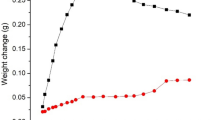
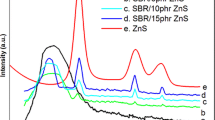
Abstract
Response surface methodology (RSM) optimized accelerator-to-sulfur (A/S) ratio was used to synthesize semi efficiently vulcanized styrene butadiene rubber (SBRSEV0) membrane possessing optimum balance between tensile strength (TS) and elongation at break (EAB). In addition, composite membranes, such as SBRSEV8, SBRSEV12 and SBRSEV24, were fabricated via incorporating 8, 12 and 24 wt% carbon black filler (CBF), respectively. The changes in physicochemical properties, as a result of crosslinking and CBF loading, were determined by analyzing CP MAS 13C-NMR, FTIR, TGA, DSC, XRD, FESEM-EDX and crosslink densities. Several bi-/poly-sulfidic products, formed by crosslinking precursors of SBR in accelerated sulfur vulcanization, were examined to ascertain the unambiguous reaction mechanism. In this regard, an extensive density functional theory (DFT) based optimization was conducted to apprehend the relative variation in stabilities of several mono-/poly-crosslinked configurations by measuring dipole moments and ground state energies. Moreover, intrinsic membrane properties, such as partial permeabilities and diffusion coefficients, were measured at varying conditions. RSM was employed to optimize membrane efficiency resulting from individual and/or interactive effects of input variables. For the first time, systematic three-stage RSM based optimization (i.e., TS/EAB, total flux (TF)/separation factor (SF) and partial permeabilities) was used to ensure excellent balance between TS/EAB (5.78 MPa/499.008% at 2.32 and 3.29 wt% of A and S, respectively), minimum TF/maximum SF (36.90 g m–2 h–1/202.46 at 35 °C, 0.97 wt% tetrahydrofuran (THF) and 24 wt% CBF) and minimum/maximum partial permeabilities of water/THF (2.94×10–8/4.64×10–8 Barrer at 35 °C, 0.97 wt% THF and 11.49 wt% CBF).
Similar content being viewed by others
References
M. Karmakar, M. Mahapatra and N. R. Singha, Korean J. Chem. Eng., 34, 1416 (2017).
N. R. Singha and S. K. Ray, J. Appl. Polym. Sci., 124, E99 (2012).
N. R. Singha, T. K. Parya and S. K. Ray, J. Membr. Sci., 340, 35 (2009).
S. Roy and N. R. Singha, Membranes, 7 (2017), DOI:10. 3390/membranes7030053.
N. R. Singha, S. Kar, S. Ray and S. K. Ray, Chem. Eng. Process., 48, 1020 (2009).
N. R. Singha, S. B. Kuila, P. Das and S. K. Ray, Chem. Eng. Process., 48, 1560 (2009).
N. R. Singha, S. Ray, S. K. Ray and B. B. Konar, J. Appl. Polym. Sci., 121, 1330 (2011).
N. R. Singha, P. Das and S. K. Ray, J. Ind. Eng. Chem., 19, 2034 (2013).
M. Mahapatra, M. Karmakar, B. Mondal and N. R. Singha, RSC Adv., 6, 69387 (2016).
M. D. Kurkuri, J. N. Nayak, M. I. Aralaguppi, B. V. K. Naidu and T. M. Aminabhavi, J. Appl. Polym. Sci., 98, 178 (2005).
M. Khayet, C. Cojocaru and G. Zakrzewska-Trznadel, J. Membr. Sci., 321, 272 (2008).
M. Catarino, A. Ferreira and A. Mendes, J. Membr. Sci., 341, 51 (2009).
V. García, J. L. Aguirre, E. Pongrácz, P. Perämäki and R. L. Keiski, J. Membr. Sci., 338, 111 (2009).
S. B. Kuila, S. K. Ray, P. Das and N. R. Singha, Chem. Eng. Process., 50, 391 (2011).
P. Das, S. K. Ray, S. B. Kuila, H. S. Samanta and N. R. Singha, Sep. Purif. Technol., 81, 159 (2011).
N. R. Singha, A. Dutta, M. Mahapatra, M. Karmakar, H. Mondal, P. K. Chattopadhyay and D. K. Maiti, ACS Omega, 3, 472 (2018).
N. R. Singha, M. Mahapatra, M. Karmakar, H. Mondal, A. Dutta, M. Deb, M. Mitra, C. Roy, P. K. Chattopadhyay and D. K. Maiti, ACS Omega, 3, 4163 (2018).
H. S. Samanta, S. K. Ray, P. Das and N. R. Singha, J. Chem. Technol. Biot., 87, 608 (2012).
N. Valentínyi, E. Cséfalvay and P. Mizsey, Chem. Eng. Res. Des., 91, 174 (2013).
N. R. Singha, S. Kar and S. K. Ray, Sep. Sci. Technol., 44, 422 (2009).
S. Ray, N. R. Singha and S. K. Ray, Chem. Eng. J., 149, 153 (2009).
N. R. Singha and S. K. Ray, Sep. Sci. Technol., 45, 2298 (2010).
G. Socrates, Infrared and Raman Characteristic Group Frequencies: Tables and Charts, Wiley, New York (2001).
A. S. Z. Naseri and A. J. Arani, Radiat. Phys. Chem., 115, 68 (2015).
S. Gunasekaran, R. K. Natarajan and A. Kala, Spectrochim. Acta A, 68, 323 (2007).
X. Liu, S. Zhao, X. Zhang, X. Li and Y. Bai, Polymer, 55, 1964 (2014).
V. Pouchaname, A. Tinabaye, R. Madivanane and Dr. Renukadevi, IRACST-Engineering Science and Technology: An International Journal, 2, 752 (2012).
