Abstract
Bupleurum chinense DC is an important medicinal plant with many active ingredients that are used for the treatment of different types of diseases and valued in pharmaceutical markets. In vitro shoot regeneration can efficiently contribute to the improvement of B. chinense. In the present study, we investigated the effects of the explant type and plant growth regulators (PGRs) on embryogenic callus induction and plant regeneration in B. chinense. Our investigation demonstrated that 2 mg/L 2,4-dichlorophenoxyacetic acid (2,4-D) combined with 1 mg/L thidiazuron (TDZ) played a major role in promoting callus induction from leaf, hypocotyl and stem 2 explants, whereas the most effective treatment for stem 1 callus formation was Murashige and Skoog (MS) medium supplemented with 1 mg/L 2,4-D, 0.5 mg/L 6-benzyladenine (BA) and 0.5 mg/L kinetin (Kin). The highest shoot regeneration rate (57.14%) was obtained from hypocotyl-induced calli in MS medium with 0.5 mg/L Kin after 12 weeks of cultivation. This regeneration protocol can be used in large-scale cultivation and may be useful for future genetic modifications of B. chinense.
Similar content being viewed by others
Introduction
Bupleurum is a genus of the family Umbelliferae (Apiaceae), represented by 200 species, which are widely distributed in the Northern Hemisphere, Eurasia and North Africa. This genus was first recorded under the name Chai Hu (saiko in Japanese and shiho in Korean) more than 2000 years ago in Shen-Nong’s Herbal (Shen Nong Ben Cao Jing), and the earliest professional pharmaceutical book described the properties, flavor, efficacy and clinical applications of Bupleurum in detail (Yang et al. 2017). In addition, the latest research shows that a lung cleansing and detoxifying decoction (Qing Fei Pai Du Tang), composed of 21 kinds of traditional Chinese medicines, such as Bupleuri Radix, Ephedra sinica and Pogostemon cablin, exerts obvious curative effects on mild COVID-19 (Cao et al. 2020; Zhong et al. 2020).
As early as the beginning of 2000, the annual consumption of Radix Bupleuri in China reached 8000 tons, and the gross proceeds of prescription medicines containing saiko reached 27 billion yen in Japan. The demand for Chinese herbal medicine resources is still increasing worldwide (Cao et al. 2021; Pan 2006). Although 20 species of Bupleurum can be made into Chinese herbal medicines, only the dry roots of B. chinense and B. scorzonerifolium are the official botanical origins of Bupleuri Radix specified in the Chinese Pharmacopoeia (Chinese Pharmacopoeia Commission 2020). Furthermore, due to years of excessive and indiscriminate digging and inappropriate use to meet the raw material demands for Bupleuri Radix, natural populations of these plants have declined sharply. Sichuan Province, China, has a long history of cultivating Bupleurum. Currently, the cultivation area of Bupleurum is approximately 47,500 acres (data unpublished), but excellent cultivars for industrialized planting are still lacking. A commercial variety of B. chinense “Zhong Chai No. 2” with high yield and good quality was introduced to Sichuan. However, this genotype is intolerant to the soil waterlogging resulting from the very rainy weather during summer in Southwest China. Although the improved variety Chuanbeichai No. 1 showed moderate waterlogging tolerance after years of rigorous selection from the population originating from Zhong Chai No. 2, its flowering and seed setting are still negatively affected by the summer rainy season, resulting in poor seed production, which has greatly hindered its popularization (Gao et al. 2020; Xu et al. 2018). Therefore, with the typical introduction and domestication processes of traditional breeding, combined with modern biotechnology to improve the existing medicinal Bupleurum varieties, enhancing the stress resistance and environmental adaptability of these plants for their further development is a future breeding goal. A stable and efficient plant regeneration system is not only useful in the micropropagation and conservation of endangered Bupleurum varieties but also opens up alternative ways of introducing novel traits via genetic transformation.
There are several previous reports of in vitro studies on B. chinense (Hao et al. 2008; Xu et al. 2013; Yang et al. 2011). However, in previous reports on the induction of calli of Chuanbeichai No. 1, the induction rate was very low, and the callus browned easily. Furthermore, the regeneration efficiency of calli largely depends on genotype and explant type. Thus, a protocol that is suitable for one cultivar may not be applicable for other cultivars. Hence, there is a need to develop an in vitro tissue regeneration system for “Chuanbeichai No. 1” to meet the demands for planting material for cultivation and to improve this species through trait-specific genetic manipulation. Therefore, we focused on developing a more efficient regeneration protocol for mass multiplication and commercial cultivation of B. chinense.
