Abstract
Cobalt bismuth nano-ferrite (Co/Bi) with the chemical formula CoBi0.02Fe1.98O4 was produced using a simple flash auto-combustion method at three different temperatures: as-prepared, 600°C, and 800°C. A single-phase spinel structure was confirmed using X-ray diffraction, and the nano-scale morphology was examined using AFM (atomic force microscopy). Magnetic measurements demonstrated that increasing the annealing temperature increased the saturation magnetization Ms by 1.3 times. However, the coercivity Hc changed from semi-hard ferrite (as-prepared sample) to soft ferrite (Co/Bi nano-ferrite at 800°C) and reduced 10.7 times that of as-prepared nanoparticles. Therefore, the 800°C Co/Bi nano-ferrite with a low coercive field is recommended for transformers, recording heads, inductor cores, magnetic shielding, and microwave devices. The as-prepared sample and that at 600°C displayed super-high microwave frequency (SHF) in the X band in high-frequency applications calculated from magnetic measurement. The 800°C sample also has an extremely high microwave frequency in the Ku band, which is utilized in radar and satellite communications. Antimicrobial characterization showed that raising the annealing temperature increased the effectiveness of the samples against tested microorganisms. Thus, the samples under investigation are highly suggested for ultra-high microwave frequency applications and biological antibacterial nanomaterials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Researchers have developed considerable interest in studying the dielectric, magnetic, and structural properties of metal nanoparticles.1,2,3,4,5,6,7,8 Particularly, nanoscale materials with diameters between 1 and 100 nm exhibit unique physicochemical characteristics.9,10 It is established that the size effects of nanoparticles result in a higher surface area to volume ratio.11,12 Each nanoparticle may be thought of as a separate magnetic domain, which leads to a significant alteration of several magnetic characteristics, including the appearance of superparamagnetic phenomena.13,14 Studying nano-ferrite materials is of great interest because of their high mechanical hardness, resistivity, chemical stability, high coercivity, and moderate and high saturation magnetization.15,16 AB2O4 is the general formula for any metal ferrite crystal structure, where A denotes the tetrahedral site, B is the octahedral site, and O is the anion site. Spinel ferrites are distinctive because of their distribution over tetrahedral and octahedral sites.17,18 Many scientists study the magnetic properties of magnetic nanoparticles by doping them with nonmagnetic elements to enhance their properties.19,20 Thus, fabricating nano-ferrites with the substitution of magnetic and nonmagnetic ions significantly alters the magnetic moments, lattice parameters, and exchange interactions.
A well-known hard magnetic material, cobalt ferrite has a cubic-spinel structure and strong coercivity and moderate magnetization. The microstructure and composition of ferrite materials significantly affect their properties, including their structural, magnetic, optical, and electrical characteristics.21,22,23,24 Cobalt nano-ferrite can be modified by adding one or more metals with different magnetic behavior, which can alter its physical and chemical properties.25,26,27,28,29 It may occupy tetrahedral and octahedral sites and has an inverted spinel structure. It has an inverted spinel structure and can occupy tetrahedral and octahedral sites. Bismuth ferrite is a single-phase multiferroic material with low saturation magnetization and high electrical resistivity. Its preferred location is the octahedral B-site.30,31 Increasing the annealing temperature has been found to enhance crystallinity in the sample.
Cobalt bismuth nano-ferrite has unique features and strong thermal stability that can be used in magnetic recording.32,33 Moreover, bismuth nanoparticles can enhance ferrites' magnetic and electrical characteristics to satisfy the needs of applications. Both the sintering temperature and the preparation method affect the physical, magnetic, and antimicrobial properties of the Co/Bi nano-ferrite. By increasing the annealing temperature of Co/Bi nano-ferrite in the present study, this research might have significant uses for high microwave frequency applications and biomedical applications. It has been shown that the distribution of cations in the spinel lattice, namely, the tetrahedral and the octahedral sites, influences the physical characteristics of various ferrites.34,35 Therefore, many physical features of spinel ferrites can only be understood after first studying their cation distribution.
