Abstract
Soft confections can serve as a reservoir for bioactive peptide delivery in both functional food and food supplement applications. In this study, pectin-based soft confections were fortified with proteolytic plant protein hydrolysates and the residual bioactivity of hydrolysates was studied after processing or simulated digestion. Cold press sunflower or hazelnut cakes were used in the manufacture of protein isolates based on an alkali extraction-isoelectric precipitation (AE-IP) method. Trypsin or bromelain were utilized in the proteolysis of the isolates and thus prepared liquid hydrolysates were used in confectionery manufacture. DPP-IV (dipeptidyl peptidase-IV) inhibitory activity (i.e., in vitro antidiabetic activity) and antioxidative activities were measured. In addition, sensory and textural attributes were investigated. In all cases, a significant concentration of hydrolysates were added to the confections (27%), which lead to significant changes in color, texture and sensory acceptance. The peptide profile and size distribution mostly altered such characteristics, while observed bioactivity was significant after processing. Simulated digestion enhanced DPP-IV inhibitory activity up to approx. 40%, whereas antioxidative performance decreased. While the applicability of the current findings is limited by hydrolysate solubility, ingredient interactions, and processing costs, the relevance of degree of hydrolysis (DH%), peptide characteristics and phenolic-peptide interactions on product quality and eventual bioactivity are being discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Confections can be defined as products that usually contain sweetener(s), a gelling ingredient, colorants, and acids [1]. Although confectionery products are commonly criticized for having low nutritional values, they are widely consumed by both children and adults [2]. Currently, various studies are being carried out on functional confectionery that are enriched in bioactive compounds [3]. For example, bioactives with potential antioxidative activities or antidiabetic capabilities may be considered in this regard. Due to its biological activities, Dipeptidyl peptidase-IV (DPP-IV) has been associated with the development of various diseases including or affecting glucose metabolism, intestinal motility, immune system, and diabetes. Consequently, inhibition of DPP-IV is a target in the treatment of diabetes and natural ingredients that inhibit DPP-IV activity may be considered as antidiabetic agents [4].
Recently, foods with high protein content have also become increasingly popular among the consumers. While gelatin-based confections demonstrate relatively high protein contents (mostly 5–10% gelatin) [5], plant protein isolate or hydrolysate bearing confections suitable for vegans or vegetarians is quite limited in the global market. In any case, the extent of plant protein or protein hydrolysate fortification remains to be mostly < 1% in recent commercial products.
Protein fortification could enhance the nutritional and bioactive characteristics of confections, whereas the interactions between protein molecules and water or other gelling agents could affect the sensory and textural attributes, especially due to the competition for water. This can be primarily attributed to the pronounced water holding capacities (WHC) of protein ingredients [6]. Although some opposite arguments are available in the literature, protein hydrolysates may be anticipated to demonstrate a lower WHC compared to their parent proteins [7] and hence, minimally affect textural attributes. While plant protein hydrolysates may exert various bioactivities due to their bioactive peptide contents, the presence of other bioactive components such as phenolic compounds may further influence their bioactivities. The presence of phenolic compounds was clearly shown to affect the behavior of both hazelnut and sunflower proteins in the previous literature [8, 9]. Consequently, protein-phenolic or peptide-phenolic interactions need to be carefully investigated [10].
In the current study, the fortification of soft confections with hazelnut and sunflower protein hydrolysates demonstrating prominent bioactive properties is examined. The potential changes in bioactive (antidiabetic and antioxidative), sensory and textural attributes were investigated throughout manufacture and digestion processes.
Materials and methods
Materials
Confectionery supplies were acquired from a local vendor (Smart Chemistry Ltd., İzmir, Türkiye) at industrial grade. Laboratory chemicals were purchased from Sigma-Aldrich (Schnelldorf, Germany) at reagent grade. Bromelain (B4882), trypsin (T4799), pepsin (P6887), and DPP-IV (D4943) were acquired from the same company, whereas pancreatin was purchased from Bio Basic (PB0681).
