Abstract
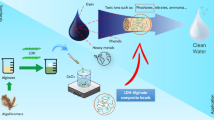
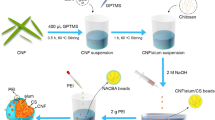
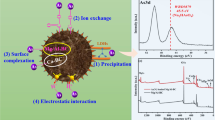
Ni-Al-CO3-layered double hydroxide (LDH) with Ni:Al ratio (3:1) and their nanocomposites with alginate and chitosan beads were prepared and examined for their efficiency in removal of Cd2+ and Cu2+ ions from wastewater. Different parameters such as contact time, pH value, adsorbent weight, and heavy metal ion concentration on the removal efficiency were examined and reported. The prepared beads were examined using X-ray diffraction (XRD), TEM, SEM, and FTIR. Our results revealed a successful preparation of the LDH in rhombohedral hexognal crystal form and the alginate-LDH-chitosan beads. The optimized batch experiment conditions in ambient room temperature were found to be 2 g/L adsorbent dose, 50 mg/L initial concentration of meal, contact time of 2 h, and pH ~ 5 and 6 for removal of Cu2+ and Cd2+, respectively. The adsorption process was well fitted with Langmuir and Freundlich isotherm models (higher R2), with trivial advantage for Freundlich approach. Kinetic studies showed that the adsorption of both Cd2+ and Cu2+ followed the pseudo-second-order. The current study demonstrated that the Ni-Al-CO3 LDH and their novel alginate-chitosan-based nanocomposite could be further tailored and used as efficient adsorbents for the uptake of heavy metals from wastewater.












Similar content being viewed by others
References
Ayawei N, Ekubo AT, Wankasi D, Dikio ED (2015) Synthesis and application of layered double hydroxide for the removal of copper in wastewater. Int J Chem 7(1):122
Bahgat M, Farghali AA, El Rouby W, Khedr M, Mohassab-Ahmed MY (2013) Adsorption of methyl green dye onto multi-walled carbon nanotubes decorated with Ni nanoferrite. Appl Nanosci 3(3):251–261
Benettayeb A, Guibal E, Morsli A, Kessas R (2017) Chemical modification of alginate for enhanced sorption of Cd (II), Cu (II) and Pb (II). Chem Eng J 316:704–714
Cataldo S, Gianguzza A, Merli M, Muratore N, Piazzese D, Turco Liveri ML (2014) Experimental and robust modeling approach for lead (II) uptake by alginate gel beads: influence of the ionic strength and medium composition. J Colloid Interface Sci 434:77–88
Cullity BD, Stock SR (1956) The directions of diffracted beams, Elements of X-ray diffraction. Addison-Wesley Publishing Co., USA, pp 78–103
DeMarco MJ, SenGupta AK, Greenleaf JE (2003) Arsenic removal using a polymeric/inorganic hybrid sorbent. Water Res 37(1):164–176
El Hassani K, Beakou BH, Kalnina D, Oukani E, Anouar A (2017) Effect of morphological properties of layered double hydroxides on adsorption of azo dye methyl orange: a comparative study. Appl Clay Sci 140:124–131 https://scholar.googleusercontent.com/schol
Elgiddawy N, Essam TM, El Rouby WMA, Raslan M, Farghali AA (2017) New approach for enhancing chlorella vulgaris biomass recovery using ZnAl-layered double hydroxide nanosheets. J Appl Phycol 29(3):1399–1407
Esmaeili A, Ghasemi S (2009) Evaluation of the activated carbon prepared of algae marine Gracilaria for the biosorption of Ni (II) from aqueous solutions. World Appl Sci J 6(4):515–518
Esmat M, Farghali AA, Khedr MH, El-Sherbiny IM (2017) Alginate-based nanocomposites for efficient removal of heavy metal ions. Int J Biol Macromol 102:272–283
