac.2018.09.025
B.I. Kharisov, H.V.R. Dias, O.V. Kharissova, Mini-review: ferrite nanoparticles in the catalysis. Arab. J. Chem. 12(7), 1234–1246 (2019). https://doi.org/10.1016/j.arabjc.2014.10.049
M. Amiri, K. Eskandari, M. Salavati-Niasari, Magnetically retrievable ferrite nanoparticles in the catalysis application. Adv. Colloid Interface Sci. 271, 101982 (2019). https://doi.org/10.1016/j.cis.2019.07.003
Ł Klapiszewski, J. Zdarta, T. Jesionowski, Titania/lignin hybrid materials as a novel support for α-amylase immobilization: a comprehensive study. Colloids Surf. B Biointerfaces 162, 90–97 (2018). https://doi.org/10.1016/j.colsurfb.2017.11.045
K. Hisamatsu et al., α-Amylase immobilization capacities of mesoporous silicas with different morphologies and surface properties. J. Porous Mater. 19(1), 95–102 (2012). https://doi.org/10.1007/s10934-011-9452-2
R. Das, M. Talat, O.N. Srivastava, A.M. Kayastha, Covalent immobilization of peanut β-amylase for producing industrial nano-biocatalysts: a comparative study of kinetics, stability and reusability of the immobilized enzyme. Food Chem. 245, 488–499 (2018). https://doi.org/10.1016/j.foodchem.2017.10.092
P.C. Ashly, M.J. Joseph, P.V. Mohanan, Activity of diastase α-amylase immobilized on polyanilines (PANIs). Food Chem. 127(4), 1808–1813 (2011). https://doi.org/10.1016/j.foodchem.2011.02.068
K. Singh, G. Srivastava, M. Talat, O.N. Srivastava, A.M. Kayastha, α-Amylase immobilization onto functionalized graphene nanosheets as scaffolds: its characterization, kinetics and potential applications in starch based industries. Biochem. Biophys. Rep. 3, 18–25 (2015). https://doi.org/10.1016/j.bbrep.2015.07.002
M. Dhiman, S. Rana, M. Singh, J.K. Sharma, Magnetic studies of mixed Mg–Mn ferrite suitable for biomedical applications. Integr. Ferroelectr. 202(1), 29–38 (2019). https://doi.org/10.1080/10584587.2019.1674821
Z. Emami Bistgani, S. A. Siadat, A. Bakhshandeh, A. Ghasemi Pirbalouti, M. Hashemi, Interactive effects of drought stress and chitosan application on physiological characteristics and essential oil yield of Thymus daenensis Celak. Crop J. 5(5), 407–415 (2017). https://doi.org/10.1016/j.cj.2017.04.003.
P. Bernfeld, Amylases, alpha and beta. Methods Enzymol. I I(540), 149–158 (1955)
H. Lineweaver, D. Burk, The determination of enzyme dissociation constants. J. Am. Chem. Soc. 56(3), 658–666 (1934). https://doi.org/10.1021/ja01318a036
M. Soleimani, A. Khani, K. Najafzadeh, α-Amylase immobilization on the silica nanoparticles for cleaning performance towards starch soils in laundry detergents. J. Mol. Catal. B Enzym. 74(1–2), 1–5 (2012). https://doi.org/10.1016/j.molcatb.2011.07.011
M. Defaei, A. Taheri-Kafrani, M. Miroliaei, P. Yaghmaei, Improvement of stability and reusability of α-amylase immobilized on naringin functionalized magnetic nanoparticles: A robust nanobiocatalyst. Int. J. Biol. Macromol. 113(2017), 354–360 (2018). https://doi.org/10.1016/j.ijbiomac.2018.02.147
S. Rana, A. Sharma, A. Kumar, S.S. Kanwar, M. Singh, Utility of silane-modified magnesium-based magnetic nanoparticles for efficient immobilization of bacillus thermoamylovorans lipase. Appl. Biochem. Biotechnol. 192(3), 1029–1043 (2020). https://doi.org/10.1007/s12010-020-03379-7
A. N. Ananth, A. N. Ananth, S. P. Jose, S. Umapathy, T. Mathavan. Influence of α-amylase template concentration on systematic entrapment of highly stable and monodispersed colloidal gold nanoparticles. AIP Adv. 6(1) (2016). https://doi.org/10.1063/1.4939849.
M. V. Nikolic, M. D. Lukovic. Influence of SnO2 content on the humidity dependent impedance of the MgFe2O4-Fe2O3-SnO2 compound. Chemosensors 8(2). https://doi.org/10.3390/CHEMOSENSORS8020039.
M. Defaei, A. Taheri-Kafrani, M. Miroliaei, P. Yaghmaei, Alpha-amylase immobilized on polycaprolactone-grafted magnetic nanoparticles: improving stability and reusability. J. Chem. Technol. Biotechnol. 95(8), 2243–2250 (2020). https://doi.org/10.1002/jctb.6412
Z.M. Milani, R. Jalal, E.K. Goharshadi, Carbodiimide for covalent α-amylase immobilization onto magnetic nanoparticles. Int. J. Nanosci. 16(5–6), 1–8 (2017). https://doi.org/10.1142/S0219581X17500156
M. Talebi, S. Vaezifar, F. Jafary, M. Fazilati, S. Motamedi, Stability improvement of immobilized α-amylase using nano pore zeolite. Iran. J. Biotechnol. 14(1), 33–38 (2016). https://doi.org/10.15171/ijb.1261
A. Sharma, T. Sharma, K.R. Meena, A. Kumar, S.S. Kanwar, High throughput synthesis of ethyl pyruvate by employing superparamagnetic iron nanoparticles-bound esterase. Process Biochem. 71(April), 109–117 (2018). https://doi.org/10.1016/j.procbio.2018.05.004
N. Antony, S. Balachandran, P.V. Mohanan, Immobilization of diastase α-amylase on nano zinc oxide. Food Chem. 211, 624–630 (2016). https://doi.org/10.1016/j.foodchem.2016.05.049
F. Eslamipour, P. Hejazi, Evaluating effective factors on the activity and loading of immobilized α-amylase onto magnetic nanoparticles using a response surface-desirability approach. RSC Adv. 6(24), 20187–20197 (2016). https://doi.org/10.1039/c5ra26140f
K. Salem et al., Enzyme storage and recycling: Nanoassemblies of α-amylase and xylanase immobilized on biomimetic magnetic nanoparticles. ACS Sustain. Chem. Eng. 9(11), 4054–4063 (2021). https://doi.org/10.1021/acssuschemeng.0c08300
Funding
No funding was provided.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rana, S., Sharma, A., Batoo, K.M. et al. Fabrication and characteristic studies of doped metal oxide-silane magnetic nanocomposite for enhancement of stability of α-amylase. Appl. Phys. A 128, 729 (2022). https://doi.org/10.1007/s00339-022-05882-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-022-05882-6