Production of protein isolates from hazelnut and sunflower press cakes
Protein isolate manufacture was based on Göksu et al. [11]. In order to obtain protein from cold press sunflower or hazelnut cakes, the samples were mixed with distilled water at a ratio of 1:15 until dispersion was complete. Then, the medium pH was adjusted to pH 9.5 using 1 N NaOH. The dispersion was mixed with a magnetic stirrer for 1 h at 500 rpm. Once, protein dissolution was ensured, the dispersion was centrifuged at 11,000xg (25 °C) for 30 min with a high-speed centrifuge (Himac CR22N, Hitachi, Japan). The supernatant was filtered and medium pH was adjusted to 4.5 in order to encourage the precipitation of proteins. The centrifugation step was repeated and precipitated pellets were collected and frozen at -20 °C. The frozen samples were dried using a lyophilizer (TRS 2/2V, Teknosem, İstanbul, Türkiye).
Proteolysis
Trypsin or bromelain were used in the preparation of hazelnut or sunflower protein hydrolysates [12]. The sample coding for trypsin hydrolysates (TH) and bromelain hydrolysates (BH) of hazelnut (H) samples were HTH and HBH, respectively. Similar coding was also utilized for sunflower seed (S) samples. The dispersion for trypsin was prepared at a ratio of 1:100 (enzyme: protein). As a buffer solution, 100 mM Tris-HCl (pH 8) was used. Then, the enzymatic hydrolysis of prepared dispersion was carried out for 18 h (overnight) using a water-bath at 37 °C. The sample dispersion for bromelain treatment was prepared at a ratio of 1:100 (enzyme: protein). As a buffer solution, 30 mM sodium acetate buffer (pH 4.5) was used. Then, the enzymatic hydrolysis of prepared dispersion was carried out at 45 °C using a water bath for 150 min. The hydrolysates were transferred to a water bath at 95 °C for 5 min to stop the enzymatic hydrolysis. Then, the hydrolysates were quickly placed in an ice bath and cooled to ambient temperature. The cooled hydrolysates were centrifuged (Himac CR22N, Hitachi, Japan) for 30 min at 5000xg to remove insoluble particles. Finally, the samples were filtered through a 0.45 μm PVDF syringe filter.
Degree of hydrolysis (%DH)
The degree of hydrolysis (%DH) was based on the TNBS (2,4,6-Trinitrobenzenesulfonic acid) method [13]. For this test, 400 µl hydrolysate, 400 µl SDS solution (% 0.44) and 190 µl 0.2 M sodium phosphate buffer (pH 8.2) was mixed. Then, 300 µl of TNBS (0.1%) solution was added to the mixture. The reaction was continued in a water bath at 50 °C for 1 h. Afterwards, the reaction was stopped by adding 1 ml 0.1 M HCl. Sample absorbance was measured at 340 nm. In this assay, L-leucine (0–2 mM) was used as the reference reagent.
Manufacture of confectionery products
The formulation of the pectin-based soft confections included crystalline sugar, apple pectin (high methoxyl pectin, HMP, with a %DE (degree of esterification) value of 58–64%), glucose syrup with dextrose equivalency of 38–42%, and 50% citric acid solution. The concentration of liquid hydrolysates varied between 0 and 30% (w/w%) in the trials. Table sugar, pectin, glucose syrup, hydrolysate samples and citric acid were mixed, where acid addition was carried dropwise using a conventional stove and the ingredients were cooked on low heat until they reached 105 °C. The mixture was then poured into silicone cups, left at room temperature for 24 h, and then removed from the molds (dimensions: 2.3 × 2.3 and 1.3 cm) covered with granulated crystalline table sugar to limit stickiness.
Texture profile analysis (TPA)
Instrumental analysis of sample texture was carried out at TÜBİTAK-MAM (Kocaeli, Türkiye) using the institutional protocols. Samples removed from the molds were used to generate at least 10 different sub-samples from each batch. Using Stable Micro Systems TA.XT plus texture analyzer supplied with a P/100 probe and a 100 mm compression plate setup, the samples were analyzed for hardness, springiness, cohesiveness, gumminess, chewiness and resilience parameters along with appropriate controls.