More research is needed on the physical characteristics of Co/Bi nano-ferrite via heat treatment, although they have several uses. Few researchers have attempted to replace bismuth in cobalt nano-ferrites.36,37 It is reported that doping bismuth with 1% (0.02) to cobalt ferrite enhanced the physical properties and possessed the best catalytic performance out of all bismuth doping.38 Thus, the authors chose this doping ratio of bismuth 1% (0.02) to apply it to other different applications. Various methods exist to synthesize nano-ferrites, including solid-state reaction, co-precipitation, sol-gel, flash, and citrate.39,40,41,42,43 Among these, the flash auto-combustion technique is the quickest and easiest way to produce uniform crystals when synthesizing complex materials.44,45 This method involves the flash auto-combustion of Co/Bi, a cost-effective way to create nanostructured materials with desired physicochemical properties.44 Compared to classic synthesis methods such as sol-gel, co-precipitation, and hydrothermal processes, this technique offers several benefits, including rapid synthesis kinetics, precise particle size and shape manipulation, and the absence of harmful byproducts and harsh reaction conditions.45 Synthesis of nano-ferrites may be accomplished using several methods,39,40,41,42,43 including solid-state reaction, co-precipitation, sol-gel, flash, and citrate. The flash auto-combustion technique is one of the quickest and easiest ways to produce uniform crystals when synthesizing complicated materials.44,45 Flash auto-combustion of Co/Bi is a fast and cost-effective method for creating nanostructured materials with the desired physicochemical properties.44 This technique offers numerous benefits over classic synthesis methods such as sol-gel, co-precipitation, and hydrothermal processes. These advantages include rapid synthesis kinetics, precise particle size and shape manipulation, and the absence of harmful byproducts and harsh reaction conditions.45 Nano-ferrites possess unique chemical and magnetic properties that show potential in fighting bacteria, fungi, and viruses due to their antibacterial qualities.7 These substances can cause oxidative stress, harm cell membranes, and obstruct essential cell processes in microorganisms, leading to inhibition or death.8 Cobalt (Co) and bismuth (Bi) nano-ferrites have distinct physicochemical characteristics that make them promise antibacterial agents, and hence, more research in this field is necessary.36,37 Cobalt ferrites exhibit strong magnetic susceptibility and stability, allowing for efficient magnetic separation and contributing to their antibacterial properties and recyclability.34 On the other hand, bismuth ferrites are ideal for use in biomedicine because of their low toxicity and essential biocompatibility.35
This research aimed to improve the cation distribution, structure, and magnetic and antibacterial characteristics of Co/Bi nano-ferrite with the formula CoBi0.02Fe1.98O4 nano-ferrite by heat treatment (as prepared, 600°C, and 800°C) for use in a variety of industrial applications.
Experimental Technique
Synthesis of Nano-Ferrite
The auto-combustion method of Flash, illustrated in Fig. 1a, was utilized to create CoBi0.02Fe1.98O4 at three different annealing temperatures—as-prepared, 600°C, and 800°C. The initial ingredients included 14.5515 g of cobalt nitrate (Co (NO3)2. 6H2O), 0.4851 g of bismuth nitrate (Bi (NO3)3. 5H2O), 39.996 g of iron III nitrate (Fe (NO3)3. 9H2O), and 20.03 g of urea from the Fisher company. These ingredients were mixed in a beaker for 30 min with a small amount of distilled water while continuously stirring. The mixture was then heated on a heater at 500°C until a fine powder was produced. The powder was divided into two parts. The first one, as-prepared sample, was ground for 30 min, while the other part was subjected to annealing at 600°C and 800°C for 2 h. After annealing, the sample was ground for an additional 30 min.
Characterization
X-ray diffraction (XRD) analysis was performed using the PANalytical XPert PRO instrument. The IR spectra were studied using Fourier transform (FTIR) on a Jasco FTIR 300 E spectrometer. Additionally, atomic force microscopy was performed using a Non-Contact Mode Wet - SPM-9600. The vibrating sample magnetometer Lake Shore 7410 (VSM) was used for magnetic measurements.
Antimicrobial Test Preparation
The in vitro antibacterial activity of nanometric CoBi0.02Fe1.98O4 was tested against a wide variety of gram-positive, gram-negative, and fungal pathogens using a modified Kirby-Bauer disc diffusion method.46 After incubating the tested microorganisms and the target drug at 30°C for 24 to 48 h, the diameters of the inhibitory zones were determined. Ampicillin (an antibiotic) and amphotericin B (an antifungal) standards were used to evaluate several antimicrobial discs; 10 ml solvent was used to saturate filter discs for negative control (deionized water, chloroform, DMSO).
