Abstract
Laser powder bed fusion (LPBF) additive manufacturing (AM) is a promising route for the fabrication of oxide dispersion strengthened (ODS) steels. In this study, 14YWT ferritic steel powders were produced by gas atomization reaction synthesis (GARS). The rapid solidification resulted in the formation of stable, Y-containing intermetallic Y2Fe17 on the interior of the powder and a stable Cr-rich oxide surface. The GARS powders were consolidated with LPBF. Process parameter maps identified a stable process window resulting in a relative density of 99.8%. Transmission electron microscopy and high-energy x-ray diffraction demonstrated that during LPBF, the stable phases in the powder dissociated in the liquid melt pool and reacted to form a high density (1.7 × 1020/m3) of homogeneously distributed Ti2Y2O7 pyrochlore dispersoids ranging from 17 to 57 nm. The use of GARS powder bypasses the mechanical alloying step typically required to produce ODS feedstock. Preliminary mechanical tests demonstrated an ultimate tensile and yield strength of 474 MPa and 312 MPa, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxide dispersion strengthened (ODS) ferritic steel with a high density of nano-scale oxides in the order of 1023/m3 offers a number of high-temperature properties, including creep strength at high temperatures,1 radiation resistance,2,3 and helium management.4,5 As such, ODS steels are desirable for improvements in fusion reactor operational lifetime and reliability, neutron radiation damage resistance, higher thermodynamic efficiency, and reduced construction costs.1 However, the current processing of ODS steels typically requires the forced dissolution of Y2O3 powder into the steel matrix by mechanical alloying (MA). This step facilitates the subsequent precipitation of Y-Ti-O nano-scale oxides during hot consolidation and thermomechanical processing,6 but the extensive MA (> 40 h) and limited machinability7,8 and, often, ductility present an impediment to the continued commercial viability of ODS steel alloys.9
Additive manufacturing (AM) has attracted interest as a potential processing method that would facilitate the direct fabrication of complex, near-net-shape ODS steel components. Only a limited number of studies have been reported for AM of ODS steel. Many studies using laser powder bed fusion (LPBF) AM have utilized ferritic or austenitic steels mechanically alloyed with Y2O3 as AM feedstock.10,11,12,13,14,15,16,17,18,19,20 Alternatively, low-energy ball milling and blending,21,22,23 along with methods to deposit oxides onto powders in situ, have been reported as alternative feedstock techniques for fabrication by AM.24,25,26,27,28 While some promising results have been reported, challenges related to heterogeneity and agglomeration of pre-existing Y2O3 remain, and these approaches typically depend on MA processing, which can be a limiting factor to scalability. Several recent studies have sought to bypass the MA step and leverage oxidation within the melt pool, observing the formation of nano-scale oxides from trace amounts of deoxidizers with favorable free energies, such as Si and Mn in austenitic steel, especially at low partial pressures of oxygen in the LPBF chamber.29,30,31,32,33,34 Early work by Liu et al. (2010) added 1.9 vol.% of O2 to the Ar LPBF chamber to process a nominal composition of 14YWT and observed the in situ formation of Y and Ti–rich oxides.35 More recent studies have experimented with varying the LPBF chamber atmosphere or the powder composition.19,36,37,38,39 While the reported data are promising, these studies highlight the need for tight control over the relative stoichiometry between oxygen and oxide formers within the LPBF melt pool. However, they are not conclusive with respect to how tightly the atmosphere must be controlled or the influence of subtle variations in local oxygen content on the oxide size, density distribution, and homogeneity. One potential approach is the use of gas atomization reaction synthesis (GARS) powders as AM feedstock. For ferritic steels like 14YWT, Cr is added to Fe as a ferrite stabilizer, along with elements such as Ti and W for particle stabilization and solid solution strengthening, respectively. Y is then added elementally to the molten alloy, and all of the components are rapidly solidified together in the resulting powders. The alloy melt is atomized with a precisely located and controlled Ar + O2 gas jet. The rapid solidification results in the entrapment of Y-containing intermetallics (typically Y2Fe17 or YFe3) at cell or dendrite interfaces from the solidification microstructure or as Y in supersaturated solid solution with Fe-Cr-Ti-W, while the interaction of the atomized droplet surfaces forms a kinetically-favored and stable oxide shell/skin, typically Cr2O3. With solid-state processing of GARS powder, the oxide layer was used as an internal metastable chemical reservoir for oxygen during hot isostatic pressing that decomposed to allow oxygen transport and formation of stable oxide dispersoids at the location of the dissolved Y and the intermetallic “template” locations.40,41,42 In recent work using GARS feedstock, oxygen was added to the LPBF atmosphere to supplement the oxidation of Y in the melt pool. These data showed a trend of decreasing oxide size and increasing oxide density with increasing atmospheric oxygen up to 5% by weight.38 In the present study, additional oxygen was supplied during atomization to increase the oxygen content in the shell of the powder. It is hypothesized that the oxide shells will provide the appropriate stoichiometry of oxygen to react with Y and Ti in the powder to form nano-scale Y2Ti2O7 pyrochlore oxides without additional oxygen in the LPBF atmosphere.
The objective of the present study was first to develop LPBF AM process maps for predicting build results ranging from porous structures to unstable/swelled structures for 14YWT GARS precursor powders to identify parameters that yield high density and process stability/repeatability. In the reported literature on the LPBF of ODS steels, only a few discuss the processing parameters used20,39,43 and, of those, it is not universally clear whether the reported oxide sizes and densities can be associated with a stable processing space. The second objective was to identify the distribution of elements and phases within the GARS powder and lastly to downselect to the stable samples with the highest density and to characterize the microstructure, material properties, and distribution of nano-scale oxides in the LPBF produced ODS steel matrix.