Sensory analysis
Sensory analysis was carried out by 9 trained panelists at SELUZ. The panelists analyzed the samples according to color and odor characteristics subjectively, whereas taste characteristics were graded on a scale of 0–5. Panels were held between 10:30 and 11:30 a.m. The panel room was a neutrally furnished environment away from all kinds of smells and sounds. Before the panels, a 1-week preliminary study was conducted with the panelists on general confectionery flavors. Within the scope of the preliminary study, sensory properties of various commercial confections purchased from the local markets were examined.
Simulated in vitro digestion tests
A dissolution device (PharmaTest PTWS 310 Dissolution Tester, Hainburg, Germany) was used to conduct simulated gastrointestinal digestion studies. Confections were placed in basket-shaped apparatus in the vessel. The system was used in accordance with USP-2 regulations and proteolytic protocols of Rivero-Pino et al. [14]. Shortly, 10 g sample was mixed with 150 ml of simulated gastric juice and 4% pepsin was added to this mixture and the contents were kept stirred (100 rpm) for 1 h at 37 °C. Afterwards, 4% pancreatin and 1 M NaOH were added to the medium in order to adjust the pH to 7.5. This step was continued for 2 h, and the aliquots were placed in a water bath set to 90 °C for 5 min to inactivate the enzymes.
Total phenolic content (TPC) and antioxidative activity
In TPC analysis, 0.1 g sample, 750 µl Folin-Ciocalteu reagent and 750 µl sodium carbonate (6%) were mixed and incubated for 90 min. Afterwards, sample absorbance was measured at 765 nm [15]. In DPPH (2,2-Diphenyl-1-picrylhydrazyl)-inhibitory activity tests, 2 ml 0.1 mM DPPH prepared in methanol was mixed with 0.1 g sample and the mixture was incubated for 30 min. Immediately afterwards, the samples were subjected to absorbance measurements at 517 nm. Methanol (100 µl) was added as the blank sample [16]. In cupric ion reducing antioxidant activity (CUPRAC) tests, 100 µl sample, 1 mL CuCl2, 1 mL neocuproine, 1 mL ammonium acetate and 1 mL distilled water were mixed and incubated at ambient temperature for 30 min. Sample absorbance was measured at 450 nm [17].
DPP-IV (Dipeptidyl peptidase) inhibitory activity test
Fifty µl sample was mixed with 50 µl Gly-Pro-pNA substrate (0.8 mmol.L− 1) and kept pre-incubated for 10 min at 37 °C using a shaker incubator. Immediately afterwards, 100 µl DPP-IV (0.01 U. mL− 1) was added to the mixture to initiate the reaction, and incubation was continued for 1 h. Afterwards, the reaction was stopped with 200 µl sodium acetate buffer (pH 4.0). Diprotin A (Ile-Pro-Ile, CAS 90614-48-5) was used as the reference inhibitor [12].
Results and discussion
Degree of hydrolysis (%DH)
The degree of hydrolysis (%DH) is defined as the total percentage of peptide bonds that has been cleaved during proteolysis. While partial hydrolysis has been shown to improve the foaming and emulsifying properties of proteins, higher DH values may increase the solubility of food proteins or peptides [18]. The degree of hydrolysis values for HBH, SBH and STH samples analyzed using the TNBS method was approx. 11, 13 and 15%, respectively. DH value for STH was higher than hydrolysates obtained using bromelain. Martinez et al. [19] found that DH values of sunflower protein hydrolysates were in the range of 1.5–9.5%. The reason for the higher DH values obtained here may be due to the variety of seeds or amount of proteases used, as well as the differences in proteolytic protocols.