Farghali AA, Bahgat M, ElRouby WMA, Khedr MH (2013) Decoration of multi-walled carbon nanotubes (MWCNTs) with different ferrite nanoparticles and its use as an adsorbent. J Nanostruct Chem 3(1):50
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57(385471):1100–1107
Ghoneim MM, el-Desoky HS, el-Moselhy KM, Amer A, Abou el-Naga EH, Mohamedein LI, al-Prol AE (2014) Removal of cadmium from aqueous solution using marine green algae, Ulva Lactuca. Egypt J Aquat Res 40(3):235–242
Goher ME, Hassan AM, Abdel-Moniem IA, Fahmy AH, Abdo MH, el-sayed SM (2015) Removal of aluminum, iron and manganese ions from industrial wastes using granular activated carbon and Amberlite IR-120H. Egypt J Aquat Res 41(2):155–164
Goher ME-S, Emara MM, Abdo MH, Refaat Mah NM, Abdel-Sata AM, el-Shamy AS (2017) Cadmium removal from aqueous solution using superparamagnetic iron oxide nanosorbents on Amberlite IR 120 H support. J Appl Sci 17:296–305
Gokila S, Gomathi T, Sudha PN, Anil S (2017) Removal of the heavy metal ion chromiuim (VI) using chitosan and alginate nanocomposites. Int J Biol Macromol 104:1459–1468
Gotoh T, Matsushima K, Kikuchi K-I (2004) Preparation of alginate-chitosan hybrid gel beads and adsorption of divalent metal ions. Chemosphere 55(1):135–140
Han Y-U, Lee W-S, Lee C-G, Park S-J, Kim K-W, Kim S-B (2011) Entrapment of Mg-Al layered double hydroxide in calcium alginate beads for phosphate removal from aqueous solution. Desalin Water Treat 36(1–3):178–186
He X, Qiu X, Chen J (2017) Preparation of Fe (II)-Al layered double hydroxides: application to the adsorption/reduction of chromium. Colloids Surf A Physicochem Eng Asp 516:362–374
Ho S-H, Chen C-Y, Lee D-J, Chang J-S (2011) Perspectives on microalgal CO2-emission mitigation systems: a review. Biotechnol Adv 29(2):189–198
Jeon C, Holl WH (2003) Chemical modification of chitosan and equilibrium study for mercury ion removal. Water Res 37(19):4770–4780
Jitianu M, Gunness DC, Aboagye DE, Zaharescu M, Jitianu A (2013) Nanosized Ni-Al layered double hydroxides-structural characterization. Mater Res Bull 48(5):1864–1873
Juang R-S, Shao H-J (2002) A simplified equilibrium model for sorption of heavy metal ions from aqueous solutions on chitosan. Water Res 36(12):2999–3008
Kameda T, Takeuchi H, Yoshioka T (2008) Uptake of heavy metal ions from aqueous solution using Mg-Al layered double hydroxides intercalated with citrate, malate, and tartrate. Sep Purif Technol 62(2):330–336
Kameda T, Takeuchi H, Yoshioka T (2011) Ni-Al layered double hydroxides modified with citrate, malate, and tartrate: preparation by coprecipitation and uptake of Cu2+ from aqueous solution. J Phys Chem Solids 72(6):846–851
Karthik R, Meenakshi S (2015) Removal of Pb (II) and Cd (II) ions from aqueous solution using polyaniline grafted chitosan. Chem Eng J 263:168–177
Komarneni S, Kozai N, Roy R (1998) Novel function for anionic clays: selective transition metal cation uptake by diadochy. J Mater Chem 8(6):1329–1331
Kumar N, Reddy L, Parashar V, Ngila JC (2017) Controlled synthesis of microsheets of ZnAl layered double hydroxides hexagonal nanoplates for efficient removal of Cr (VI) ions and anionic dye from water. J Environ Chem Eng 5(2):1718–1731
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. Part I. solids. J Am Chem Soc 38(11):2221–2295