Materials and Methods
Preparation and Characterization of Precursor GARS Powder
The powder was prepared by gas atomization of a nominal composition of Fe-14Cr-0.3Y-3 W-0.4Ti (wt.%). Fe-Cr-Ti-W and elemental Y were melted in an inert argon atmosphere and superheated to homogenize the alloy. The molten stream was then atomized with a mixture of Ar + 1760 ppm wt. O2. All of the elements were rapidly solidified in the resulting powders. After cooling, the powder was screened to 20 µm and 53 µm (− 270/ + 625 mesh). The oxygen content of the powder was measured by inert gas fusion using a LECO OH 836 referencing ASTM E1019-18 standard for Fe-base alloys. The elemental composition of the powder was determined by inductively coupled plasma mass spectrometry (ICP-MS). The particle size distribution (PSD) of the powder was measured using a Microtrac S3500 laser diffraction particle analyzer following a wet method protocol with ultrasonic agitation. Powder morphology was observed using a JEOL 6010LA scanning electron microscope (SEM). Transmission electron microscopy (TEM) liftouts were from powder particles prepared using an FEI Quanta field emission gun (FEG) with a focused ion beam (FIB). Scanning transmission electron microscopy (STEM) and TEM was accomplished using a ThermoFisher Talos F200X with energy-dispersive x-ray spectroscopy (EDS) and selected area electron diffraction (SAED). A PANalytical Empyrean Diffractometer using a standard Bragg–Brentano geometry was used to identify the phases present in the powder. The XRD parameters were 45 kV acceleration voltage, 40 mA current, kα wavelength of 1.5406 Å, and a step size of 0.04° and 0.013°, over 2θ ranges 10°–90°, and 30°–44°, respectively. It was expected that the fraction of the anticipated Y-containing phases (e.g., Y2Fe17) would be relatively small, so in addition, high-energy x-ray diffraction (HE-XRD) was carried out at the Advanced Photon Source (APS), Argonne national laboratory-USDOE, beamline 11B using synchrotron radiation. Data were collected at a wavelength of 0.458 Å (30 keV), at room temperature (298 K), over 2θ range 0.5°–50°, with a step size of 0.001°.
LPBF Sample Fabrication
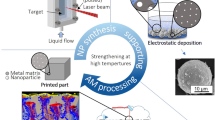
Figure 1a illustrates the premise of the GARS-LPBF approach. This study hypothesizes that the Cr-rich oxide shell and the Y-containing intermetallics, typically Y2Fe17, YFe3, or Y retained in supersaturated solid solution, dissociate and react within the LPBF molten pool to form a homogeneous distribution of nano-scale oxide dispersoids. The oxide shell on the powder acts as an oxygen reservoir to provide the correct stoichiometry in the melt pool for the reaction synthesis of the oxides, ideally Y2Ti2O7.
Illustration of the experimental approach showing the dissolution of the Cr-rich oxide shell and stable Y-containing phases in the LPBF melt pool and the in situ reaction to form a homogeneous distribution of nano-scale Y–Ti rich oxides (a). Photograph of the LPBF chamber during the processing of the 15 mm × 7.5 mm × 4 mm samples (b). Schematic of the sub-size tensile bar geometry, units in mm (c).
All test specimens were fabricated using a modified concept laser M100R with a 100-W Nd:YAG fiber laser at ~ 1060 nm wavelength and 50 µm spot size. The standard steel build substrate measuring 90 mm × 90 mm × 10 mm was CNC machined with three slots 6.35 mm deep and 20.32 mm wide across the plate to accept matching precision-ground steel inserts that served as the substrate upon which the samples were produced.
Samples were printed only on the first insert to avoid re-introducing spatter into the sample during melting. Three different sample geometries were fabricated. Process parameter mapping was carried out using 7.5 mm × 15.0 mm × 4.0 mm samples. Figure 1b shows a photograph of these samples in the LPBF chamber during processing. After down-selecting to the stable parameters that resulted in high sample density, a larger sample measuring 15 mm × 25 mm × 15 mm was produced for microstructure characterizations and a sample 14 mm × 80.0 mm × 8.0 mm for harvesting sub-size tensile samples from the as-fabricated specimen. All samples were produced using a Grade 5 Ar chamber atmosphere with an O2 content of less than 0.5% as measured by the built-in system oxygen sensor (± 0.1%). All samples were fabricated with 10 mm × 10 mm scan patches with an angle of rotation at 45°.38 These patches were shifted from layer to layer by 2.5 mm. The layer thickness for the study was fixed at 0.025 mm, and the beam power was fixed at 95 W.44 The hatch spacing was varied in discrete steps (0.03 mm, 0.05 mm, and 0.08 mm). The beam speed was then selected to target a wide range of volumetric energy density, shown in Table I. The order in which the samples were fabricated and the sample position on the steel insert was randomly assigned using a random number generator in Microsoft Excel. Table 1 lists the LPBF process parameters used in this study.
Characterization of LPBF consolidated 14YWT
The processing space was analyzed by measuring the 2D relative density of cross-sections of the samples and assessed in terms of density as a function of the volumetric energy density, E (J/mm3). This term is often represented by the expression E = P/vht, where P is the laser beam power, v is the beam speed (mm/s), h is the hatch offset (mm), and t is the layer displacement (mm). Using a low-speed liquid-cooled diamond saw, each sample was cross-sectioned perpendicular to the long dimension (resulting in a 7.5 mm × 4 mm area cross-section for each test condition). Samples were cold-mounted in epoxy and were progressively ground and polished using a Buehler Ecomet 30 Automatic polisher (600 grit, 1200 grit, followed by 1 µm, 0.3 µm, and 0.05 µm diamond polishing). The cross-sections were imaged and tiled on a digital optical microscope (Hirox KH-7700) at 70 × magnification. The images were thresholded using ImageJ (± 15% variation in the thresholding was also carried out, resulting in ~ ± 2% variation in the relative density measurements). The imageJ particle analysis toolpak was then used to calculate the area fraction of the observed porosity.45,46 Vickers hardness measurements of the as-fabricated solids were made using a LECO M400 microhardness tester with a diamond indenter (500 g-force, 10-s dwell). General microstructure, EDS, and electron backscatter diffraction (EBSD) characterization were accomplished using a ThermoFisher Helios 5 Hydra CX and plasma Focused Ion Beam (pFIB). TEM sample extraction was carried out by a lift-out technique with an FEI Quanta 3D Field Emission Gun (FEG) equipped with a FIB. XRD of solid specimens was performed on a PANalytical Empyrean x-ray diffractometer. Nano-scale oxides were measured from images acquired from several regions on the liftouts using a ThermoFisher Talos F200X TEM utilizing TEM, STEM, and EDS. Electron energy loss spectroscopy (EELS) was used to generate thickness maps of the region used to calculate dispersoid sizes to calculate the volume distribution of the dispersoids.