Manufacture of the confectionery products
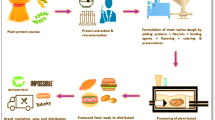
First of all, how the confections held their form and how neatly they were removed from the molds were visually examined, and the formulation was updated accordingly to generate samples that could be intact and sellable in a commercial setting (Fig. 1). As a result of the preliminary experiments, the amount of liquid hydrolysate added to the confections was determined to be 27% (w/w) to ensure intact structure and enhanced bioactivity, whereas HTH samples did not meet the expectations (data not shown for brevity). pH value is a critical factor in structuring pectin-based confections and citric acid were utilized in order to control gelation. Consequently, pectin and citric acid concentrations were adjusted to maintain the sample geometry and integrity.
Textural analysis
In order to investigate the influence of hydrolysate addition, confections were analyzed for textural characteristics (Table 1). In all cases, hydrolysate addition increased the hardness, gumminess and chewiness score of the confections, while trypsin treated sunflower protein hydrolysates (STH) increased these parameters to a lesser extent. For STH samples, the changes in springiness, cohesiveness and resilience were relatively small, even when significant. Firstly, it is anticipated that a higher protein or peptide content may improve structural bonding [20], which in turn could lead to increased hardness. Although the hydrolysate concentrations in the confections were identical, the changes in protein composition (i.e., sunflower vs. hazelnut) and DH values (i.e., SBH vs. STH) affected the textural characteristics. As detailed earlier, since DH values were higher for STH, the average molecular weight of thus formed peptides may be anticipated to be lower. Therefore, the presence of smaller peptides lowered the hardness values. Consequently, the increased DH values could serve 2 specific purposes in functional confections: the hardness is less pronounced and secondly, smaller peptides are more likely to be characterized with various bioactivities [11], which might further improve the bioactive attributes.
Springiness measures how quickly a sample that has been distorted returns to its original shape when the deforming force has been removed. The springiness values for confections were previously determined to vary between 0.9 and 1.5 [21], and the findings from this study fall slightly below this range (Table 1). The cohesiveness is related to the degree of deformation of the food under mechanical influence. The cohesiveness and flexibility of products are affected by the elastic network structure developed by proteins in food formulations [22]. According to Mutlu et al. [23], the cohesiveness of jelly candies ranged between 0.54 and 0.82, which was similar to the current results. Furthermore, gumminess and chewiness were stated to increase with hardness. In general, fortification with protein hydrolysates generated harder, more flexible, and chewable structure, where the extent of which could be affected by DH values and peptide profile.
Sensory analysis
To complement textural data, hydrolysate bearing confections were analyzed for sensory attributes by a professional team of panelists. In the current studies, no extra efforts were made to enhance color or flavor characteristics of the confections per se and it was thought that negatively perceived colors or odors could be readily masked in industrial production. In the evaluation of color performance, the controls were the most preferred sample by the panelists (data not shown). In order of preference in color evaluation control, HBH, SBH and STH samples were preferred, respectively, where the sample with the most negative color perception was STH. This could be due to the greenish colors imparted by sunflower ingredients [24].
In the evaluation of odors, HBH sample was evaluated negatively by all the panelists. In the STH sample, on the other hand, burnt, caramel and sugary odors were dominant. While the control sample was evaluated as the most neutral sample in terms of odor, the second most acceptable sample in terms of odor was SBH. Consequently, differing peptide profiles in the samples could lead to varying characteristics such as surface hydrophobicity, increasing alkalinity or acidity, which in turn could affect the perception of odors. In addition, the likelihood of Maillard reaction to generate burnt and caramel flavors increases with DH. The most balanced sample in terms of overall flavor balance was the control sample, followed by SBH, HBH and STH samples, respectively (Table 2). The highest aftertaste and off-taste perception was perceived in the STH sample, which was the sample with the weakest overall taste balance as graded by all panelists.
Consequently, based on the findings, while objective hardness determined by TPA was relatively favorable for STH, some of its sensory attributes were relatively unacceptable. This finding may be due to the interactions of peptides present in hydrolysates with other ingredients including pectin and water, which could also point to differences in the macro- and microstructure of the confections. As proteinaceous materials are added to the confectionery matrices, competition for water becomes a critical issue. Peptide contents of hydrolysate bearing confections affect sensory, textural and potentially bioactive attributes, which remains to be investigated in the following sections.