Results and Discussions
XRD Study
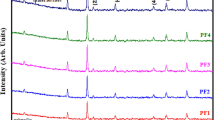
The X-ray diffraction (XRD) patterns of CoBi0.02Fe1.98O4 at various annealing temperatures are shown in Fig. 1b. The powder X-ray diffraction analysis revealed prominent diffraction peaks along planes 311, 220, 400, 511, and 440, while weaker diffraction was observed along planes 222 and 422. The ICDD card number 22-1086 suggests that all samples exhibit a cubic spinel phase, with only a minimal presence of the secondary phase Bi2O3 along the planes 120, 121, and 113, as indicated by ICDD card 76-1730. The peaks' intensity and sharpness are improved with higher annealing temperatures, as shown in Fig. 1b, indicating an increase in crystallinity. The intensities of peaks 220 and 440 vary with the annealing temperature because of the presence of cations on the tetrahedral site. In addition, raising the annealing temperature resulted in larger crystallites, resulting in a decrease in peak width.
Table I provides the calculated average crystallite sizes ranging from 21.6 to 88.6 nm, using Debye–Scherrer's equation.47
where (λ = 1.54) is the wavelength of Cukα radiation, (l) is the shape factor, and (β) is the full width at half maximum in radians. As the annealing temperature increased, the size of the crystallites increased. During annealing, the redistribution of cations between different sites could potentially explain the decrease in lattice parameters as the temperature increases.
The theoretical lattice parameter (ath) is obtained by utilizing the predicted cation distribution of the system, as presented in Table II.48,49
where rA, rB, and Ro denote the ionic radius of the tetrahedral, octahedral sites, and oxygen ion, respectively. Figure 3b and Table II show a strong correlation between the experimental value of the lattice parameter and the theoretical value predicted by the literature.50 The lattice parameter decreased as the temperature increased because of the redistribution of the cations, as shown in Table I.
The X-ray density relationship was as follows:51
where Z represents the number of molecules, Mwt represents the molecular weight, N represents Avogadro's number, and a is the experimental lattice parameter. The annealing temperature was increased, leading to a slightly higher X-ray density Dx for the samples compared to the as-prepared one. This can be attributed to the fact that the atomic weight of Co2+ ions is greater than that of Fe3+ ions. The X-ray density for Co/Bi increased at 800°C because of the higher concentration of Co2+ ions in the octahedral site, as indicated by the expected cation distribution.
The increase in the annealing temperature of the Co/Bi nano-ferrite led to a significant rise in the X-ray density Dx, suggesting a notable enhancement in the densification of the materials under investigation.
The relation of the tolerance factor is as follows:52
Table II shows that the tested samples exhibited tolerance factors (T) that were nearly identical to unity, as anticipated for an ideal spinel structure.53,54,55,56,57,58 The finding matches the creation of a cubic spinel structure without any distortions.
The hopping length in the tetrahedral A-site (LA) and octahedral B-site (LB) was calculated using the following relations based on the experimental lattice parameter aexp.59,60,61,62,63
Table II shows that the decrease in LA and LB may be explained by a decrease in the lattice parameter caused by increasing the annealing temperature.
The effect of annealing temperature of Co/Bi nano-ferrite has been found to have significant benefits for various technological applications. To make informed decisions about the most suitable applications, it is important to gain a deeper understanding of the cation distribution and other features of this material.
AFM Study
Figure 2 demonstrates the utilization of atomic force microscopy to investigate the impact of different annealing temperatures on the morphology and size of the particles in the Co/Bi nano-ferrite. Grain size and surface roughness values were found to vary between samples,64 indicating that the preparation procedure influences these variables. A stronger interaction between magnetic nanoparticles and a higher calcination temperature caused the agglomeration of the resultant ferrite particles. Furthermore, the morphology aggregates, suggesting an absence of surfactant during the preparation process. The particle size distribution is presented as a histogram in Fig. 3a, c based on AFM analysis. Figure 3b, d shows the roughness histogram obtained from AFM analysis. The surface of the nano-ferrite sample was the roughest at a temperature of 600°C. The nano-ferrite sample annealed at 600°C has larger particles compared to the as-prepared sample, which is in accordance with the findings in the literature.65 The increase in the annealing temperature leads to an increase in the size of the crystallites. Finally, the confirmation of the materials' nanoscale nature was achieved through XRD and AFM morphological testing.
By increasing the annealing temperatures, the grain size can be increased because of the improved atomic mobility at higher temperatures, which promotes grain growth. The surface became rougher because of the increased annealing temperature of 600°C, which caused the nano-ferrite to exhibit strong surface activity.