Materials and methods
Plant material
The seeds of “Chuanbeichai No. 1” used in this study were chosen from a new variety of Bupleurum chinense. DC, which was jointly bred by Sichuan Agricultural University, Southwest University of Science and Technology, Institute of Medicinal Plant Development & Chinese Academy of Medical Sciences and Sichuan De Pei Yuan Traditional Chinese Medicine Science & Technology Development Co., Ltd. from farm-holding populations through systemic and purification selection methods.
In early 2019, stem segments were collected from B. chinense “Chuan Bei Chai No 1” plants at the jointing stage grown in the Chai Hu Demonstration Base of College of Life Science and Engineering, Southwest University of Science and Technology.
Explant sterilization
Seed sterilization and germination
The seeds were maintained in water (at 24 ± 1 °C) that was changed every 4 h for 24 h. Then, seeds were rinsed with flowing water for 2 h, placed in 75% ethanol for 30 s and washed with sterilized water three times, followed by 10% (w/v) NaClO for 15 min and 0.1% HgCl2 for 5 min. After the surface sterilization process, the seeds were washed with sterile distilled water to remove any trace of the sterilization agents. The seeds were then planted on MS (Murashige and Skoog 2006) seed germination medium supplemented with 3.0% (w/v) sucrose, 0.4% (w/v) plant gel, 1.5 mg/L 6-benzylaminopurine (BA) and 1.0 mg/L indole-3-acetic acid (IAA) (pH 5.8). All culture plates were kept in darkness at 24 ± 1 °C in an incubator for 14 days. When the seeds germinated, the culture conditions were changed to a 16-h light/8-h dark cycle at 23 ± 1 °C, and the light intensity was 2000 lx (25 μmolm−2 s−1). From 25 to 60 days, we collected hypocotyls and leaves and used them as explants.
Stem sterilization
After removing leaves, the fresh stems were rinsed with flowing water for 2 h, and then the stems were placed in 75% ethanol for 30 s and washed with distilled water. After that, the stems were sterilized with 0.1% (w/v) HgCl2 for 7 min and washed 5 ~ 6 times with distilled water. Surface-sterilized stems were cut into 3–5 cm segments, inoculated on MS medium and maintained at 24 ± 1 °C in the dark. The basic medium used in this study was MS medium containing 3.0% (w/v) sucrose and 0.4% (w/v) plant gel (pH 5.8).
Embryogenic callus induction
Leaf callus induction
Fully expanded green leaves were excised from the in vitro shoots (30 days old) and scratched with a surgical blade perpendicular to the vein at an interval of 0.5 cm. The treated leaves were soaked in 0.6% (w/v) polyvinylpyrrolidone for 10 min to remove the phenolic substances secreted from the wound and reduce browning and blotted with sterilized filter paper. Each treatment had 30 explants and was repeated three times. The cultures were kept in the dark for two weeks and then transferred to the light conditions described above. The callus induction rate and mortality were determined after four weeks.
Previous studies showed that the induction of Bupleurum callus is mainly accomplished with plant growth regulators (PGRs), such as 2,4-dichlorophenoxyacetic acid (2,4-D), BA, thidiazuron (TDZ), α-naphthaleneacetic acid (NAA) and kinetin (Kin) (Hao et al. 2008; Park et al. 1994; Yang et al. 2011; Zhao and Xi 1993). Therefore, according to tissue culture studies of Bupleurum plants and preliminary experiments, different hormone combinations were designed with leaves as explants, and induction formulas for embryogenic calli of B. chinense were preliminarily screened. The callus induction MS medium included 6 different concentrations and combinations of PGRs (Table 1).