Antioxidative activity tests
In the previous literature, hazelnut and sunflower protein hydrolysates were found to demonstrate antioxidative activity (for example, Göksu et al. [11]). Consequently, here, TPC (total phenolic content), DPPH (2,2-diphenyl-1-picrylhydrazyl), and CUPRAC (copper (II) reducing antioxidant capacity) assays were performed in order to determine to what extent these hydrolysates preserved their antioxidative content and/or activity during processing or simulated digestion. CUPRAC, DPPH and TPC values of the hydrolysate bearing confections and their respective post-digestion products are given in Table 3.
Post-digestion antioxidative activity decreased for each fortified confection in antioxidative tests. CUPRAC, DPPH, and TPC values of the controls were lower than each of the post-digestion samples. As anticipated, however, antioxidative activity was observed in the fortified confections. In addition to peptides, the antioxidant property of the hydrolysates could potentially be due to the phenolic compounds remaining in the cold press cakes and protein isolates used as raw materials in confections. For example, sunflower seeds are known to be rich in antioxidants including phenolic compounds and have been reported to contain 4.2 g of total phenolic components and about 3 g of chlorogenic acid in the kernel [24]. In the case of hazelnuts, protein-phenolic interactions were observed to yield complexes between the two moeities [8]. As detailed in the earlier sections, the color of confections could also be affected by phenolic compounds.
Total phenolic content and antioxidative activity values of confections bearing trypsin hydrolysates were significantly higher than the bromelain counterparts. In addition, samples bearing hydrolysates with higher %DH were found to generate higher antioxidative activities, which once again pointed towards higher bioactivity of smaller peptides. Previously bromelain hydrolysates were observed to demonstrate the lowest DPPH inhibitory activities among a number of proteases [25], which could be attributed to peptide characteristics.
Dipeptidyl peptidase-IV (DPP-IV) inhibitory activity test
DPP-IV inhibitory activity of hydrolysates and digested confectionery was investigated and the results were summarized on Table 4. DPP-IV inhibitory activities of HBH and SBH were measured as approx. 20.5 and 8.1%, respectively. STH did not demonstrate significant DPP-IV inhibitory activity. Meanwhile, DPP-IV inhibitory activities of post-digestion samples D-STH, D-SBH and D-HBH were measured as approx. 40.6, 42.8, and 41.2%, respectively.
When the results of hydrolysates and post-digestion products were compared, DPP-IV inhibitory (i.e., in vitro anti-diabetic) activities were observed to increase after simulated digestion. In the studies of our team with hazelnut protein hydrolysates, DPP-IV inhibitory activity was found to increase as a result of simulated digestion in functional hazelnut cocoa cream [26]. Martini et al. [27] also demonstrated that DPP-IV inhibitory activities of various animal protein hydrolysates increased after simulated digestion. The authors attributed the increase in activity to the formation of small peptides that were mostly < 500 Da. In addition, more bioactive peptides emerged as a result of multiple protease treatments during simulated digestion, which in turn could enhance DPP-IV inhibitory activities of the digesta [28]. DPP-IV inhibitory activities of various dietary proteins, including milk, fish, hemp, bean, and cricket proteins, also increased after in vitro digestion [29]. This may be due in part to the known preference of pepsin for hydrophobic and aromatic amino acids such as leucine, phenylalanine, tryptophan and tyrosine located at the C-terminus of its substrates, which provides the emergence of many potent DPP-IV inhibitory peptide chains that contain aromatic amino acids (especially tryptophan) and/or a polar group at their N-terminals.
Conclusion
In this study, the influence of production and digestion processes was examined on the stability of bioactive peptides in soft confections. Hazelnut and sunflower seed protein hydrolysates imparted varying degrees of antidiabetic and antioxidative activity to the confections and their corresponding digesta. While in vitro antidiabetic effect was enhanced by the digestion protocols, there was a reduction in the antioxidative capacity. The textural and sensory attributes were significantly affected by the peptide concentration, size and proteases used. While product characteristics require optimization for enhanced acceptability, confections represent a suitable platform to stabilize bioactive peptides both as an enjoyable food products and an alternative delivery tool for food supplements.
