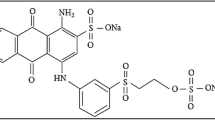
Abstract
Contamination of surface water and groundwater by organic pollutants is a serious problem due to their persistence, bioaccumulation and biomagnification through food webs. Since the removal of dyes from wastewater is considered an environmental challenge and government legislation requires textile wastewater to be treated, therefore there is a constant need to have an effective process that can efficiently remove these dyes. The aim of the present study is to evaluate the potentiality of dried Carpobrotus edulis plant as low-cost adsorbent for the removal of the industrial acid blue 113 dye from aqueous solutions using the batch equilibration technique. The effects of different physicochemical parameters such as adsorbent dose, contact time, initial dye concentration, solution pH and temperature on adsorption rate of anionic AB113 dye on microparticles of dried C. edulis plant were investigated. The experimental data were analyzed by using mathematical models to determine the thermodynamic parameters. The negative values of free energy change indicated the spontaneous nature of the adsorption and negative value of enthalpy change suggested the exothermic nature of the adsorption process. These results indicate that dried C. edulis plant as an environmentally friendly adsorbent could be potentially used for the removal of anionic dyes from aqueous solutions.






Similar content being viewed by others
References
Akar T, Demir TA, Kiran I, Ozcan A, Ozcan AS, Tunali S (2006) Biosorption potential of Neurospora crassa cells for decolorization of Acid Red 57 dye. J Chem Technol Biotechn 81:1100–1106
Attia AA, Rashwan WE, Khedr SA (2006) Capacity of activated carbon in the removal of acid dyes subsequent to its thermal treatment. J Dyes Pig 69:128–136
Benhima H, Chiban M, Sinan F, Seta P, Persin M (2008) Removal of Cd(II) and Pb(II) ions from aqueous solution by adsorption onto micro-particles of dry plants. Colloids Surf B: Biointer 61:10–16
Bulut Y, Gozubenli N, Aydın H (2007) Equilibrium and kinetics studies for adsorption of direct blue 71 from aqueous solution by wheat shells. J Hazard Mater 144:300–306
Chiban M (2007) Adsorption study in a batch and column systems of heavy metals, anions and other pollutants from model and experimental wastewaters by solid biomaterials” (Etude de l’adsorption en système statique et dynamique d’ions métalliques, minéraux et autres polluants sur des Biomatériaux Inertes Solides (végétaux secs) à partir de solutions modèles et d’eaux usées de la région d’Agadir). Thesis of Ibn Zohr University, Agadir, Morocco
Chiban M (2011) Élaboration et Évaluation d'un Nouveau Procédé d'Épuration des Eaux: Application à des solutions modèles et d'eaux usées domestiques et industrielles de la région d'Agadir., Editions Universitaires Européennes, ISBN: 978–613–1-58836-5, 272p
Chiban M, Amzeghal A, Benhima H, Sinan F, Tahrouch S, Seta P (2007) Phytochemical study of some inert plants from the south-western region of Morocco (Etude phytochimique de certaines plantes inertes du sud marocain). Rev. Biol. Biotech 6:40–43
Chiban M, Soudani A, Sinan F, Persin M (2011a) Single, binary and multi-component adsorption of some anions and heavy metals on environmentally friendly Carpobrotus edulis plant. Colloids Surf. B: Biointer. 82:267–276
Chiban M, Soudani A, Sinan F, Tahrouch S, Persin M (2011b) Characterization and application of dried plants to remove heavy metals, nitrate and phosphate ions from industrial wastewaters in a batch system. Clean - Soil, Air, Water 39:376–283
Chiban M, Zerbet M, Sinan F (2012a) Low-cost materials for phosphate removal from aqueous solutions, Chapter 1. In: Handbook of Phosphates: Sources, Properties and Applications, Nova Science Publishers, Inc. USA, pp.1–41
Chiban M, Soudani A, Sinan F (2012b) Removal of nitrate ions by using low-cost adsorbents: Equilibrium isotherm, kinetics and thermodynamic study. Chapter 3. In: Handbook of Nitrate: Occurrence, characteristics and health considerations, Nova science publishers, Inc. USA, pp. 31–48
