Abstract
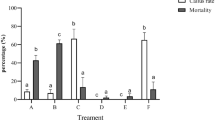
This study aimed to investigate the callus induction and shoot regeneration to produce rootstock from stems and leaves explants of the Ohadi and UCB-1 pistachio cultivars. Plant growth regulators (PGRs), including combinations of BAP, Kin, IAA, NAA, and 2, 4-D, were added to Murashige and Skoog (MS) or Driver and Kuniyuki Walnut (DKW) media to induce callus induction and regeneration. Obtained calli were transferred to the DKW medium containing three different concentrations, 2 and 4 mg L−1 of BAP and 1 mg L−1 + 2 mg L−1 of BAP + NAA, to compare the frequency of shoot regeneration. The highest callus induction rate (96%) was observed in the DKW medium supplemented with 2 mg L−1 Kin + 0.5 mg L−1 NAA in the Ohadi stems. The highest callus induction frequency for leaves was obtained in the DKW medium supplemented with 2 mg L−1 BAP + 0.1 mg L−1 2, 4-D concentrations for both UCB-1 (73%) and Ohadi cultivar (71%). The highest shoot regeneration rate in Ohadi stems (41%) and leaves (20%) was observed in the medium supplemented with 2 mg L−1 NAA + 1 mg L−1 BAP. It resulted in significant differences (P ≤ 0.05) in callus induction and shoot regeneration between stems and leaves; stems generated more calli and shoots than leaves, and Ohadi significantly (P ≤ 0.05) inducted calli and generated shoots more than UCB-1. Altogether, these regeneration protocols, achieved from stems and leaves of the Ohadi and UCB-1 cultivars, may be helpful in future pistachio genetic transformation.






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Aghaei P, Bahramnejad B, Mozafari A (2013) Effect of different plant growth regulators on callus induction of stem explants in’Pistacia atlantica’subsp. kurdica. Plant Knowl J 2(3):108–112
Ahmed MGU, Shin SL, Lee CH (2011) In vitro culture responses of Cratoneuron decipiens (Brid.) G. roth gametophyte for micropropagation. Hortic Environ Biotechnol 52(6):614–620
Arias E, González J, Oria R, Lopez-Buesa P (2007) Ascorbic acid and 4-hexylresorcinol effects on pear PPO and PPO catalyzed browning reaction. J Food Sci 72(8):C422–C429
Barghchi M, Alderson P (1983) In vitro propagation of Pistacia vera l. from seedling tissues. J Hortic Sci 58(3):435–445
Barghchi M, Alderson P (1985) In vitro propagation of Pistacia vera l. and the commercial cultivars ohadi and kalleghochi. J Hortic Sci 60(3):423–430
Barghchi M, Alderson P (1982) In vitro propagation of Pistacia species. Acta Hortic 131:49–60
Barghchi M, A Martinelli (1984) In vitro propagation of mature Pistacia vera varieties of Kerman (female) and Peter’s (male) pistachio. Proceedings of the 41st easter school symposium on plant tissue culture and its agricultural applications, University of Nottingham, Abstract.
Barghchi M (1986) In vitro micropropagation of Pistacia rootstocks. Proc Int Plant Prop Soc.
Benmahioul B, Kaid-Harche M, Daguin F (2016) In vitro regeneration of Pistacia vera L. from nodal explants. J for Sci 62(5):198–203
Bhagwat B, Lane WD (2004) In vitro shoot regeneration from leaves of sweet cherry (Prunus avium)’Lapins’ and’Sweetheart’. Plant Cell, Tissue Organ Cult 78(2):173–181
Caglar S, Kaska N (1994) A study on the supplemental pollination of pistachios in the mediterranean region. I Inter Symp Pistachio. 419:55–60
D’Onofrio C, Morini S (2006) Somatic embryo, adventitious root and shoot regeneration in in vitro grown quince leaves as influenced by treatments of different length with growth regulators. Sci Hortic 107(2):194–199
Dolcet-Sanjuan R, Claveria E (1995) Improved shoot-tip micropropagation of Pistacia vera L. and the beneficial effects of methyl jasmonate. J Am Soc Hortic Sci 120(6):938–942
Driver JA, Kuniyuki AH (1984) In vitro propagation of paradox walnut rootstock. HortScience 19(4):507–509
Gaba VP (2005) Plant growth regulators in plant tissue culture and development. Plant development and biotechnology. CRC Press, Boca Raton, pp 87–99
Gharyal PK, Maheshwari SC (1990) Differentiation in explants from mature leguminous trees. Plant Cell Rep 8(9):550–553
Giri C, Shyamkumar B, Anjaneyulu C (2004) Progress in tissue culture, genetic transformation and applications of biotechnology to trees: an overview. Trees 18(2):115–135