Li C (2008) Batch and bench-scale fixed-bed column evaluations of heavy metal removals from aqueous solutions and synthetic landfill leachate using low-cost natural adsorbents
Li S-S, Jiang M, Jiang T-J, Liu J-H, Guo Z, Huang X-J (2017) Competitive adsorption behavior toward metal ions on nano-Fe/Mg/Ni ternary layered double hydroxide proved by XPS: evidence of selective and sensitive detection of Pb (II). J Hazard Mater 338:1–10
Liu Y-g et al (2013) Biosorption of copper (II) from aqueous solution by Bacillus subtilis cells immobilized into chitosan beads. Trans Nonferrous Metals Soc China 23(6):1804–1814
Liu X-J, Zeng H-Y, Xu S, Chen C-R, Zhang Z-q, du J-Z (2016) Metal oxides as dual-functional adsorbents/catalysts for Cu2+/Cr (VI) adsorption and methyl orange oxidation catalysis. J Taiwan Inst Chem Eng 60:414–422
Malesu VK, Sahoo D, Nayak PL (2011) Chitosan – sodium alginate nanocomposites blended with Cloisite 30b as a novel drug delivery system for anticancer drug curcumin. Int J Appl Biol Pharm Technol 2(3):402–411
Negrea P, Caunii A, Sarac I, Butnariu M (2015) The study of infrared spectrum of chitin and chitosan extract as potential SOURCES of BIOMASS. Dig J Nanomater Biostruct 10(4)
Nejati K, Keypour H, Nezhad PDK, Rezvani Z, Asadpour-Zeynali K (2015) Preparation and characterization of cetirizine intercalated layered double hydroxide and chitosan nanocomposites. J Taiwan Inst Chem Eng 53(3):168–175. https://doi.org/10.1016/j.jtice.2015.02.035
Ngah WSW, Fatinathan S (2008) Adsorption of Cu (II) ions in aqueous solution using chitosan beads, chitosan-GLA beads and chitosan-alginate beads. Chem Eng J 143(1):62–72
Papageorgiou SK, Kouvelos EP, Katsaros FK (2008) Calcium alginate beads from Laminaria Digitata for the removal of Cu+2 and Cd+2 from dilute aqueous metal solutions. Desalination 224(1–3):293–306
Peligro FR, Pavlovic I, Rojas R, Barriga C (2016) Removal of heavy metals from simulated wastewater by in situ formation of layered double hydroxides. Chem Eng J 306:1035–1040
Perez MR et al (2006) Uptake of Cu2+, Cd2+ and Pb2+ on Zn-Al layered double hydroxide intercalated with EDTA. Appl Clay Sci 32(3–4):245–251
Pfeiffer H, Martínez-dlCruz L, Lima E, Flores J, Vera MA, Valente JS (2010) Influence of Mg/Al ratio on the thermokinetic rehydration of calcined Mg- Al layered double hydroxides. J Phys Chem C 114(18):8485–8492
Puranik PR, Paknikar KM (1999) Biosorption of lead, cadmium, and zinc by citrobacter strain MCM B 181: characterization studies. Biotechnol Prog 15(2):228–237
Rahmanian O, Maleki MH, Dinari M (2017) Ultrasonically assisted solvothermal synthesis of novel Ni/Al layered double hydroxide for capturing of Cd (II) from contaminated water. J Phys Chem Solids 110:195–201
Rojas R (2016) Effect of particle size on copper removal by layered double hydroxides. Chem Eng J 303:331–337
Ruan X, Chen Y, Chen H, Qian G, Frost RL (2016) Sorption behavior of methyl orange from aqueous solution on organic matter and reduced graphene oxides modified Ni-Cr layered double hydroxides. Chem Eng J 297:295–303
Sikder MT, Tanaka S, Saito T, Kurasaki M (2014) Application of zerovalent iron impregnated chitosan-caboxymethyl- -cyclodextrin composite beads as arsenic sorbent. J Environ Chem Eng 2(1):370–376