Chiban M, Soudani A, Zerbet M, Sinan F (2013) Wastewater treatment processes, Chapter 10. In: Handbook of Wastewater Treatment: Biological Methods, Technology and Environmental Impact, Nova Science Publishers, inc. USA, pp.249–262
Christie RM (2007) Environmental aspects of textile dyeing. Boca Raton, Cambridge, Woodhead
Das A, Pal A, Saha S, Maji SK (2009) Behaviour of fixed-bed column for the adsorption of malachite green on surfactant-modified alumina. J Environ Sci Health, Part A 44(3):265–272
Dávila-Jiménez MM, Elizalde-González MP, Hernández-Montoya V (2009) Performance of mango seed adsorbents in the adsorption of anthraquinone and azo acid dyes in single and binary aqueous solutions. J Biores Technol 100:6199–6206
Errais E, Duplay J, Darragi F, M'Rabet I, Aubert A, Huber F, Morvan G (2011) Efficient anionic dye adsorption on natural untreated clay: kinetic study and thermodynamic parameters. Desalination 275:74–81
Golka KS, Kopps S, Myslak ZW (2004) Carcinogenicity of azo colorants: influence of solubility and bioavailability. Toxicol Lett 151(1):203–210
Gupta VK, Suhas (2009) Application of low-cost adsorbents for dye removal: a review. J Environ Manag 90(8):2313–2342
Gupta VK, Gupta B, Rastogi A, Agarwal S, Nayak A (2011) A comparative investigation on adsorption performances of mesoporous activated carbon prepared from waste rubber tire and activated carbon for a hazardous azo dye-acid blue 113. J Hazard Mater 186:891–901
Gupta VK, Suhas, Tyagi I, Agarwal S, Singh R, Chaudhary M, Harit A, Kushwaha S (2016) Column operation studies for the removal of dyes and phenols using a low cost adsorbent. Global J Environ Sci Manage 2(1):1–10
Jain AK, Gupta VK, Bhatnagar A, Suhas (2003) Utilization of industrial waste products as adsorbents for the removal of dyes. J Hazard Mater B101:31–42
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. Part I. Solids. J Am Chem Soc 38:2221–2295
Lee LY, Gan S, Yin Tan MS, Lim SS, Lee XJ, Lam YF (2015) Effective removal of acid blue 113 dye using overripe Cucumis sativus peel as an eco-friendly biosorbent from agricultural residue. J Clean Prod. doi:10.1016/j.jclepro.2015.11.016
Mahmoodi NM, Hayati B, Arami M, Lan C (2011) Adsorption of textile dyes on pine cone from colored wastewater: kinetic, equilibrium and thermodynamic studies. Desalination 268:117–125
Mohan D, Pittman CU Jr (2007) Arsenic removal from water/wastewater using adsorbents—a critical review. J Hazard Mater 142:1–53
Mui ELK, Cheung WH, Valix M, McKay G (2010) Dye adsorption onto char from bamboo. J Hazard Mater 177:1001–1005
Ofomaja AE, Ho YS (2007) Equilibrium sorption of anionic dye from aqueous solution by palm kernel fibre as sorbent. Dyes Pigments 74:60–66
Ong ST, Khoo EC, Hii SL, Ha ST (2010) Utilization of sugarcane bagasse for removal of basic dyes from aqueous environment in single and binary systems. Desalin Water Treat 20:86–95
Shirzad-Siboni M, Jafari SJ, Giahi O, Kim I, Lee SM, Yang JK (2014) Removal of acid blue 113 and reactive black 5 dye from aqueous solutions by activated red mud. J Ind Eng Chem. doi:10.1016/j.jiec.2013.07.028
Tabak A, Eren E, Afsin B, Caglar B (2009) Determination of adsorptive properties of a Turkish sepiolite for removal of reactive blue 15 anionic dye from aqueous solutions. J Hazard Mater 161:1087–1094
Valliammai S, Nagaraja KS, Jeyaraj B (2015) Removal of acid blue 113 dyes from aqueous solution by activated carbon of Varagu millet husk: equilibrium, kinetics and thermodynamic studies. Int J ChemTech Res 8(12):329–341
Weber TW, Chakkravorti RK (1974) Pore and solid diffusion models for fixed bed adsorbers. Am Institute Chem Engin J 20:228–232
Author information
Authors and Affiliations
Corresponding authors
Additional information
This article is part of the Topical Collection on Water Resources in Arid Areas
Rights and permissions
About this article
Cite this article
Aziam, R., Chiban, M., Eddaoudi, E. et al. Factors controlling the adsorption of acid blue 113 dye from aqueous solution by dried C. edulis plant as natural adsorbent. Arab J Geosci 9, 659 (2016). https://doi.org/10.1007/s12517-016-2675-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-016-2675-4