Magnetic Study
The magnetic measurements were used to analyze the behavior of the investigated nanomaterials, as depicted by the magnetic hysteresis loop (M-H) in Fig. 4. The magnetic properties of CoBi0.02Fe1.98O4 nano-ferrite were measured at room temperature under an applied magnetic field of 20 kG using a vibrating sample magnetometer (VSM). Figure 4 shows magnetic hysteretic loops, which indicate that the samples changed from being semi-hard ferrimagnetic (at 600°C and as-prepared) to soft ferrimagnetic (at 800°C) behavior. The magnetic characteristics, including coercivity (Hc), saturation magnetization (Ms), and retentivity (Mr), were determined by magnetic measurements and are presented in Table III. As the crystallite size of Co/Bi nano-ferrite increased the saturation magnetization (Ms) increased by 1.1 times for the investigated sample (at 600°C) and 1.3 times at 800°C. The Bi3+ ions (4f14 5d10) have no magnetic properties, while the Fe3+ ions (3d5) have a higher magnetic moment (5.91 BM) compared to the Co2+ ions (3d7) (3.87 BM). As a result, the Co2+ ions are more likely to occupy the B-site when the annealing temperatures are increased, as predicted by the cation distribution. Table I shows that the predicted cation distribution was responsible for the decrease in saturation magnetization of the as-prepared sample compared to that at 800°C. This reduction can be attributed to the super-exchange interaction of the magnetic ions between A and B sites.
The coercivity of a material can be affected by several factors, such as the magnetocrystalline anisotropy k, the shape of the magnetic particles, the size of the magnetic domains, the microstrain, and the distribution of sizes. The annealing temperature strongly affects the magnetocrystalline anisotropy constant k, as shown in Table III. The magnetocrystalline anisotropy constant k undergoes a decrease of 8.6 times for the nano-ferrite sample when heated to 800°C compared to the as-prepared sample. This reduction in k leads to a 10.7-fold decrease in coercivity due to the increased crystallite size of the Co/Bi nano-ferrite. In addition, as shown in Table I, the increase in k could be attributed to the growth of the crystallite size. The coercivity of the Co/Bi nano-ferrite increases by 1.2 times at 600°C compared to the as-prepared form. This is due to the increase in the magnetocrystalline anisotropy constant k, which also increases by 1.2 times. The calculation of the magnetocrystalline anisotropy constant is based on the saturation magnetization and coercivity, as demonstrated in the equation below:
Increasing the annealing temperature, the shape of the (M–H) loop converts from semi-hard to soft ferrimagnetic behavior. The nano-ferrite of the as-prepared sample and at 600°C with semi-hard ferrimagnetic behavior Hc = 1018.5 and 1181.2 G and Ms = 51.5 and 55.08 emu/g can be used in permanent magnets, magnetic recording, and memory devices. The nano-ferrite sample at 800°C exhibiting soft ferrimagnetic behavior with narrow hysteresis loop Hc = 95.5 G and Ms = 64.1 emu/g can be used in motor cores and transformers.
The following relation provides an experimental magnetic moment (η):67,68,69
where the molecular weight of the sample is denoted by Mwt. The results show that the magnetic moment increases at 800°C compared to the as-prepared value because of the increase in saturation magnetization Ms. Several grain-group exchanges were indicated by squareness values between zero and one.70 All samples had squareness values of < 0.5 at different annealing temperatures due to the interaction between nanoparticles being magnetostatic.
The metal ions in ferrites are often found in either the tetrahedral A-site or the octahedral B-site, depending on the geometrical arrangement of the oxygen's nearest neighbors. The A-A and B-B parallel arrangements, as well as the A-B antiparallel configuration, provide the ferrimagnetic ordering. Increasing the annealing temperature raised the saturation magnetization (Ms) and the experimental magnetic moment (η). In contrast, when the nano-ferrite sample was annealed at 800°C, its coercivity (Hc) dropped by 10.7 fold compared to the as-prepared sample. Thus, the Co/Bi nano-ferrite at 800°C with a low coercive field could be applied at transformers, recording heads, inductor cores, magnetic shielding, and microwave devices.71 Consequently, the saturation magnetization Ms is highest in the 800°C nano-ferrite sample because of the largest crystallites (88.6 nm).
As shown in Table III, by increasing the annealing temperature to 800°C, the energy loss decreased by 3.2 fold, decreasing the area under the loop and converting from a semi-hard behavior to a soft behavior. It is well known that the X-ray density is inversely related to porosity; hence, the hysteresis loops get smaller as the annealing temperature is raised, which is suggestive of the better ordering of good crystalline material, as shown in the AFM morphology.