Two sub-size flat tensile coupons were harvested from the 14.0 mm × 80.0 mm 8.0 mm sample by wire electric discharge machining. The gauge cross-section of the coupons was 3.18 mm × 3.18 mm, and the gauge length was 9.53 mm with a 12.7-mm radius fillet. The specimens were subjected to uniaxial loading in tension at room temperature based on ASTM E8/E8M standard on an ATS model 1620C by Applied Test Systems Inc. with a crosshead speed of 0.1 mm/s47,48 and a 5-kN autocorrected load cell. Digital image correlation (DIC) was performed to map surface displacement fields during testing and calculate strain49 using GOM Correlate (2021 Hotfix 3, Rev 144,264). A black and white speckle pattern was applied to the gauge section, and images were recorded using a Sony IMX74 monochromatic grasshopper3 USB3 (Model: GS3-U3-23S6M-C) with a 2.3-MP camera at a rate of 15 frames per s.50,51
Results and Discussion
Powder Characterization
Powder characterization of the 14YWT GARS powder was performed to determine the composition, morphology, size, and internal structure to ascertain the potential role of these characteristics in the reaction synthesis of nano-scale oxides during LPBF processing. The composition was determined as (wt.%): Fe-bal., Cr-14.03, W-3.06, Ti-0.4, Y-0.35, O-981 ppm (± 53 ppm). Figure 2a shows an SEM of the gas atomized powder morphology, which consists primarily of spherical particles and what would appear to be two size distributions. The volumetric size measurements (Fig. 2b) made with laser diffraction indicated a Gaussian distribution with a median size of 35 µm and a range of 26 to 51 µm (10th–90th percentile.). However, the numeric (count) size data revealed a bimodal distribution with a significant fraction of fine particles with a median size of 7 µm and a range of 4–34 µm (10th–90th percentile).
Despite the bimodal distribution and presence of fines, the powder was observed to spread without apparent irregularities in the formation of the powder bed. Powder morphology and size distribution are well-known factors that influence interparticle friction and, hence, spreadability and uniformity.52,53,54 Finer particles generally fill the void between relatively larger particles and thus result in a higher packing density which affects the spread.48,55 As discussed by Kishimoto et al., Fe–Cr–Ti powders mechanically alloyed with Y2O3 to produce 14YWT exhibit irregular, ‘asteroid’ shaped morphology.56 The AM literature has predominantly focused on LPBF of MA compositions like PM2000,10 MA956,11 and other Fe-Cr-W-Ti compositions mechanically alloyed with Y2O3.12,13,14,15,20,43 Additionally, LPBF has been applied to austenitic stainless steel powders mechanically alloyed with Y2O3.16,17,18,19
While it has not been directly addressed in this study, it would be expected that the general sphericity of powders produced by gas atomization would be well suited for consistent bed formation during LPBF application of ODS powders compared with equivalent MA powders due to the resistance to flow caused by mechanical interlocking between irregular and satellite particles.54,55,57
The internal structure of the powder particles was examined by TEM to determine the distribution of elements and to confirm the absence of Y-Ti dispersoids in the precursor powder. Figure 3a shows a TEM image of a liftout from a typical median-sized particle. The green and red arrows point to regions along grain boundaries where pockets rich in Y were observed. These were often associated with discontinuous regions rich in Y with spherical and elongated morphologies. Previous studies using similar ferritic steel GARS compositions identified Y as elemental Y at the grain boundaries, in solution in the matrix, or as a stable intermetallic compound Y2Fe17.38,40,41,42 The labeled boxes in Fig. 3a refer to regions inspected with high-angle annular dark-field (HAADF) imaging and EDS maps of Fe, Cr, Ti, Y, and O in Fig. 3b, c, and d. Figure 3b shows a region through the surface of the powder particle. The oxide crust was observed to be continuous and consisted of a top layer of Cr and O, followed by a thin subsurface layer of Ti, Y, and O, where both layers combined, were less than 50 nm as measured by an EDS line scan in Fig. 3e. During gas atomization, the interaction of the O2-rich gas stream with the liquid droplet resulted in the formation of a kinetically favored oxide shell/skin rich in Cr2O3. Since GARS powders are intentionally oxidized during atomization, the oxide crust is often slightly thicker than traditional steel powder. In previous GARS compositions, the continuous surface layer of Cr-O was typically < 150 nm.40
TEM image of a liftout harvested from a powder particle (a). The green arrows indicate larger areas rich in Y, and the red arrows indicate discontinuous regions rich in Y. The labeled boxes indicate areas analyzed with STEM/EDS (b, c, & d). The STEM/EDS images highlight the distribution of elements in the shell of the powder (b) and the interior (c & d). An EDS line scan shows a Cr-rich oxide shell with Y, Ti, and O beneath (e).
