Abstract
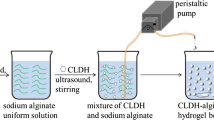
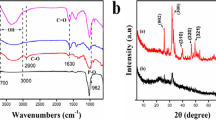
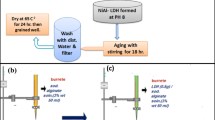
Recently, layered double hydroxides (LDH) have attracted a lot of interest due to their characteristics and biocompatibility for various applications. Although they have advantages, LDHs often have several limitations regarding application requirements, such as aggregation due to their high surface properties and are difficult to recover from aqueous solutions. In addressing these issues, the recent developments in the fabrication of intelligent LDHs composites based on biopolymer. The objective of this study was to investigate the phosphate removal. Using successfully synthesized double layered hydroxide (LDH) and alginate composite beads. The sample was characterized by X-ray diffraction and Scanning Electron Microscopy coupled with Energy Dispersive X-ray (SEM–EDX). SEM images confirm the successful incorporation of sodium alginate into LDH. They indicate that the surface of the LDH/alginate beads has a heterogeneous topography and that the LDH powder has been mixed with the alginate polymers. The efficiency and percentage removal were examined along with various influencing factors like adsorbent dose, solution pH, kinetic time, initial phosphate concentration, temperature and salt concentration also carried out (adsorbent dose = 0.02 g in 40 ml solution; initial phosphate concentration = 100 mgP/l; reaction time = 2 h). The results proved that the composites were effective in removing phosphate with a sorption capacity of 73% under the given experimental conditions. The phosphate removal was not sensitive to initial solution pHs between 2 and 12 and was not affected by the presence of salt. The adsorption process follows the Langmuir isotherm. The pseudo-second order model provides the best description of the kinetic data. Furthermore, the thermodynamic of adsorption was spontaneous and endothermic. All these results prove that the used adsorbent is environmentally friendly and effective for phosphate adsorption.
Graphical abstract





















Similar content being viewed by others
Availability of data and materials
Not applicable.
Abbreviations
- T:
-
Absolute temperature (K)
- pHpzc :
-
Point of zero-charge
- t :
-
Contact time (min)
- pHi :
-
Initial pH
- R(m/V):
-
Ratio mass/volume (g/L)
- pHf :
-
Final pH
- C 0 :
-
Initial phosphate concentration (mg/L)
- ∆H°:
-
Enthalpy change (kJ mol−1)
- C e :
-
Equilibrium phosphate concentration (mg/L)
- ∆G°:
-
Gibb’s free energy change (kJ mol−1)
- Cr :
-
Adsorbed phosphate concentration at any time (mg/L)
- ∆S°:
-
Entropy change (J K−1 mol−1)
- q e :
-
Equilibrium uptake of phosphate adsorbed (mg/g)
- R:
-
Universal gas constant (8.314 J K−1 mol−1)
References
Ahmed MA, Mohamed AA (2022) A systematic review of layered double hydroxide-based materials for environmental remediation of heavy metals and dye pollutants. Inorg Chem Commun. https://doi.org/10.1016/j.inoche.2022.110325
Al-Degs YS, El-Barghouthi MI, El-Sheikh AH, Walker GM (2008) Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dyes Pigments 77(1):16–23
Aziam R, Chiban M, Eddaoudi E, Soudani A, Zerbet M, Sinan F (2016) Factors controlling the adsorption of acid blue 113 dye from aqueous solution by dried C. edulis plant as natural adsorbent. Arab J Geosci 9(15):1–7. https://doi.org/10.1007/s12517-016-2675-4
Aziam R, Chiban M, Eddaoudi H, Soudani A, Zerbet M, Sinan F (2017) Kinetic modeling, equilibrium isotherm and thermodynamic studies on a batch adsorption of anionic dye onto eco-friendly dried Carpobrotus edulis plant. Eur Phys J Spec Top 226(5):977–992. https://doi.org/10.1140/epjst/e2016-60256-x
Aziam R, Boukarma L, Zaghloul A, Benhiti R, Eddaoudi E, Zerbet M, Chiban M (2021) Factor design methodology for modelling and optimization of carcinogenic acid dye adsorption onto Moroccan prickly pear cactus peel. In: E3S Web of conferences. EDP Sciences, vol 240 p 02005 https://doi.org/10.1051/e3sconf/202124002005
Bachmann SAL, Calvete T, Féris LA (2021) Caffeine removal from aqueous media by adsorption: an overview of adsorbents evolution and the kinetic, equilibrium and thermodynamic studies. Sci Total Environ 767:144229. https://doi.org/10.1016/j.scitotenv.2020.144229