The squareness R is an essential material property dependent on the anisotropy,72 showing the ease with which the magnetization direction is reoriented to the next easy axis magnetization direction once the magnetic field is removed. The following relation demonstrates the squareness:73
The lower the material's R-value, the more isotropic it will be. The drop in R-value when the annealing temperature was raised is in good agreement with the reduction in X-ray density that increased porosity. As porosity grows, a stronger field is required to push the domain wall or rotation, i.e., Hc rises. Also, the retentivity Mr is related to porosity.74 Comparing the investigated samples with previous literature,38,75,76,77 the saturation magnetizations Ms of the present study give a fascinating enhancement and increase compared with that of the previous literature38,75,76,77 that could be used in various applications.
Figure 5 depicts the magnetic susceptibility (χ = dM/dH) vs. applied field for Co/Bi nano-ferrite at various annealing temperatures. As shown in Table III, heating nano-ferrite to 800°C leads to an increase in Ms of 1.3 fold compared to as-prepared nanoparticles. In addition, the as-prepared nano-ferrite and that at 600°C have a larger width than nanoparticles at 800°C because of the high coercivity (10.7 fold compared with as-prepared Co/Bi nano-ferrite). It is known that an increase in annealing temperature influences the spin-spin interaction (dipolar and exchange).78 Consequently, the double peaks appearing in Fig. 5 for both as-prepared and 600°C nano-ferrite have been explained.
Finally, the nano-ferrite sample at 800°C is the best examined. It has the greatest Ms and the lowest Hc, making it applicable to various technical applications.
High-Frequency Application
The magnetic data were used to estimate the operating frequency response, which is shown in Fig. 6 and Table III. The operating frequency of the nanoparticles at 800°C was much greater than that of as-prepared nanoparticles. Several factors influence the operational frequencies, including the nanomaterials' magnetism and the devices' shapes. The equipment's operating frequency is a valuable measure of its performance. The following relationship79,80 was used to get the operating frequency (ω):
where M represents the sample's magnetization and γ = 2.8 MHz/G represents the gyromagnetic ratio. The operating frequencies of the samples under study are shown in Table III. By raising the annealing temperature, the operating frequency of the nano-ferrite under study increased. The as-prepared sample and the 600°C sample are therefore highly recommended for usage in super high microwave frequency (SHF) in the X band, which is utilized in radio frequency communications for spacecraft. Moreover, the nano-ferrite sample at 800°C is strongly recommended for use in the Ku band in radar and satellite communications.81
Antimicrobial Applications
Figure 7a–c and Table IV show the antimicrobial studies performed on Co/Bi nanoparticles at different annealing temperatures of 600 and 800°C with gram-positive, gram-negative, and fungal strains. The materials exhibited no antifungal efficacy against the lab-grown pathogens. The tested gram-negative bacteria were Neisseria gonorrhoeae (ATCC 19424), Escherichia coli (ATCC 11775), and Pseudomonas aeuroginosa (ATCC 10145). Moreover, the tested gram-positive bacteria used in this study were Bacillus subtilis (ATCC 6051), Streptococcus faecalis (ATCC 19433), and Staphylococcus aureus (ATCC 12600). The nanoparticles were more effective against all tested strains when annealed at 800°C than at 600°C. This is because the increased crystallinity of the nanoparticles at higher temperatures makes the ions (Bi3+, Co2+, and Fe3+) more effective in destroying bacteria and causing damage to their DNA yyyyyyy.18,82,83,84,85,86 Moreover, there is a correlation between saturation magnetization (Ms) and the strong efficacy against tested bacteria, where the larger the saturation magnetization, the stronger the efficacy against tested bacteria. Thus, the nanoparticles annealed at 800°C exhibited higher Ms values and higher antibacterial properties compared to those annealed at 600°C. Therefore, the results suggest that the studied nanoparticles can be used as an alternative to various drugs as antibacterial nanomaterials.
Conclusion
All the tested samples of Co/Bi nano-ferrite at different annealing temperatures were successfully synthesized using the flash auto-combustion technique. They have a single-phase cubic spinel structure. XRD analysis showed that raising the annealing temperature resulted in larger crystallites. Atomic force microscopy (AFM) analysis confirmed the nanoscale, low-aggregation character of the materials. Increasing the annealing temperature resulted in a high-saturation magnetization Ms at 800°C. However, the coercivity of Hc was reduced, allowing the Co/Bi nano-ferrite to be used in a variety of technological applications. The magnetic measurements are used to compute high-frequency applications, which demonstrate that the samples under study have the potential to be used in super high microwave frequency (SHF) at X band (as-prepared sample and at 600°C) for radio frequency applications in spacecraft and at Ku band (Co/Bi nano-ferrite at 800°C) for radar and satellite communications. Moreover, antimicrobial applications were measured, and it was shown that both samples had a strong efficacy against the tested bacteria and were recommended to be antibacterial nanomaterials, especially Co/Bi nano-ferrite at 800°C.