Figure 3c shows an area near the surface and reveals the presence of Y near a grain boundary. From the EDS maps, it would appear that these same regions are lacking in Fe, Cr, and O. Figure 3d shows a region on the interior of the particle. The EDS maps show that the internal features, indicated by the red arrows, are depleted in Cr, Ti, and O, indicating mixed Y and Fe-Y rich phases regions. These regions were identified as Y2Fe17. The bulk of the reactive Y & Ti elements were observed to be available in submicron sizes individually or as intercellular Y-containing intermetallic, which is critical to the process where they may be available for reaction with the Cr-oxide shell, which acts as a chemical reservoir during LPBF to form nano-scale oxides in the melt pool.
Characterization of LPBF consolidated 14YWT
Figure 4a shows the tiled optical images of the sectioned and polished samples. The images are arranged in columns by the hatch spacing (0.03 mm, 0.05 mm, and 0.08 mm). The beam speed variable decreases from the top row to the bottom row. This series of samples was intended to span the entire process space with the maximum power of the LPBF system. The numerical value in the bottom left corner of each tile in Fig. 4 is the volumetric energy density. The plot in Fig. 4b shows the measured relative density of each cross-section as a function of the volumetric energy density and the beam speed for the three hatch-spacing conditions. Lines of constant hatch spacing are plotted, and the experimental data are colored according to the measured relative density. These data provide a compiled visual representation of the entire processing space and identify the most promising process window for further evaluation. The minimum volumetric energy density leading to parts > 95% dense was 80 J/mm3. Samples processed below this threshold exhibited a high degree of lack-of-fusion type defects. The porosity at higher energy densities was the result of sample swelling that led to uneven/unstable powder-bed formation. The highest optical relative density was observed in the samples produced with 0.080 mm hatch spacing with 507 mm/s (93.75 J/mm3) and 0.050 hatch with 608 mm/s (125 J/mm3) (99.6% and 99.8%, respectively). The 608 mm/s and 0.050 mm hatch parameters were down-selected to produce the 15 mm × 15 mm × 25 mm sample for metallographic analysis and the 14.0 mm × 80.0 mm × 8.0 mm sample for tensile testing because the sample surfaces appeared (qualitatively) more planar during processing.
Figure 5a shows a photograph of the 15 mm × 15 mm × 25 mm 14YWT specimen produced with the down-selected stable parameters. The sectioned and polished surface, shown in Fig. 5b, is free of prominent or visible porosity. The entire cross-section was imaged with computer-aided SEM tiling of 504 images in backscatter mode to identify defects such as porosity, inclusions, or presence of large-scale oxides (< 5 µm), of which only several were observed. The typical microstructure is shown in Fig. 5c. Overall, the sample exhibited only occasional micron-scale porosity. As the microstructure revealed a highly dense surface, the same stable parameters were used to fabricate the 14 mm × 80 mm × 8 mm tensile specimen.
Photograph showing the as-fabricated 15 mm × 15 mm × 25 mm sample using a beam speed of 608 mm/s, hatch spacing of 0.05 mm, and beam power of 95 W before sectioning (a) and after sectioning and polishing (b). Backscatter SEM image showing general microstructure (c). EBSD inverse pole figure (ipf) map showing a strong texture in the < 100 > direction (d) and a high magnification backscatter SEM image of a region enlarged from C showing a distribution of low-density particles indicated by the white arrows (e).
Figure 5d is an inverse pole figure (ipf) map of a representative low magnification area accompanied by a pole figure showing crystallographic texture in the < 100 > direction, and is typical of AM-fabricated materials with epitaxial grain growth. The average grain length was observed to be ~ 343 ± 177 μm with an average grain width of ~ 24 ± 14 μm. Figure 5e is a backscatter SEM image that reveals a matrix with structures of slender grains and nano-scale spherical particles indicated by the white arrows; these were later identified as Y2Ti2O7 by STEM/EDS and SAED. These particles were observed to be non-agglomerated and homogeneously distributed throughout the matrix and not preferentially located along grain boundaries. However, it was difficult to determine the size of the particles in the SEM images due to the sectioned specimen surface. Particle measurement was instead carried out by TEM.
X-ray diffraction patterns of the GARS powder and LPBF fabricated solid are shown in Fig. 6a from 10 to 80° 2θ with 0.013 step size. The diffraction patterns were analyzed on the face along the Z-axis or along the XZY plane as defined by ISO/ASTM 52921 standard. The ferrite Fe peaks dominate the spectra, and the effect of the laser PBF on the solid spectrum is observed in the peak positions and relative intensity. The solid sample exhibited peak shifts of − 0.09, − 0.22, and − 0.25°, for (110), (200), and (211), respectively, as well as the increased intensity in the (200) peak. These effects are most likely related to residual stress in the solid samples from thermal cycling during fabrication. The XRD pattern of the powder showed minor changes compared with powder diffraction standards [00-006-0696]. The inset in Fig. 6a shows a scan between 30° and 44° 2θ that highlights a peak at 40.2° that could possibly be associated with un-melted W in the powder (possibly a trace amount of W left over from the original, pre-atomized liquid melt) and, at 42.8 degrees, is one of the two main peaks associated with Y2Fe17. These small and broad peaks associated with small phase fractions are difficult to resolve from background signal with standard Bragg Brentano XRD setups, even with long scans and small step sizes. Therefore, the presence of the Y2Fe17 intermetallic peaks was confirmed using HE-XRD. Figure 6b shows the APS HE-XRD data between 8° and 30° 2θ. Here, the main peaks for Y2Fe17, located at 42.507° (220) and 42.795° (303), are clearly visible against the background BCC Fe. These data corroborate the previous TEM powder characterization and confirm the presence of Y-containing phases that then dissociate in the LPBF melt pool. In order to investigate the formation of nano-scale oxides from the GARS precursor powders, TEM analysis was conducted to identify the nano-dispersoid elemental composition and size.
XRD results between 10° and 80° 2θ for the GARS powders and LPBF fabricated solids. Black arrow on inserted block represents the face that was analyzed (a). The inset in (a) shows a higher resolution scan between 30° and 44° 2θ. HE-XRD scans from APS beamline 11B confirm the presence of the Y2Fe17 in the powder (b).