Song TE, Wilde G, Peterlechner M (2014) Morphology and aspect ratio of bismuth nanoparticles embedded in a zinc matrix. Appl Phys Lett 105(24):241902
Sulekha MS (2016) Nanotechnology for waste water treatment. Int J Chem Stud 4(2):22–24
Sun M, Xiao Y, Zhang L, Gao X, Yan W, Wang D, Su J (2015) High uptake of Cu2+, Zn2+ or Ni2+ on calcined MgAl hydroxides from aqueous solutions: changing adsorbent structures. Chem Eng J 272:17–27
Tamahrajah J, Goncharova I, Pytskii I, Shiryaev AA, Averin AA, Kiselev MR, Shaphigulina A, Basirun WJ, Buryak AK, Pethukova GA, Roessner F (2017) X-ray coupled laser desorption ionization assessment of layered double hydroxides for nitrate adsorption based on ion size, affinity and compromised layer height. Appl Clay Sci 143:134–141
Vijayalakshmi K, Devi BM, Latha S, Gomathi T, Sudha PN, Venkatesan J, Anil S (2017) Batch adsorption and desorption studies on the removal of Lead (II) from aqueous solution using Nanochitosan/sodium alginate/microcrystalline cellulose beads. Int J Biol Macromol 104:1483–1494
Wang Y-y et al (2016) Synthesis of phosphate-embedded calcium alginate beads for Pb (II) and Cd (II) sorption and immobilization in aqueous solutions. Trans Nonferrous Metals Soc China 26(8):2230–2237
Wu M, Zhang J, Peng Y, Zhou J, Ruan X, Liu J, Liu Q, Xi Y, Frost R, Qian G (2017) An investigation into mechanism of cation adsorption by reconstruction of calcined layered double hydroxide. Microporous Mesoporous Mater 242:182–189
Xu ZP, Lu GQ (2005) Hydrothermal synthesis of layered double hydroxides (LDHs) from mixed MgO and Al2O3: LDH formation mechanism. Chem Mater 17(5):1055–1062
Yu S, Liu Y, Ai Y, Wang X, Zhang R, Chen Z, Chen Z, Zhao G, Wang X (2018) Rational design of carbonaceous nanofiber/Ni-Al layered double hydroxide nanocomposites for high-efficiency removal of heavy metals from aqueous solutions. Environ Pollut 242:1–11
Yurekli Y (2016) Removal of heavy metals in wastewater by using zeolite nano-particles impregnated polysulfone membranes. J Hazard Mater 309:53–64
Zhou X, Xue X, Jiang W (2011) Study on adsorption of heavy metal ion in metallurgical wastewater by Sepiolite. In: 2011 2nd International Conference on Environmental Science and Development IPCBEE, vol. 4
Zhou H, Jiang Z, Wei S (2018) A new hydrotalcite-like absorbent FeMnMg-LDH and its adsorption capacity for Pb2+ ions in water. Appl Clay Sci 153:29–37
Zubair M, Daud M, McKay G, Shehzad F, Al-Harthi MA (2017) Recent progress in layered double hydroxides (LDH)-containing hybrids as adsorbents for water remediation. Appl Clay Sci 143:279–292
Acknowledgments
Our study depends on our author̕s personal efforts, as well as on Materials Science and Nanotechnology Department, Faculty of Postgraduate Studies for Advanced Sciences (PSAS), Beni-Suef University (BSU), Egypt, together with the Chemistry Lab., Fresh water and Lakes Division, National Institute of Oceanography and Fisheries (NIOF), Cairo, Egypt.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Angeles Blanco
Rights and permissions
About this article
Cite this article
El Rouby, W.M.A., El-Dek, S.I., Goher, M.E. et al. Efficient water decontamination using layered double hydroxide beads nanocomposites. Environ Sci Pollut Res 27, 18985–19003 (2020). https://doi.org/10.1007/s11356-018-3257-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3257-7