Hypocotyl and stem segment callus induction
Hypocotyls were obtained from 15-day-old seedlings in vitro under sterile conditions; the cotyledons and roots were cut off, and 3–5 mm hypocotyls were reserved and soaked in 0.6% (w/v) polyvinylpyrrolidone for 10 min. The stems, which were cut into 1–2-cm segments, were sterilized and pre-cultured for seven days, and those without browning or contamination were soaked in 0.6% (w/v) polyvinylpyrrolidone for 10 min. The stems were then divided into two parts based on their maturity: the slightly younger part was Stem 1, and the slightly lignified part was Stem 2. All the explants were transferred to three different types of MS media, including different types and combinations of PGRs (1 mg/L2,4-D + 0.5 mg/L BA + 0.5 mg/L Kin, 2 mg/L 2,4-D + 1 mg/L TDZ or 0.5 mg/L BA + IAA 0.5 mg/L). The explants were subjected to darkness for 14 days of callus incubation at 24 ± 1 °C and then cultured under a 16 h photoperiod and 2000 lx light intensity. The induction rate and callus growth status were recorded 4 weeks after the inoculation of explants.
Shoot induction
Stem explants were taken from B. chinense planted in the field at the jointing stage. The acquisition of materials was limited by the growth cycle of the plant. Therefore, we only selected the hypocotyl calli and leaf calli of sterile seedlings as differentiation materials. Calli cultured for more than eight weeks were transferred to 9 different MS media with 3 different PGRs, zeatin (ZEA), Kin and abscisic acid (ABA), and each had three different concentrations (0.5, 1.0, and 1.5 mg/L ZEA; 0.5, 1.0, and 1.5 mg/L Kin; and 0.5, 1.0, and 1.5 mg/L ABA). All flasks were cultured at 24 ± 1 °C under a 16 h photoperiod and 2000 lx light intensity. The regeneration efficiency was calculated by counting the shoots or roots that formed from at least one out of the total number of shoots and roots cultivated on MS media after 4 weeks.
Rooting and plantlet acclimatization
In reference to our previous study (Li et al. 2018), the in vitro regenerated shoots of B. chinense (3 – 4 cm) were transferred to MS medium supplemented with indole-3-butyric acid (IBA) 0.5 mg/L for rooting. All the shoots for rooting were held at 22 ± 1 °C with a 12 h photoperiod and 1800 lx light intensity.
After 30 days of culture, the bottles with tube-rooted plantlets were opened under natural light for 5 ~ 7 days, the root medium was washed off, and the plants were planted into seedling substrate. The bottles were prepared by mixing peat soil and sandy loam soil at a ratio of 2:1.
Statistical analysis
The effects of different media on callus induction rate of different explants were modeled via the PROC MIXED procedure in SASv.9.2. (SAS Institute Inc., Cary, NC), similar to an analysis of variance for repeated measures, referring to the studies by Wiklund et al. (2012) and Ranganathan et al. (2014). The callus differentiation data are expressed as the means ± standard errors of the mean (SE) and were subjected to analysis of variance (ANOVA), and significant differences were selected by Duncan’s multiple range test with SPSS v.23 (SPSS Inc., Chicago, IL, USA) (Denis 2018). The histograms were plotted with GraphPad Prism v.8.0 (GraphPad Software Inc., La Jolla, CA, USA) (Motulsky 2007).
Results
Callus induction
Initially, we drew lessons from previous reports on Bupleurum plants, and six induction media were used for callus induction of B. chinense leaves for the first round of screening. Since whole leaves were the easiest and most abundant to obtain, they were cultured on the six media to test the induction rate. In contrast to other media, media C (MS + 2 mg/L 2,4-D + 1 mg/L TDZ) and F (MS + 0.5 mg/L IAA + 0.5 mg/L BA) showed good callus induction and a low death rate (P < 0.05), with callus induction rates of 66.43% and 65.30%, respectively (Fig. 1). It was observed that the callus in medium F grew only at the wound and was small and easily browned, while medium C could induce the whole leaf into callus with a compact texture and strong proliferation ability. A previous study showed that medium A can result in a high induction rate of calli of different explants, particularly floral buds (Hao et al. 2008), but in this study, the callus ratio from young leaves was not high (only 8.63%). This different result may be caused by the different genotypes. Therefore, other explants, such as stems and hypocotyls, should be used for further screening.
The mortality results showed a significant difference (P < 0.05) between the six media. The mortality of medium B was the highest (61.29%), whereas that of medium F and C was lower than 14%. Although the mortality under treatments D and E was significantly lower than that under the other treatments, they did not induce callus formation.