Figure 7a is a HAADF image showing the FIB liftout used and one of several locations used for oxide measurement identified by the rectangle in B. The STEM/EDS images shown in Fig. 7b show that spherical oxide particles are depleted of Fe and rich in Ti and Y. No Fe-Y phases were observed in any TEM liftouts or in any SEM analyses in the fabricated solids. Figure 7c shows thickness maps of the TEM lift-outs which were obtained using Electron Energy Loss Spectroscopy (EELS) used to calculate particle densities. Thickness was calculated using the zero loss method using the Digital Micrograph script developed by Malis et al.58 The electron Mean Free Path (MFP) was obtained for the regions of interest and converted into a thickness using the method which relies on the mean atomic number based on the composition. The error in thickness measurement is estimated to be ~ 10%. Figure 7d shows the size distribution of measured particles by TEM, where the 10th and 90th size percentiles range from 17 to 57 nm with a median value of 34 nm and oxide density of 1.7 × 1020/m3. The inset SAED maps in Fig. 7e and f identify the BCC Fe matrix (E) and the nano-oxide pyrochlore phase, Ti2Y2O7 (F), which also captures a small amount of the matrix symmetry mixed within the pattern. This oxide phase is consistent with studies in Fe-14YWT and similar alloys where the MA alloyed yttria and rutile particles transform into complex Ti-Y-O oxides during high-temperature processing such as thermo-mechanical processing and hot isostatic processing (HIP). Studies by Rieken et al. reported that GARS powders with similar compositions formed Ti2Y2O7 after HIP40,41,42 and, by Jia et al. after additively printing the sample, due to favorable free energy of formation at high temperature.39 In our study, the observation of spherical oxides in the fabricated solid indicates that the intermetallic Y2Fe17 and Cr-rich oxide crust, initially observed in the GARS powder, successfully disassociated in the laser PBF melt pool and reacted to form the intended nano oxide particles rich in Ti and Y.
HAADF image of the liftout (a), the red box indicates the region of the STEM/EDS analysis shown in (b). EELS mfp map used to calculate the liftout thickness in the region of interest (b). The size distribution of oxides combined from multiple measurements on the liftout (d), and SAED patterns identifying the BCC Fe matrix and the Ti2Y2O7 particles (e & f, respectively).
The oxide size and density in this study are comparable to what has been reported for recent AM processing of ODS steels which has been compiled in detail in recent reviews.38 Among the smallest reported oxide sizes and highest oxide densities for LPBF are those produced by in situ synthesis with reactive atmospheres, but these data, while compelling, are insufficient to draw broad conclusions regarding an optimal processing pathway and further studies are needed. Horn et al. recently reported an average oxide size of 12.2 nm and density of 3.4 × 1021/m3 produced with LPBF in an atmosphere of Ar + 5 wt.% O2.38 Jia et al. reported a mean oxide size of 21.09 nm with an oxide density of 4.1 ± 0.5 × 1021/m3 for GA powder consolidated by LPBF in a chamber atmosphere containing 3500 ppm oxygen.39 Nevertheless, these results are still significantly different from what is expected from more conventional ODS steel processing routes with oxide sizes in the order of 1–5 nm and densities in the order of ~ 1023/m3.59,60,61 However, it is also important to highlight that the well-documented processing limitations that have hindered the adoption of ODS steels, including microstructural heterogeneity, limited scalability, and difficulties in producing complex assemblies and weldments have motivated recent research on the in situ synthesis of nano-oxides regardless of the manufacturing process.
The methods for in situ oxide synthesis reported in the literature can be described by two general categories in which oxygen is incorporated; either by oxidizing the surface of the powder using processes such as GARS,40,41,42,62 or STARS (surface treatment of gas atomized powder followed by reaction synthesis)63,64,65 or by enriching the processing atmosphere with oxygen during hot processing. Both of these methods have been recently explored for LPBF. Many of the recent data reported are centered not on the application to ODS steels, but rather on the understanding of the origin of Si-Mn nano-scale oxides observed within stainless steels produced by LPBF.30,31,32 By substituting Si and Mn with desired oxidizers like Y, Ti, or Al into powder compositions it is possible to produce compositions more typical of ODS steels. For instance, Haines et al. studied the formation of oxides during LPBF under various concentrations of CO2 in an Ar atmosphere for Fe-Cr-Al-Ti stainless steel and formed nano-scale Al-Ti oxides.36 In a reactive atmosphere, the melt pool encounters different thermal boundary conditions compared to the same material under a non-reactive shielding gas such as pure argon.66,67 Babu et al. have discussed the effect of factors such as alloy composition, melt pool temperature, cooling, and solidification rates as having a significant role in the nucleation of oxides. Through early modeling and experimental work, data by Babu et al. on welding of low-alloy steels suggests an increase in inclusion diameter and a decrease in inclusion density with an increase in the local heat input. The amount of oxygen that is absorbed by the melt pool is dependent on the time the melt spends in the liquid phase.68
Horn et al. used a precursor GARS powder and studied the formation of Y2Ti2O7 rich oxides during LPBF under various concentrations of O2 (such as 1%, 5% by wt.), and did not observe Cr-O rich phases in the solid components at these levels.38,40 Mirzababaei et al. demonstrated the in situ formation of oxides during LPBF under an atmosphere of < 400 ppm oxygen. They observed predominantly nano-scale Y-O, Al-O, and Y-Al-O oxides ranging from 10 to 100 nm with an oxide density 1.48 × 1019/m3, but also larger Fe–Cr–O or Cr–Al–O oxides ranging from 80 to 220 nm.69 Jia et al. used a similar approach in which GA ODS precursor powder was consolidated by LPBF in an atmosphere containing 3500 ppm oxygen. They reported the formation of predominantly Ti2O3 nano-oxides with a smaller amount of Y-Ti-O oxides.39 In the present study, the oxygen content of the LPBF chamber atmosphere was kept to a minimum and the stoichiometric quantity of oxygen needed to fuel in situ reaction synthesis of nano-oxides was instead supplied by the stable Cr-rich oxide shell of the powder.