M. J. Fernandez-Berridi, N. Gonzalez, A. Mugica and C. Bernicot, Thermochim. Acta, 444, 65 (2006).
Y. S. Lee, W. Lee, S. Cho, I. Kim and C. Ha, J. Anal. Appl. Pyrolysis, 78, 85 (2007).
H. Li, H. Kang, W. Zhang, S. Zhang and J. Li, Int. J. Adhes. Adhes., 66, 59 (2016).
G. Mertz, F. Hassouna, V. Toniazzo, A. Dahoun and D. Ruch, J. Eng. Mater. Technol., 134, 0109031 (2012).
P. A. Ajibade and B. C. Ejelonu, Spectrochim. Acta A, 113, 408 (2013).
L. Pellicioli, S. K. Mowdood, F. Negroni, D. D. Parker and J. L. Koenig, Rubber Chem. Technol., 75, 65 (2002).
N. R. Singha, M. Karmakar, M. Mahapatra, H. Mondal, A. Dutta, C. Roy and P. K. Chattopadhyay, Polym. Chem., 8, 3211 (2017).
N. R. Singha, S. Kar and S. K. Ray, Sep. Sci. Technol., 44, 1970 (2009).
A. Arockiasamy, H. Toghiani, D. Oglesby, M. F. Horstemeyer, J. L. Bouvard and R. L. King, J. Therm. Anal. Calorim., 111, 535 (2013).
S. J. Lue, W. W. Chen and S. F. Wang, Sep. Sci. Technol., 44, 3412 (2009).
P. Li, L. Yin, G. Song, J. Sun, L. Wang and H. Wang, Appl. Clay. Sci., 40, 38 (2008).
S. Ray and S. K. Ray, J. Membr. Sci., 270, 132 (2006).
N. R. Singha, M. Karmakar, M. Mahapatra, H. Mondal, A. Dutta, M. Deb, M. Mitra, C. Roy and P. K. Chattopadhyay, J. Mater. Chem. A, 6, 8078 (2018).
P. K. Chattopadhyay, N. C. Das and S. Chattopadhyay, Compos. Part A: Appl. Sci. Manuf., 42, 1049 (2011).
R. Guo, C. Hu, B. Li and Z. Jiang, J. Membr. Sci., 289, 191 (2007).
M. Karmakar, M. Mahapatra, A. Dutta, P. K. Chattopadhyay and N. R. Singha, Int. J. Biol. Macromol., 102, 438 (2017).
M. Mahapatra, M. Karmakar, A. Dutta, H. Mondal, J. S. D. Roy, P. K. Chattopadhyay and N. R. Singha, J. Environ. Chem. Eng., 6, 289 (2018).
R. V. Kumar, I. G. Moorthy and G. Pugazhenthi, RSC Adv., 5, 87645 (2015).
N. R. Singha, M. Mahapatra, M. Karmakar, A. Dutta, H. Mondal and P. K. Chattopadhyay, Polym. Chem., 8, 6750 (2017).
P. Das and S. K. Ray, J. Ind. Eng. Chem., 34, 321 (2016).
S. Claes, P. Vandezande, S. Mullens, P. Adriaensens, R. Peeters, F. H. J. Maurer and M. K. V. Bael, J. Membr. Sci., 389, 459 (2012).
Y. Nagase, T. Ando and C. M. Yun, React. Funct. Polym., 67, 1252 (2007).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
11814_2018_99_MOESM1_ESM.pdf
Fabrication of composite membranes for pervaporation of tetrahydrofuran-water: Optimization of intrinsic property by response surface methodology and studies on vulcanization mechanism by density functional theory
Rights and permissions
About this article
Cite this article
Mahapatra, M., Karmakar, M., Dutta, A. et al. Fabrication of composite membranes for pervaporation of tetrahydrofuran-water: Optimization of intrinsic property by response surface methodology and studies on vulcanization mechanism by density functional theory. Korean J. Chem. Eng. 35, 1889–1910 (2018). https://doi.org/10.1007/s11814-018-0099-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-018-0099-4
Keywords
- Filled and/or Crosslinked Organoselective Styrene Butadiene Rubber Membrane
- Optimization of Sulfur-accelerator-filler of Composite Membrane by RSM
- CP MAS 13C NMR
- FTIR
- TGA
- DSC
- XRD
- FESEM
- EDX and Crosslink Density Analyses
- Permeability
- Diffusion Coefficient
- Activity Coefficient and Solubility Parameter of Synthetic Rubber Membrane
- Analysis of Vulcanization Mechanism by DFT
- Organic-water Separation