According to the results of the first round of tests, we chose the three media (C, F, A) with the highest leaf callus induction abilities to carry out the second-round screening on different organs of B. chinense; the explants were from hypocotyls, leaves and stems. As shown in Fig. 2, there were significant differences in the callus rate among different explants in the same medium (P < 0.05), and the percentage of explants producing calli was in the range of 8.64 – 83.95%. To further compare the difference in callus formation among different explants in different media, the mixed model was employed for the following analysis. The fixed effect test showed that a single factor of explant type or medium had no significant effect on the induction of callus, but the interaction between explant and medium was statistically significant (Table 2, P < 0.05). To compare the differences among the 12 interactions (Explant * Medium), we performed a t test using the least square mean of interactions to check whether the difference was significant (Table 3). A total of 66 groups of interactions were compared in pairs, and 17 of these groups exhibited significant differences (P < 0.05). From the comparisons, hypocotyls were found to be the most suitable explants for callus induction, and the highest induction rate (83.97%) was achieved in C medium supplemented with TDZ (1 mg/L) and 2,4-D (2 mg/L).
Shoot induction
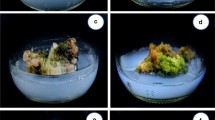
The calli were inoculated with individual cytokinin (ZEA, Kin) and ABA in MS media and tested for shoot regeneration. After 10 days of cultivation, the hypocotyl calli were first to initiate differentiation to produce cluster shoots and roots. Roots were formed in leaf calli after approximately 15 days, but after 4 weeks, they still failed to produce regenerated shoots. Therefore, we extended the induction time and recorded data every 4 weeks (Table 4). When leaf calli were cultured on MS media supplemented with different concentrations of PGRs, new purple or yellow soft and fragile calli were formed on the surface of compact green calli, white growth spots appeared in the cells of these purple calli, and they gradually developed into adventitious roots. In the three investigation stages, the highest root differentiation rate of leaf calli was in MS medium supplemented with 0.5 mg/L Kin (Table 5). While the hypocotyl callus was softer than the leaf callus, white globular embryogenic calli and dense green globular calli were incubated on protocalli during induced differentiation. The white spheroids can be transformed into somatic embryo structures and produced a green separate bud from each structure loosely attached to the parental tissue (Fig. 3a). These independent buds further developed radicles and hypocotyls in the basal callus and became complete plants (Fig. 3b, c). After another 20 days of culture, the plant height of the regenerated seedlings reached approximately 8 cm (Fig. 3d). The dense green granular callus gradually transformed into spherical embryos, heart-shaped embryos and torpedo embryos, differentiated into leaf primordia and finally developed into a new shoot (Fig. 4a-d). Furthermore, these regenerated shoots were densely distributed on the surface of the callus, and this effect was accompanied by the emergence of adventitious roots, as shown in Fig. 4e. After eight weeks of culture, the callus still maintained high differentiation ability and became a chimera containing dense cluster buds and adventitious roots (Fig. 4f). To avoid affecting the next cycle of data investigation and the further differentiation of calli, we did not destructively count the number of adventitious buds per callus. Instead, the hypocotyl calli that produced new shoots were graded and counted (Tables 6, 7, 8). We divided the differentiated calli into three grades: calli with fewer than 5 regenerated buds were defined as level-1 differentiated calli (Fig. 5a), calli with 5–10 regenerated buds were defined as level-2 differentiated calli (Fig. 5b), and calli with more than 10 regenerated buds were defined as level-3 differentiated calli (Fig. 5c). Among the PGRs, Kin more efficiently promoted hypocotyl callus differentiation. ANOVA revealed that different kinds and contents of PGRs in the medium had significant effects (P < 0.05) on the induction of regeneration shoots from calli of B. chinense. (Table 4) at the 4th and 12th week. At the 4th and 8th week, the hypocotyl callus was best induced in MS medium containing 1 mg/L Kin, and the adventitious shoot regeneration rates were 28.57% and 35.71%, respectively. After 12 weeks of culture, the highest regeneration rate was observed in hypocotyl calli cultivated on medium supplemented with 0.5 mg L−1 Kin, and the adventitious bud and root regeneration rates were 57.12% and 97.92%, respectively.