Tensile Tests
Sub-sized tensile specimens were harvested from the 14 mm × 80 mm × 8 mm sample and tested to failure. The 14 mm × 80 mm × 8 mm sample was fabricated horizontally on the LPBF build platform (along the YXZ orthogonal orientation as per ISO/ASTM 52921) due to the limited quantity of powder. As a result, the columnar grains on the tensile sample are oriented perpendicular to the loading direction. Figure 8 shows the progression of DIC strain development for the entire test phase from the start, middle (45 min), late (70 min), and before fracture at 95 min. The average ultimate tensile strength (UTS) and yield strength (YS) were 474 MPa and 312 MPa, respectively, and the total elongation was 26.8%. A sample coupon was cut along the build direction to measure the microhardness value of the as-fabricated sample in multiple locations. The average Vickers hardness value of the sample was measured at 236.6 HV.
To put these results in context, ODS steels produced by solid-state consolidation and subsequent rolling can provide some insight. Alam et al. reported a UTS of 966 ± 6 MPa, YS of 1053 ± 4 MPa, and microhardness value of 352 ± 39 HV for an SSJ-2 sample for a 14YWT composition produced using MA powders and extracted from the transverse direction of a hot-rolled sample. Here the grain length was 0.799 ± 0.833 µm and grain width was 0.296 ± 0.180 µm with an aspect ratio of 2.7 ± 1.3.70 Kim et al. reported a UTS of 1749 MPa and YS of 1600 MPa for an SS-3 type sample extracted from the longitudinal direction of a hot-rolled sample. They reported near-equiaxed grains with diameters in the range of 100–300 nm with a maximum aspect ratio of 5:1.71 McClintock et al. reported on a YS of 1450 MPa and a UTS of ~ 1600 MPa along the longitudinal direction for an SS-3 type bar produced by extrusion and rolling using a14YWT composition.72
In contrast, ferritic ODS steels produced with AM methods tend to result in lower mechanical properties. Vasquez et al. reported a YS of 392 MPa and a UTS of 447 MPa.13 Jia et al. reported a UTS of 725 MPa and YS of 673 MPa for samples extracted along the longitudinally fabricated direction in an LPBF AM process (VED—104 J/mm3).39 Shi et al. reported a UTS of 370 MPa for a sample extracted along the transverse directions.15 For LPBF samples produced using commercially available MA956 powder, Hunt et al. reported a UTS of 651 MPa.11
Summary and Conclusion
This study demonstrated the in situ formation of nano-scale oxides in the LPBF melt pool from precursor 14YWT GARS powders. TEM analysis and HE-XRD of the GARS precursor powders showed that Y was present in the powder in the form of Y2Fe17 intermetallic. During LPBF, the Cr-rich oxide layer on the surface of the powder served as a chemical reservoir, providing a controlled stoichiometry of oxygen for the reaction of Y and Ti. The parameter map produced in this study identified a stable process space (95 W, 608 mm/s, 0.050 mm hatch, and 0.025 layer displacement) that resulted in a dense microstructure (~ 99.8% optical relative density) that was free of large-scale microstructural defects. For this parameter set, high magnification backscatter SEM and STEM/EDS analysis of the LPBF-produced solids confirmed that this reaction resulted in a homogeneous distribution of nano-scale Ti2Y2O7 oxides 17–57 nm. EELS thickness maps were used in combination with the TEM results to estimate the oxide density of 1.7 × 1020/m3. Preliminary mechanical tests were also conducted. The UTS and yield strengths were 474 MPa and 312 MPa, respectively, and the Vickers hardness was 236.6 HV.
The oxide sizes measured in our study were larger than those often reported in more traditional MA ODS alloys, usually ranging from 3 to 25 nm.5,6 However, the benefits of circumventing the MA step warrant further investigation. In contrast to previous AM trials on MA-processed ODS particulate that resulted in agglomeration of pre-existing Y2O3, the GARS powder used in this study was demonstrated to resist nano-oxide agglomeration by separating dispersed oxygen sources (metastable oxides on powder surfaces) from nano-oxide formation sites (dissolved intermetallic fragments). The mechanisms leading to the observed oxide size and density are not fully understood, and further research will be required to elucidate the influence of LPBF process parameters, powder size, and GARS composition on the size and distribution of oxides.
References
S.J. Zinkle, J.L. Boutard, D.T. Hoelzer, A. Kimura, R. Lindau, G.R. Odette, M. Rieth, L. Tan, and H. Tanigawa, Nucl. Fusion 57, 092005. (2017).
S. Ukai, in Comprehensive Nuclear Materials. ed. by R.J.M. Konings (Elsevier, Oxford, 2012), pp. 241–271.
G.R. Odette, M.J. Alinger, and B.D. Wirth, Annu. Rev. Mater. Res. 38, 471. (2008).
P.D. Edmondson, C.M. Parish, Y. Zhang, A. Hallén, and M.K. Miller, J. Nucl. Mater. 434, 210. (2013).
G.R. Odette, P. Miao, D.J. Edwards, T. Yamamoto, R.J. Kurtz, and H. Tanigawa, J. Nucl. Mater. 417, 1001. (2011).
G.R. Odette, N.J. Cunningham, T. Stan, and M.E. Alam, and Y. De Carlan, in Structural Alloys for Nuclear Energy Applications. ed. by G.R. Odette, and S.J. Zinkle (Elsevier, Boston, 2019), pp. 529–583.