Gonzalez A, Frutos D (1990) In vitro culture of Pistacia vera L. embryos and aged trees explants. Plant Aging 186:335–338
Huetteman CA, Preece JE (1993) Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell, Tissue Organ Cult 33(2):105–119
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15(3):473–497
Nezami SR, Yadollahi A, Hokmabadi H, Eftekhari M (2015) Control of shoot tip necrosis and plant death during in vitro multiplication of pistachio rootstock UCB1 (Pistacia integrima× P. atlantica). J Nuts 6(01):27–35
Nicholson RL, Hammerschmidt R (1992) Phenolic compounds and their role in disease resistance. Annu Rev Phytopathol 30(1):369–389
Onay A (2000a) Micropropagation of pistachio from mature trees. Plant Cell, Tissue Organ Cult 60(2):159–163
Onay A (2000b) Somatic embryogenesis from mature seed cultures of Pistacia atlantica. Turk J Agric for 24(4):465–474
Onay A, Jeffree C, Yeoman M (1995) Somatic embryogenesis in cultured immature kernels of Pistachio, Pistacia vera L. Plant Cell Rep 15(3):192–195
Onay A, Jeffree C, Yeoman M (1996) Plant regeneration from encapsulated embryoids and an embryogenic mass of pistachio, Pistacia vera L. Plant Cell Rep 15(9):723–726
Onay A, Jeffree C, Theobald C, Yeoman M (2000) Analysis of the effects of maturation treatments on the probabilities of somatic embryo germination and plantlet regeneration in pistachio using a linear logistic method. Plant Cell, Tissue Organ Cult, Dordrecht 60(2):121–129
Onay A, Pirinç V, Tilkat E, Aktürk Z, Yildirim H (2004) Somatic embryogenesis of pistachio from female flowers. J Hortic Sci Biotechnol 79(6):960–964
Onay A, Jeffree C (2000) Somatic embryogenesis in pistachio (Pistacia Vera L). Somatic embryogenesis in woody plants. Springer, Dordrecht, pp 361–390
Parfitt D, Almehdi A (1994) Use of high CO2 atmosphere and medium modifications for the successful micropropagation of pistachio. Sci Hortic 56(4):321–329
Ramming DW (1985) In ovulo embryo culture of early-maturing Prunus. HortScience (USA) 20:419–420
Stamler RA, Kilcrease J, Kallsen C, Fichtner EJ, Cooke P, Heerema RJ, Randall JJ (2015) First report of rhodococcus isolates causing pistachio bushy top syndrome on ‘UCB-1’rootstock in california and arizona. Plant Dis 99(11):1468–1476
Tang W, Newton RJ (2004) Increase of polyphenol oxidase and decrease of polyamines correlate with tissue browning in Virginia pine (Pinus virginiana Mill.). Plant Sci 167(3):621–628
Tang H, Ren Z, Reustle G, Krczal G (2002) Plant regeneration from leaves of sweet and sour cherry cultivars. Sci Hortic 93(3–4):235–244
Tilkat E, Onay A (2009) Direct shoot organogenesis from in vitro-derived mature leaf explants of pistachio. In Vitro Cell Develop Biol-Plant 45(1):92–98
Tilkat E, Isikalan C, Onay A (2005) In vitro propagation of khinjuk pistachio (Pistacia khinjuk Stocks) through seedling apical shoot tip culture. Propag Ornam Plant 5(3):124–128
Tilkat E, Onay A, Yıldırım H, Ayaz E (2009) Direct plant regeneration from mature leaf explants of pistachio, Pistacia vera L. Sci Hortic 121(3):361–365
Turakhia D, Kulkarni A (1988) In vitro regeneration from leaf explants of Ladebouria Hyacinthiana, Roth. (Scilla Indica, Bak.). Curr Sci 57(4):214–216
Walden R, Wingender R (1995) Gene-transfer and plant-regeneration (techniques). Trends Biotechnol 13(9):324–331
Yang Z, Lüdders P (1994) In vitro propagation of Pistachio (Pistacia vera L.). Gartenbauwissenschaft 59(1):30–34
Yıldırım H (2012) Micropropagation of Pistacia lentiscus L. from axenic seedling-derived explants. Sci Hortic 137:29–35
Acknowledgements
The current study was extracted from a thesis that earned a Master of Science degree (Project Number: 53169). The authors appreciate the assistance of the vice chancellor for research at the Ferdowsi University of Mashhad. The authors also thank Seyed MohammadReza Aboutorabzadeh for improving the manuscript’s English.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nadalizadeh Ghannad, A., Marashi, H., Seifi, A. et al. Optimization of callus induction and shoot regeneration in leaf and stem of Pistacia vera L. and UCB-1 (P. atlantica × P. integerrima). Plant Biotechnol Rep 17, 605–613 (2023). https://doi.org/10.1007/s11816-022-00798-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-022-00798-2