Baliarsingh N, Parida KM, Pradhan GC (2013) Influence of the nature and concentration of precursor metal ions in the brucite layer of LDHs for phosphate adsorption—a review. RSC Adv 3(46):23865–23878. https://doi.org/10.1039/C3RA42857E
Barkhordari S, Alizadeh A (2022) Fabrication of pH-sensitive chitosan/layered double hydroxide (LDH)/Fe3O4 nanocomposite hydrogel beads for controlled release of diclofenac. Polym Bull. https://doi.org/10.1007/s00289-021-03761-3
Barrak I, Ayouch I, Kassab Z, El Achaby M, Barhoun A, Draoui K (2022) Sodium alginate encapsulated Moroccan clay as eco-friendly and efficient adsorbent for copper ions from aqueous medium. Mater Today Proc 51:2040–2046. https://doi.org/10.1016/j.matpr.2021.07.392
Benhiti R, Ait Ichou A, Zaghloul A, Aziam R, Carja G, Zerbet M, Chiban M (2020) Synthesis, characterization, and comparative study of MgAl-LDHs prepared by standard coprecipitation and urea hydrolysis methods for phosphate removal. Environ Sci Pollut Res 27(36):45767–45774. https://doi.org/10.1007/s11356-020-10444-5
Benjelloun M, Miyah Y, Evrendilek GA, Zerrouq F, Lairini S (2021) Recent advances in adsorption kinetic models: their application to dye types. Arab J Chem 14(4):103031. https://doi.org/10.1016/j.arabjc.2021.103031
Chen BQ, Xia HY, Mende LK, Lee CH, Wang SB, Chen AZ, Kankala RK (2022) Trends in layered double hydroxides-based advanced nanocomposites: recent progress and latest advancements. Adv Mater Interfaces 9(22):2200373. https://doi.org/10.1002/admi.202200373
Chiban M, Carja G, Lehutu G, Sinan F (2016) Equilibrium and thermodynamic studies for the removal of As (V) ions from aqueous solution using dried plants as adsorbents. Arab J Chem 9:S988–S999
De Castro GF, Mattiello EM, Ferreira JA, Zotarelli L, Tronto J (2020) Synthesis, characterization and agronomic use of alginate microspheres containing layered double hydroxides intercalated with borate. New J Chem 44(24):10066–10075. https://doi.org/10.1039/C9NJ06042A
Deng Y, Li M, Zhang Z, Liu Q, Jiang K, Tian J, Ni F (2021) Comparative study on characteristics and mechanism of phosphate adsorption on Mg/Al modified biochar. J Environ Chem Eng 9(2):105079. https://doi.org/10.1016/j.jece.2021.105079
El-Habacha M, Dabagh A, Lagdali S et al (2023) An efficient and adsorption of methylene blue dye on a natural clay surface: modeling and equilibrium studies. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-023-27413-3
Freundlich H (1906) Over the adsorption in solution. J Phys Chem 57(385471):1100–1107
Gao C, Yu XY, Luo T, Jia Y, Sun B, Liu JH, Huang XJ (2014) Millimeter-sized Mg–Al-LDH nanoflake impregnated magnetic alginate beads (LDH-n-MABs): a novel bio-based sorbent for the removal of fluoride in water. J Mater Chem A 2(7):2119–2128. https://doi.org/10.1039/C3TA13526
Han YU, Lee WS, Lee CG, Park SJ, Kim KW, Kim SB (2011) Entrapment of Mg-Al layered double hydroxide in calcium alginate beads for phosphate removal from aqueous solution. Desalin Water Treat 36(1–3):178–186
Iftekhar S, Srivastava V, Sillanpää M (2017) Synthesis and application of LDH intercalated cellulose nanocomposite for separation of rare earth elements (REEs). Chem Eng J 309:130–139. https://doi.org/10.1016/j.cej.2016.10.028
Iftekhar S, Srivastava V, Ramasamy DL, Naseer WA, Sillanpää M (2018) A novel approach for synthesis of exfoliated biopolymeric-LDH hybrid nanocomposites via in-stiu coprecipitation with gum Arabic: application towards REEs recovery. Chem Eng J 347:398–406. https://doi.org/10.1016/j.cej.2018.04.126
Karthikeyan P, Meenakshi S (2019) Synthesis and characterization of Zn–Al LDHs/activated carbon composite and its adsorption properties for phosphate and nitrate ions in aqueous medium. J Mol Liq 296:111766. https://doi.org/10.1016/j.molliq.2019.111766
Karthikeyan P, Meenakshi S (2021a) Development of sodium alginate@ ZnFe-LDHs functionalized beads: adsorption properties and mechanistic behaviour of phosphate and nitrate ions from the aqueous environment. Environ Chem Ecotoxicol 3:42–50. https://doi.org/10.1016/j.enceco.2020.11.003