The differentiation process of white globular embryogenic callus obtained from hypocotyl explant. a Shoot initiation from globular embryogenic callus (Scale bar indicates 2 mm). b Hypocotyl and radicle produced from globular embryogenic callus (Scale bar indicates 2 mm). c Embryoids develop into complete plants (Scale bar indicates 2 mm). d Plant produced by embryoids elongate further (Scale bar indicates 1 cm)
The differentiation process of green embryogenic calli obtained from hypocotyl explant. a-d Embryogenic callus at globular, heart-shaped, torpedo, and cotyledonary stages (Scale bar indicates 500 μm). e General view of green embryogenic callus obtained on differentiation medium (Scale bar indicates 1 mm). f Shoots induced from hypocotyl callus 8 weeks after initiating differentiation, the shoots and root elongated further (Scale bar indicates 1 cm)
Discussion
Callus induction
Adjusting PGRs in culture media is one of the most common approaches used in developing regeneration protocols, such as somatic embryogenesis and shoot proliferation. Previous studies mostly explored the synergistic effect of 2,4-D with auxins or cytokinins on the induction of calli. These studies demonstrated that 2,4-D in combination with other PGRs could modify the frequency of embryogenic callus induction and growth. This kind of PGR combination has also been widely used in regeneration systems of some other Bupleurum plants, such as Bupleurum scorzonerifolium and Bupleurum latissimum (Bae 2015; Cheng et al. 2015; Xia et al. 1992). Therefore, according to previous reports, we designed 5 PGR combinations containing 2,4-D to induce calli of B. chinense. In addition, we also found that the adventitious bud proliferation medium (MS + IAA 0.5 mg/L + BA 0.5 mg/L) could induce calli in the roots of tube plantlets; hence, we also tried to use other explants to induce calli on this medium.
In the present study, different explants showed different states in the process of callus formation. After 14 days of dark culture, the whole hypocotyl dedifferentiated into soft white calli, the stem explants formed green granular calli at both ends of the wound, while the dedifferentiation of whole leaves into tender green calli took 20 days. Initial calli grew slowly from all explants. However, once stable callus growth had been established, the growth rate increased. The results showed that the calli of B. chinense were induced successfully from aseptic leaf, hypocotyl and stem explants. The best PGR combination for inducing calli varied according to the type of explant, and the hypocotyl had the highest rate of callus induction. The callus induced by different explants varied in texture, color and size.
Shoot induction
During the process of callus differentiation, cytokinins are key regulators used to induce somatic embryogenesis and play very important roles in the success of plant regeneration (Bidabadi and Jain 2020; Jiménez 2005). Among the protocols in which cytokinins were used as the sole PGR for the induction of somatic embryogenesis, Kin and ZEA were the most frequently used cytokinins (Phillips and Garda 2019). In addition, ABA can induce somatic embryogenesis and regulate somatic embryo development in certain cases (Lü et al. 2013; Liu et al. 2021; Nishiwaki et al. 2000; Su and Zhang 2014). Therefore, we utilized ABA, ZEA and Kin to test their effects on the differentiation of leaf and hypocotyl calli of B. chinense. We also set up a blank control group, and some calli could also produce cluster buds, but a large number of calli gradually browned and died after 2 weeks. On the medium containing cytokinin and ABA, the callus showed active proliferation ability, the volume of the callus increased rapidly, and the differentiation rate also increased. This finding indicates that the medium without PGRs is not conducive to the survival and proliferation of B. chinense calli. Among the three PGRs, Kin showed the best differentiation-inducing ability, so the hypocotyl callus could differentiate more shoots and roots, and their elongation rate was faster. However, regardless of the type of PGR, the leaf callus could not produce regenerated shoots, and only roots could be produced. To further dissect this cause, we tried to induce leaf callus differentiation with BA/IAA, three concentration gradients (0.5, 1.0, 1.5 mg/L BA and 0.05, 0.1, 0.15 mg/L IAA) were set up. However, the effect was not satisfactory, and the leaf callus still could not differentiate into shoots. A similar result was reported by Hao et al. (2008), they used high levels of cytokinin/auxin to induce calli differentiation, but most leaf calli died of browning during culture and that a few continued to divide and proliferate but never differentiated. And there is no report of other genus Bupleurum plants successfully utilizing leaf regeneration. These results showed that leaf calli from B. chinense may not have the ability to differentiate regenerated shoots.