Availability of Data and Material
The data are available on reasonable request.
References
H.N. Abdelhamid, A. Talib, and H.-F. Wu, RSC Adv. 5, 34594–34602 (2015).
F. Xue, Z. Liu, S. Yi, and K. Varahramyan, Micro-Electron. Eng. 83, 298 (2006).
Y. Pan, J. Phys. Chem. Solids 174, 111152 (2023).
Y. Pan, Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 281, 115746 (2022).
Y. Pan, J. Electron. Mater. 48, 5154–5160 (2019).
Y. Pan, Int. J. Eng. Res. 46, 13070–13078 (2022).
E. Yu, and Y. Pan, Int. J. Hydrogen Eng. 48, 14785–14794 (2023).
Y. Pan, Mater. Sci. Semicond. Process. 135, 106084 (2021).
A.A.H. El-Bassuony, H.K. Abdelsalam, and W.M. Gamal, JOM 74, 2656–2664 https://doi.org/10.1007/s11837-022-05170-x (2022).
S. Honary, K. Ghajar, P. Khazaeli, and P. Schalchian, Trop. J. Pharm. Res. 10, 69–74 (2011).
A. El-adly, and I. Shabana, Egypt. J. Bot. 58, 119–132 (2018).
A.A.H. El-Bassuony, W.M. Gamal, and H.K. Abdelsalam, Eur. Phys. J. Spec. Top. 232, 1339–1351 https://doi.org/10.1140/epjs/s11734-022-00759-4 (2023).
V. Pallai, and D.O. Shah, J. Magn. Magn. Mater. 163, 243–248 (1996).
J.S. Kim, E. Kuk, K.N. Yu, J.-H. Kim, S.J. Park, H.J. Lee, S.H. Kim, Y.K. Park, Y.H. Park, and C.-Y. Hwang, Nanomed. Nanotechnol. Biol. Med. 3, 95–101 (2007).
W.M. Gamal, A.H. El-Bassuony, H.K. Abdelsalam, et al., J. Mater. Sci. Mater. Electron. 32, 21590–21602 https://doi.org/10.1007/s10854-021-06667-y (2021).
L. Yin, Y. Shi, L. Lu, R. Fang, X. Wan, and H. Shi, Catalysts 6, 69 (2016).
T. Giannakopoulou, L. Kompotiatis, A. Kontogeorgakos, and G. Kordas, J. Magn. Magn. Mater. 246, 360–365 (2002).
M.A. Sayed, A.A.H. El-Bassuony, and H.K. Abdelsalam, Braz. J. Microbiol. 51, 1475–1482 https://doi.org/10.1007/s42770-020-00366-2 (2020).
A.A.H. El-Bassuony, and H.K. Abdelsalam, Phys. Scr. 98, 055919 https://doi.org/10.1088/1402-4896/acc90c (2023).
M. Pardavi-Horvath, J. Magn. Magn. Mater. 215–216, 171–183 (2000).
W.M. Gamal, A.A.H. El-Bassuony, and H.K. Abdelsalam, Polym. Bull. https://doi.org/10.1007/s00289-023-04788-4 (2023).
R.J.B. Pinto, P.A.A.P. Marques, C.P. Neto, T. Trindade, S. Daina, and P. Sadocco, Acta Biomater. 5, 2279–2289 (2009).
R.C. Bharamagoudar, A.S. Patil, S.N.M. Vijay, M.K. Laxmi, and B. Kankanawadi, Acta Chem. IASI 26, 249–262 (2018).
A.A.H. El-Bassuony, and H.K. Abdelsalam, Eur. Phys. J. Plus 135, 66 https://doi.org/10.1140/epjp/s13360-019-00025-y (2020).
Y. Slimani, M.A. Almessiere, S.E. Shirsath, et al., Inorg. Chem. Commun. 153, 110753 (2023).
R. Shitole, V.K. Barote, M.L. Mane, et al., J. Mater. Sci.: Mater. Electron. 34, 1106 (2023).
R.H. Kadam, R. Shitole, S.B. Kadam, et al., Nanomaterials 13, 1165 (2023).
J. Dhatwalia, A. Kumari, A. Chauhan, et al., Chem. Pap. 77, 1377–1393 (2023).
V.J. Angadi, K.M. Batoo, S. Hussain, et al., J. Mater. Sci.: Mater. Electron. 33, 24308–24320 (2022).