R. Lindau, M. Klimenkov, U. Jäntsch, A. Möslang, and L. Commin, J. Nucl. Mater. 416, 22. (2011).
P. Miao, G.R. Odette, J. Gould, J. Bernath, R. Miller, M. Alinger, and C. Zanis, J. Nucl. Mater. 367–370, 1197. (2007).
G.R. Odette, Scr. Mater. 143, 142. (2018).
J.C. Walker, K.M. Berggreen, A.R. Jones, and C.J. Sutcliffe, Adv. Eng. Mater. 11, 541. (2009).
R.M. Hunt, K.J. Kramer, and B. El-Dasher, J. Nucl. Mater. 464, 80. (2015).
C. Doñate-Buendía, F. Frömel, M.B. Wilms, et al., Mater. Des. 154, 360. (2018).
E. Vasquez, P.-F. Giroux, F. Lomello, M. Nussbaum, H. Maskrot, F. Schuster, and P. Castany, Powder Technol. 360, 998. (2020).
Y. Shi, Z. Lu, H. Xu, R. Xie, Y. Ren, and G. Yang, J. Alloys Compd. 791, 121. (2019).
Y. Shi, Z. Lu, L. Yu, R. Xie, Y. Ren, and G. Yang, Mater. Sci. Eng. A 774, 138937. (2020).
Y. Zhong, L. Liu, J. Zou, X. Li, D. Cui, and Z. Shen, J. Mater. Sci. Technol. 42, 97. (2020).
M. Ghayoor, K. Lee, Y. He, C. Chang, B.K. Paul, and S. Pasebani, Microsc. Microanal. 25, 2594. (2019).
M. Ghayoor, K. Lee, Y. He, C. Chang, B.K. Paul, and S. Pasebani, Mater. Sci. Eng. A 788, 139532. (2020).
M. Ghayoor, S. Mirzababaei, A. Sittiho, I. Charit, B.K. Paul, and S. Pasebani, J. Mater. Sci. Technol. 83, 208. (2021).
E. Vasquez, P.-F. Giroux, F. Lomello, A. Chniouel, H. Maskrot, F. Schuster, and P. Castany, J. Mater. Process. Technol. 267, 403. (2019).
H.J. Chang, H.Y. Cho, and J.H. Kim, J. Alloys Compd. 653, 528. (2015).
T. Boegelein, S.N. Dryepondt, A. Pandey, K. Dawson, and G.J. Tatlock, Acta Mater. 87, 201. (2015).
W. Zhai, W. Zhou, S.M.L. Nai, and J. Wei, J. Mater. Sci. Technol. 47, 162. (2020).
M.B. Wilms, R. Streubel, F. Frömel, A. Weisheit, J. Tenkamp, F. Walther, S. Barcikowski, J.H. Schleifenbaum, and B. Gökce, Procedia CIRP 74, 196. (2018).
R. Streubel, M.B. Wilms, C. Doñate-Buendía, A. Weisheit, S. Barcikowski, J.H. Schleifenbaum, and B. Gökce, Jpn. J. Appl. Phys. 57, 040310. (2018).
C. Doñate-Buendia, R. Streubel, P. Kürnsteiner, M.B. Wilms, F. Stern, J. Tenkamp, E. Bruder, S. Barcikowski, B. Gault, K. Durst, J.H. Schleifenbaum, F. Walther, and B. Gökce, Procedia CIRP 94, 41. (2020).
B.K. Paul, K. Lee, Y. He, M. Ghayoor, C. Chang, and S. Pasebani, CIRP Ann. 69, 193. (2020).
C. Doñate-Buendia, P. Kürnsteiner, F. Stern, M.B. Wilms, R. Streubel, I.M. Kusoglu, J. Tenkamp, E. Bruder, N. Pirch, S. Barcikowski, K. Durst, J.H. Schleifenbaum, F. Walther, B. Gault, and B. Gökce, Acta Mater. 206, 116566. (2021).
F. Yan, W. Xiong, E. Faierson, and G.B. Olson, Scr. Mater. 155, 104. (2018).
B. Morrow, T. Lienert, C. Knapp, J. Sutton, M. Brand, R. Pacheco, V. Livescu, J. Carpenter, and G. Gray, Metall. Mater. Trans. A 49, 3637. (2018).
K. Saeidi, L. Kvetková, F. Lofaj, and Z. Shen, RSC Adv. 5, 20747. (2015).
D.-R. Eo, S.-H. Park, and J.-W. Cho, Mater. Des. 155, 212. (2018).
X. Zhang, H. Cao, X. Yang, Y. Zhao, H. Wang, X. Mao, and Y. Zhai, Fusion Eng. Des. 164, 112213. (2021).
M. Song, X. Lin, F. Liu, H. Yang, and W. Huang, Mater. Des. 90, 459. (2016).
Y. Liu, J. Fang, D. Liu, Z. Lu, F. Liu, S. Chen, and C.T. Liu, J. Nucl. Mater. 396, 86. (2010).
M.P. Haines, N.J. Peter, S.S. Babu, and E.A. Jägle, Addit. Manuf. 33, 101178. (2020).
D. Riabov, M. Rashidi, E. Hryha, and S. Bengtsson, Mater. Charact. 169, 110582. (2020).
T. Horn, C. Rock, D. Kaoumi, I. Anderson, E. White, T. Prost, J. Rieken, S. Saptarshi, R. Schoell, M. DeJong, S. Timmins, R. Napolitano, D. Zhang, and J. Darsell, Laser Powder Bed Fusion Additive Manufacturing of Oxide Dispersion Strengthened Steel Using Gas Atomized Reaction Synthesis Powder (Social Science Research Network, Rochester, NY, 2022).
H. Jia, Z. Zhou, and S. Li, Mater. Charact. 187, 111876. (2022).
J. Rieken, Grad. Theses Diss. (2011).
J.R. Rieken, I.E. Anderson, and M.J. Kramer, Int. J. Powder Metall. 46, 17. (2010).