The present study is also an important part of our team’s systematic research on B. chinense. By analyzing the transcriptome data of B. chinense, we found some genes that may play an important role in the development of lateral roots and saponin synthesis (Yu et al. 2021; 2020). Transgenic transformation based on regeneration systems is in progress, and manipulation of these genes may improve the yield of saikosaponins.
Conclusion
The hypocotyls of B. chinense were used as explants to establish an indirect somatic embryogenesis system through calli. The hypocotyl callus proliferated and differentiated into shoots. Compared with previous methods, we obtained a higher hypocotyl embryogenic callus induction rate and produced a higher de novo shoot regeneration rate and differentiation rate. Moreover, most of the shoots produced by hypocotyl calli were dense cluster shoots. Thus, our results demonstrate that hypocotyl explants of B. chinense offer great potential for large-scale in vitro multiplication of this popular medicinal plant species. Furthermore, this improved regeneration of B. chinense can be used in genetic transformation studies to improve its qualitative traits.
Data availability
All data are available from the corresponding author on request.
References
Ake W, Malm T, Honkakangas J, Eklund B (2012) Spring development of hydrolittoral rock shore communities on wave-exposed and sheltered sites in the northern Baltic proper. Oceanologia 54:75–107
Bae KH (2015) Improvement of seed germination and in vitro propagation of Bupleurum latissimum Nakai through embryogenesis. Turk J Biol 39:732–739
Bidabadi SS, Jain SM (2020) plants cellular, molecular, and physiological aspects of in vitro plant regeneration. Plants 9:702
Cao P, Wu SL, Wu TT, Deng YH, Zhang QL, Wang KP, Zhang Y (2020) The important role of polysaccharides from a traditional Chinese medicine-Lung cleansing and detoxifying decoction against the COVID-19 pandemic. Carbohydr Polym 240:1–10
Cao P, Wang G, Wei XM, Chen SL, Han JP (2021) How to improve CHMs quality: enlighten from CHMs ecological cultivation. Chin Herb Med 13:301–312
Cheng YP, Lin JH, Li TC, Chen Q, Ma AP, Gao N, Wang B (2015) Callus induction in Bupleurum scorzonerifolium Willd. Acta Chin Med Pharmacol 43:46–48
Chinese Pharmacopoeia Commission (2020) The Pharmacopoeia of the People’s Republic of China, 2020 Edition Part I. China Medical Science, Beijing.
Denis DJ (2018) SPSS data analysis for univariate, bivariate, and multivariate statistics: SPSS data analysis for univariate, bivariate, and multivariate. Statistics. https://doi.org/10.1002/9781119465775
Gao RR, Hu YT, Dan Y, Hao LJ, Liu X, Song JY (2020) Chinese herbal medicine resources: where we stand. Chin Herb Med 12:3–13
Hao JP, Xu XF, Yang DF (2008) Condition for induction and differentiation of callus and propagation of adventitious buds in Bupleurum chinese. China Tradit Herb Drugs 39:1234–1238
Ho PC, Yeon YC, Wook KD, Kyeong CH, Ho JB (1994) Plant regeneration of bupleurum spp. through somatic tissue culture. Korean J Med Crop Sci 2:60–66
Jiménez VM (2005) Involvement of plant hormones and plant growth regulators on in vitro somatic embryogenesis. Plant Growth Regul 47:91–110
Li YC, Hou DB, Yu M, Tang ZK, Liu M (2018) Root Induction waterlogging toleratntline of HB-1601 in Buplerurum chinense DC. Seed 37:89–92
Liu Y, Wei C, Wang H, Ma X, Shen HL, Yang L (2021) Indirect somatic embryogenesis and regeneration of Fraxinus mandshurica plants via callus tissue. J for Res 32:1613–1625
Lü JF, Chen R, Zhang MH, Da S, Jaime AT, Ma GH (2013) Plant regeneration via somatic embryogenesis and shoot organogenesis from immature cotyledons of Camellia nitidissima Chi. J Plant Physiol 170:1202–1211
Motulsky H. (2007) Prism 5 statistics guide, 2007.