P. Fischer, M. Polomska, I. Sosnowska, and M. Szymański, J. Phys. C 13, 1931–1940 (1980).
S. Nakamura, S. Soeya, N. Ikeda, and M. Tanaka, J. Appl. Phys. 74, 5652–5657 (1993).
H. Yang, X.C. Zhang, A.D. Tang, and G.Z. Qiu, Chem. Lett. 33, 826–827 (2004).
L.H. Ai, and J. Jiang, Curr. Appl. Phys. 10, 284–288 (2010).
Z. Huang, X. Jiang, D. Guo, and N. Gu, J. Nanosci. Nanotechnol. 11, 9395–9408 (2011).
A.K.S. Gamerith, H. Scheiber, U. Scherf, E. Moderegger, and E.J.W. List, Adv. Funct. Mater. 17, 3111 (2007).
R. Skomski, J. Phys.: Condens. Matter 15, R841–R896 (2003).
A. Meenakshisundaram, N. Gunasekaran, and V. Srinivasan, Physica Status Solidi A 69, K15–K19 (1982).
S. Kapoor, A. Goyal, S. Bansal, and S. Singhal, New J. Chem. 42, 14965–14977 https://doi.org/10.1039/C8NJ00977E (2018).
S.H. Kim, H.S. Lee, D.S. Ryu, S.J. Choi, and D.S. Lee, J. Microbial. Biotechnol. 39, 77–85 (2011).
R. Katwal, H. Kaur, G. Sharma, M. Naushad, and D. Pathania, J. Ind. Eng. Chem. 31, 173–184 (2015).
K.S. Tan, and K.Y. Cheong, J. Nanoparticle Res. 15(4), 1–29 (2013).
Y. Lu, and K. Chou, J. Chin. Inst. Chem. Eng. 39, 673–678 (2008).
A.A.H. El-Bassuony, W.M. Gamal, and H.K. Abdelsalam, JOM 74, 2635–2644 https://doi.org/10.1007/s11837-022-05315-y (2022).
W.M. Gamal, A.A.H. El-Bassuony, R.S. Hafez, and H.K. Abdelsalam, JOM 74, 4898–4908 https://doi.org/10.1007/s11837-022-05491-x (2022).
A.A.H. El-Bassuony, W.M. Gamal, and H.K. Abdelsalam, J. Mater. Sci.: Mater. Electron. 33, 16219–16235 https://doi.org/10.1007/s10854-022-08516-y (2022).
A.W. Bauer, W.M. Kirby, C. Sherris, and M. Turck, Am. J. Clin. Pathol. 45, 493–496 (1966).
E.S. Anooj, S.J. Sreelekshmi, S.T. Gopukumar, and P.K. Praseetha, Int. J. Pharm. Sci. Rev. Res. 46, 22–26 (2017).
A.A.H. El-Bassuony, JOM 72, 1154–1162 https://doi.org/10.1007/s11837-019-03784-2 (2020).
M. Srivastava, A.K. Ojha, S. Chaubey, P.K. Sharma, and A.C. Pandey, Mater. Sci. Eng., B 175, 14–21 (2010).
R.C. Kambale, K.M. Song, Y.S. Koo, and N. Hur, J. Appl. Phys. 110, 053910 (2011).
A.A.H. El-Bassuony, and H.K. Abdelsalam, J. Therm. Anal. Calorim. 138, 81–88 (2019).
H.M. Khan, A.I. Misbah-ul-Islam, and M. Rana, Mater. Sci. Appl. 2, 1083–1089 (2011).
W.M. Gamal, A.A.H. El-Bassuony, H.K. Abdelsalam, and S.M. Abd El Wahab, J. Mater. Sci. Mater. Electron. 32, 21590–602 (2021).
X. Liu, R. Hong, and C. Tian, J. Mater. Sci. Mater. Electron. 20, 323–327 (2009).
M.Z. Ahsan, M.A. Islam, and F.A. Khan, Results Phys. 19, 103402 (2020).
M.M. da Silva Paula, C.V. Franco, M.C. Baldin, L. Rodrigues, T. Barichello, G.D. Savi, and L. da Silva, Mater. Sci. Eng. 29, 647–650 (2009).
M.J. Iqbal, and M.R. Siddiquah, J. Alloy. Compd. 453, 513–518 (2008).
R. Saravanan, Mater. Res. Found. 18, 29 (2017).
B. Mallick, T. Patel, R.C. Behera, S.N. Sarangi, S.N. Sahu, and R.K. Choudhar, Nucl. Instrum. Method Phys. Res. B 248, 305 (2006).