J.R. Rieken, I.E. Anderson, M.J. Kramer, G.R. Odette, E. Stergar, and E. Haney, J. Nucl. Mater. 428, 65. (2012).
R. Gao, L. Zeng, H. Ding, T. Zhang, X. Wang, and Q. Fang, Mater. Des. 89, 1171. (2016).
J.M. Weaver, T.J. Barton, J. Linn, D. Jenkins, M.P. Miles, and R. Smith, Rapid Prototyp. J. 25, 221. (2018).
A.B. Spierings, M. Schneider, and R. Eggenberger, Rapid Prototyp. J. 17, 380. (2011).
C. Arvieu, C. Galy, E. Le Guen, and E. Lacoste, J. Manuf. Mater. Process. 4, 83. (2020).
R. K. Nanstad, G. R. Odette, N. Almirall, J. Robertson, W. L. Server, T. Yamamoto, and P. Wells, Effects of ATR-2 Irradiation to High Fluence on Nine RPV Surveillance Materials (Oak Ridge National Lab. (ORNL), Oak Ridge, TN (United States), 2017).
S.A. Farzadfar, M.J. Murtagh, and N. Venugopal, Powder Technol. 375, 60. (2020).
M. Sutton, J.-J. Orteu, and H. Schreier, Image Correlation for Shape, Motion and Deformation Measurements. Basic Concepts, Theory and Applications (Springer, Berlin, 2009).
N.J. Karanjgaokar, C.-S. Oh, and I. Chasiotis, Exp. Mech. 51, 609. (2011).
J. Agirre, L. Galdos, E. Saenz de Argandoña, and J. Mendiguren, Mech. Mater. 124, 71. (2018).
Z. Snow, R. Martukanitz, and S. Joshi, Addit. Manuf. 28, 78. (2019).
J.A. Slotwinski, and E.J. Garboczi, JOM 67, 538. (2015).
A. Mussatto, R. Groarke, A. O’Neill, M.A. Obeidi, Y. Delaure, and D. Brabazon, Addit. Manuf. 38, 101807. (2021).
S.E. Brika, M. Letenneur, C.A. Dion, and V. Brailovski, Addit. Manuf. 31, 100929. (2020).
H. Kishimoto, M.J. Alinger, G.R. Odette, and T. Yamamoto, J. Nucl. Mater. 329–333, 369. (2004).
L. Haferkamp, L. Haudenschild, A. Spierings, K. Wegener, K. Riener, S. Ziegelmeier, and G. Leichtfried, Metals 11, 418. (2021).
T. Malis, S.C. Cheng, and R.F. Egerton, J. Electron Microsc. Tech. 8, 193. (1988).
G.R. Odette, and D.T. Hoelzer, JOM 62, 84. (2010).
R.L. Klueh, P.J. Maziasz, I.S. Kim, L. Heatherly, D.T. Hoelzer, N. Hashimoto, E.A. Kenik, and K. Miyahara, J. Nucl. Mater. 307–311, 773. (2002).
L.L. Hsiung, S.J. Tumey, D.T. Hoelzer, and M.J. Fluss, MRS Adv. 3, 1761. (2018).
D. Zhang, J.T. Darsell, J. Wang, X. Ma, G.J. Grant, I.E. Anderson, J.R. Rieken, D.J. Edwards, W. Setyawan, T.J. Horn, and G.R. Odette, J. Nucl. Mater. 566, 153768. (2022).
E. Gil, N. Ordás, C. García-Rosales, and I. Iturriza, Fusion Eng. Des. 98–99, 1973. (2015).
E. Gil, J. Cortés, I. Iturriza, and N. Ordás, Appl. Surf. Sci. 427, 182. (2018).
E. Gil, N. Ordás, C. García-Rosales, and I. Iturriza, Powder Metall. 59, 359. (2016).
C.L.A. Leung, S. Marussi, M. Towrie, R.C. Atwood, P.J. Withers, and P.D. Lee, Acta Mater. 166, 294. (2019).
L. He, H. Zhao, and W. Niu, J. Laser Appl. 30, 012003. (2018).
S.S. Babu, S.A. David, J.M. Vitek, K. Mundra, and T. DebRoy, Mater. Sci. Technol. 11, 186. (1995).
S. Mirzababaei, M. Ghayoor, R.P. Doyle, and S. Pasebani, Mater. Lett. 284, 129046. (2021).
M.E. Alam, S. Pal, K. Fields, S.A. Maloy, D.T. Hoelzer, and G.R. Odette, Mater. Sci. Eng. A 675, 437. (2016).
J.H. Kim, T.S. Byun, and D.T. Hoelzer, J. Nucl. Mater. 407, 143. (2010).
D.A. McClintock, D.T. Hoelzer, M.A. Sokolov, and R.K. Nanstad, J. Nucl. Mater. 386–388, 307. (2009).
Acknowledgements
This work was carried out under DE- AR0001379. Portions of this work were performed at the Analytical Instrumentation Facility (AIF) at North Carolina State University, which is supported by the State of North Carolina and the National Science Foundation (award number ECCS-2025064). The AIF is a member of the North Carolina Research Triangle Nanotechnology Network (RTNN), a site in the National Nanotechnology Coordinated Infrastructure (NNCI). Portions of this work were carried out with support from the Department of Energy, Office of Fossil Energy (ARM program) through Ames Laboratory contract no. DE-AC02-07CH11358, and this support is gratefully acknowledged. Use of the Advanced Photon Source at Argonne National Laboratory was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357. The authors would like to thank Angelica Lopez Morales for their assistance in carrying out TEM data acquisition.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saptarshi, S., deJong, M., Rock, C. et al. Laser Powder Bed Fusion of ODS 14YWT from Gas Atomization Reaction Synthesis Precursor Powders. JOM 74, 3303–3315 (2022). https://doi.org/10.1007/s11837-022-05418-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-022-05418-6