Karthikeyan P, Meenakshi S (2021b) Fabrication of hybrid chitosan encapsulated magnetic-kaolin beads for adsorption of phosphate and nitrate ions from aqueous solutions. Int J Biol Macromol 168:750–759. https://doi.org/10.1016/j.ijbiomac.2020.11.132
Keyikoglu R, Khataee A, Yoon Y (2022) Layered double hydroxides for removing and recovering phosphate: recent advances and future directions. Adv Colloid Interface Sci. https://doi.org/10.1016/j.cis.2021.102598
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. Part I. Solids. J Am Chem Soc 38(11):2221–2295. https://doi.org/10.1021/ja02268a002
Lin CH, Chu HL, Hwang WS, Wang MC, Ko HH (2017) Synthesis and optical properties of Mg-Al layered double hydroxides precursor powders. AIP Adv 7(12):125005. https://doi.org/10.1063/1.4990832
Lyu F, Yu H, Hou T, Yan L, Zhang X, Du B (2019) Efficient and fast removal of Pb2+ and Cd2+ from an aqueous solution using a chitosan/Mg-Al-layered double hydroxide nanocomposite. J Colloid Interface Sci 539:184–193. https://doi.org/10.1016/j.jcis.2018.12.049
Majd MM, Kordzadeh-Kermani V, Ghalandari V, Askari A, Sillanpää M (2021) Adsorption isotherm models: a comprehensive and systematic review (2010–2020). Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2021.151334
Naz A, Chowdhury A (2022) Pollutant extraction from water and soil using Montmorillonite clay-polymer composite: a rapid review. Mater Today Proc 60:1–7. https://doi.org/10.1016/j.matpr.2021.10.366
Ragadhita RIST, Nandiyanto ABD (2022) curcumin adsorption on zinc imidazole framework-8 particles: isotherm adsorption using langmuir, freundlich, temkin, and dubinin-radushkevich models. J Eng Sci Technol 17(2):1078–1089
Rajahmundry GK, Garlapati C, Kumar PS, Alwi RS, Vo D-VN (2021) Statistical analysis of adsorption isotherm models and its appropriate selection. Chemosphere 276:130176
Rodier J, Legube B, Merlet N (2009) L’analyze de l’eau 9e édition Dunod, Paris Sebastian
Sebastian S, Mayadevi S, Beevi BS, Mandal S (2014) Layered Clay-Alginate Composites for the Adsorption of Anionic Dyes: A Biocompatible Solution for Water/Wastewater Treatment. J Water Resour Prot 06(03):177–184. https://doi.org/10.4236/jwarp.2014.63023
Sepehr MN, Al-Musawi TJ, Ghahramani E, Kazemian H, Zarrabi M (2017) Adsorption performance of magnesium/aluminum layered double hydroxide nanoparticles for metronidazole from aqueous solution. Arab J Chem 10(5):611–623. https://doi.org/10.1016/j.arabjc.2016.07.003
Shan R, He Y, Zi T, Wang G, Liu X, Han Z, Zhu Y (2018) Immobilization of calcined layered double hydroxide into alginate hydrogel beads for PNP and PAP removal: kinetics, isotherms, thermodynamics, and mechanism. Water Air Soil Pollut 229(10):1–18. https://doi.org/10.1007/s11270-018-3976-x
Siwek H, Bartkowiak A, Włodarczyk M (2019) Adsorption of phosphates from aqueous solutions on alginate/goethite hydrogel composite. Water 11(4):633. https://doi.org/10.3390/w11040633
Tran HN, You SJ, Chao HP (2016) Thermodynamic parameters of cadmium adsorption onto orange peel calculated from various methods: a comparison study. J Environ Chem Eng 4(3):2671–2682. https://doi.org/10.1016/j.jece.2016.05.009
Acknowledgements
The authors are grateful to all research staff that contributed to the data collection required for this study.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
NS: conceptualization, methodology, formal analysis, data curation, writing—original draft, writing—review, editing, and visualisation. AR: Conceptualization, methodology, formal analysis, data curation, writing—original draft, writing—review, editing, and visualisation. BR: Revision, editing and data processing. GC: Supervision and project administration. SI: Revision, editing, MZ: revision, editing CM: revision, editing,validation, visualisation, supervision and project administration.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nouaa, S., Aziam, R., Benhiti, R. et al. Synthesis of LDH/Alginate composite beads as a potential adsorbent for phosphate removal: kinetic and equilibrium studies. Chem. Pap. 77, 6689–6705 (2023). https://doi.org/10.1007/s11696-023-02969-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-02969-z