Murashige T, Skoog F (2006) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Natarajan R, Pechenyak B, Vyas U, Ranganathan P, Weinberg A, Liang P, Mallappallil MC, Norin AJ, Friedman EA, Saggi SJ (2014) Randomized controlled trial of strain-specific probiotic formulation (Renadyl) in dialysis patients. Biomed Res Int 2014:568–571
Nishiwaki M, Fujino K, Koda Y, Kikuta MY (2000) Somatic embryogenesis induced by the simple application of abscisic acid to carrot (Daucus carota L.) seedlings in culture. Planta 211:756–759
Pan SL (2006) Bupleurum species: scientific evaluation and clinical applications. CRC, Boca Raton
Phillips G, Garda M (2019) Plant tissue culture media and practices: an overview. Vitro Cell Dev Biol Plant 55:242–247
Su YH, Zhang XS (2014) The hormonal control of regeneration in plants. Curr Top Dev Biol 108:35–69
Wu JN (2005) An illustrated Chinese materia medica. Oxford University, New York
Xia GM, Li ZY, Guo GQ, Chen HM (1992) Direct somatic embryogenesis and plant regeneration from protoplasts of Bupleurum scorzonerifolium Willd. Plant Cell Rep 11:155–158
Xu JS, Zhao LZ, Wei JH, Tao YW, Yang CM (2013) Studies on high efficient plant regeneration system from anther callus of Bupleurum chinense. China J Chin Mater Med 38:3661–3665
Xu DM, Yu M, Shen J, Zhang Z, Li YC, Tang ZK, Hou DB (2018) Properly breed region to Chuanbeichai No.1 of new Bepleurum chinense in Sichuan area. Chin Med Mater 41:834–838
Yang CM, Zhao YK, Wei JH, Zhao LZ, Sui C, Zhang Z, Cui LY (2011) Factors affecting embryogenic callus production and plant regeneration in anther culture of Bupleurum chinense. Chin Herb Med 03:214–220
Yang FD, Dong X, Yin XB, Wang WP, You LT, Ni J (2017) Radix bupleuri: a review of traditional uses, botany, phytochemistry, pharmacology, and toxicology. Biomed Res Int 2017:22
Yu M, Chen H, Liu SH, Li YC, Sui C, Hou DB, Wei JH (2020) Differential expression of genes involved in saikosaponin biosynthesis between Bupleurum chinense DC. and Bupleurum scorzonerifolium Willd. Front Genet 11:583245
Yu M, Chen H, Liu Q, Huang J, Semagn K, Liu D, Li YC, Yang B, He YL, Sui C, Hou DB, Wei JH (2021) Analysis of unigenes involved in lateral root development in Bupleurum chinense and B. scorzonerifolium. Planta 253:128
Zhao SL, Xi CP (1993) Somatic embryogenesis and plant regeneration of Bupleurum falcatum L. China J Chin Mater Med 018:525–526
Zhong LLD, Lam WC, Yang W, Chan KW, Sze SCW, Miao J, Yung KK, Bian Z, Wong VT (2020) Potential targets for treatment of coronavirus disease 2019 (covid-19): a review of Qing-Fei-Pai-Du-Tang and its major herbs. Am J Chin Med 48:1051–1071
Funding
This work was supported by the China Agriculture Research System of MOF and MARA, the Young Elite Scientists Sponsorship Program by CACM (2019-QNRC2-C15), the CAMS Innovation Fund for Medical Sciences (CIFMS) under grant number 2016-I2M-2–003, the Programs of Science and Technology Department of Sichuan Province (2019YFH0072), the Doctoral Research Funding of Southwest University of Science and Technology (19zx7117 and 21zx7116), Opening Project Fund of Key Laboratory of Rubber Biology and Genetic Resource Utilization, Ministry of Agriculture (RRL-KLOF202201).
Author information
Authors and Affiliations
Contributions
Y.L and J.Z wrote the manuscript. H.C and W.X seeded Chuanbeichai No.1. D.B.H and M.Y bred out the commercial varieties of Chuanbeichai No.1. Y.L, H.L, Y.Z, L.F, and Z.W conducted propagation experiments. X.Y performed the data analysis. M.Y funded the whole project and helped Y.L to complete the manuscript. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The work is presented in the manuscript with the consent of all authors. The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Y., Zhao, J., Chen, H. et al. Plant regeneration via callus-mediated organogenesis in commercial variety of Chuanbeichai No. 1 in Bupleurum chinense DC. Plant Biotechnol Rep 17, 159–169 (2023). https://doi.org/10.1007/s11816-022-00772-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-022-00772-y