M. Madani, Curr. Appl. Phys.Appl. Phys. 11, 70 (2011).
N.S. Kumar, and K.V. Kumar, World J. Nano Sci. Eng. 5, 140–151 (2015).
A. Globus, H. Pascard, and V. Cagan, J. Phys. (Paris) Colloq. 38, 168 (1977).
M.A. Islam, A.K.M. Hossain, M.Z. Ahsan, M.A.A. Ballya, M.S. Ullaha, S.M. Hoque, and F.A. Khana, RSC Adv. 12, 8502–8519 (2022).
B. Aslibeiki, Curr. Appl. Phys. 14, 1659–1664 (2014).
K.B. Modi, S.J. Shah, N.B. Pujara, T.K. Pathak, N.H. Vasoya, and I.G. Jhala, J. Mol. Struct. 1049, 250–262 (2013).
S. Qamar, S. Yasin, N. Ramzan, A. Umer, and M.N. Akhtar, Chin. J. Phys. 65, 82–92 (2020).
M. Amami, F. Jlaiel, P. Strobel, and A. Ben Salah, IOP Conf. Ser. Mater. Sci. Eng. 13, 012001 (2010).
K. Kamazawa, Y. Tsunoda, H. Kadowaki, and K. Kohn, Phys. Rev. B 68, 24412 (2003).
S.R. Naik, A.V. Salker, S.M. Yusuf, and S.S. Meena, J. Alloys Compd. 566, 54 (2013).
R. Sagayaraj, S. Aravazhi, P. Praveen, and G. Chandrasekaran, J. Mater. Sci. 29, 2151–2158 (2017).
T.G. Aminov, G.G. Shabunina, and E.V. Busheva, Inorg. Mater. 56, 771–778 (2020).
N. Sivakumar, A. Narayanasamy, K. Shinoda, C.N. Chinnasamy, B. Jeyadevan, and J.M. Greneche, J. Appl. Phys. 102, 13916 (2007).
H. Irfan, R. Ezhil Vizhi, and P. Saravanan, J. Mater. Sci. Mater. Electron. 31, 10585–10592 (2020).
S.D. Ali, S.T. Hussain, and S.R. Gilani, Appl. Surf. Sci. 271, 118 (2013).
Y. Pan, Mater. Sci. Semicond. Proc. 120, 105306 (2020).
Y. Pan, and S. Chen, Int J Energ Res. 44, 10970–10981 (2020).
A.A.H. El-Bassuony, W.M. Gamal, A.F. Ibrahim, and H.K. Abdelsalam, JOM. https://doi.org/10.1007/s11837-024-06391-y (2024).
S. Mørup, M.F. Hansen, and C. Frandsen, Compr. Nanosci. Technol. 1, 437 (2011).
K. Park, M.A. Novotny, N.S. Dalal, S. Hill, and P.A. Rikvold, Phys. Rev. B 66, 144409 (2002).
P. Akhtar, M.N. Akhtar, M.A. Baqir, A. Ahmad, M.U. Khallidoon, M. Farhan, and M. AzharKhan, J. Mater. Sci. Mater. Electron 32, 7692–7703 (2021).
M.N. Akhtar, M. Saleem, and M.A. Khan, J. Phys. Chem. Solids 123, 260–265 (2018).
You, K. Y. Emerging Microwave Technologies in Industrial, Agricultural, Medical and Food Processing. London: Intech Open, (2018) https://www.intechopen.com/chapters/58958
A.A.H. El-Bassuony, and H.K. Abdelsalam, JOM 71, 1866–1873 https://doi.org/10.1007/s11837-019-03415-w (2019).
Revie, N. M., Iyer, K. R., Robbins, N., & Cowen, L. E. Curr. Opin. Microbiol. 45 (2018).
M.A. Sayed, T.M.A.A. El-Rahman, H.K. Abdelsalam, et al., BMC Chem. 16, 39 https://doi.org/10.1186/s13065-022-00832-y (2022).
M.A. Sayed, T.M.A.A. El-Rahman, H.K. Abdelsalam, et al., Indian J Microbiol. https://doi.org/10.1007/s12088-024-01229-2 (2024).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors contributed to data analysis, drafting, and revising the article. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Bassuony, A.A.H., Hafez, R.S., Matter, N.M.S. et al. Enhancement of Cobalt Bismuth Nano-Ferrite via Heat Treatment to be Applied in High-Frequency and Antimicrobial Applications. JOM (2024). https://doi.org/10.1007/s11837-024-06564-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11837-024-06564-9