Data availability
Data will be presented upon request.
Abbreviations
- AE-IP:
-
Alkali Extraction-Isoelectric Precipitation
- BH:
-
Bromelain Hydrolysates
- CUPRAC:
-
Cupric Ion Reducing Antioxidant Activity
- DE:
-
Degree of Esterification
- DH:
-
Degree of Hydrolysis
- DPPH:
-
2,2-Diphenyl-1-Picrylhydrazyl
- DPP-IV:
-
Dipeptidyl Peptidase-IV
- HMP:
-
High Methoxyl Pectin
- TH:
-
Trypsin Hydrolysates
- TNBS:
-
2,4,6-Trinitrobenzenesulfonic Acid
- TPC:
-
Total Phenolic Content
- WHC:
-
Water Holding Capacity
References
N. Sumonsiri, P. Phalaithong, A. Mukprasirt, R. Jumnongpon, R, E3S Web of Conferences (pp. 1–8), EDP Sciences (2021)
E. Teixeira-Lemos, A. Almeida, B. Vouga, C. Morais, I. Correia, P. Pereira, R.P. Guiné, Open. Agric. 6(1), 466–478 (2021)
M.A. Abd El Latif, H. El Aziz, A. El Deen, Int. J. Fam Stud. 3, 40–63 (2020)
L. Mojica, E.G. De Mejia, M. Menjivar, M.Á. Granados-Silvestre, FASEB J. 30, 125–126 (2016)
R. Wang, R.W. Hartel, Food Hydrocoll. 124, 107132 (2022)
Ö. Coşkun, B. Çakır, B. Vahapoğlu, İ. Gülseren, J. Food Meas. Charact. 13(3), 2328–2338 (2019)
A.G. Wouters, I. Rombouts, E. Fierens, K. Brijs, J.A. Delcour, Compr. Rev. Food Sci. Food Saf. 15(4), 786–800 (2016)
D. Ceylan, H. Yılmaz, N. Adrar, D. Günal Köroğlu, B. Gultekin Subasi, E. Capanoglu, Separations. 9(12), 406 (2022)
S.R. Wildermuth, E.E. Young, L.M. Were, Compr. Rev. Food Sci. Food Saf. 15(5), 829–843
Q. Zhang, Z. Cheng, Y. Wang, L. Fu, Crit. Rev. Food Sci. Nutr. 61(21), 3589–3615 (2021)
A.G. Göksu, B. Çakır, İ. Gülseren, Food Res. Int. 161, 111865 (2022)
A.F. Çağlar, B. Çakır, İ. Gülseren, Eur. Food Res. Technol. 247, 1189–1198 (2021)
J. Adler-Nissen, J. Agric. Food Chem. 27, 1256–1262 (1979)
F. Rivero-Pino, F.J. Espejo-Carpio, E.M. Guadix, Food Res. Int. 137, 109572 (2020)
V.L. Singleton, J.A. Rossi, Am. Soc. Enol. Vitic. 16, 144–158 (1965)
A. Kumaran, R.J. Karunakaran, Pharm. Biol. 44(2), 146–151 (2006)
R. Apak, K. Güçlü, M. Özyürek, S.E. Karademir, J. Agric. Food Chem. 52, 7970–7981 (2004)
D. Spellman, E. McEvoy, G. O’cuinn, R.J. FitzGerald, Int. Dairy. J. 13(6), 447–453 (2003)
K.D. Martinez, R.I. Baeza, F. Millan, A.M.R. Pilosof, Food Hydrocoll. 19, 361–369 (2005)
M. Shafiur Rahman, A.I. Al-Mahrouqi, Int. J. Food Sci. Nutr. 60(sup7), 229–242 (2009)
H.A. Khouryieh, F.M. Aramouni, T.J. Herald, J. Food Qual. 28(2), 179–190 (2005)
M.M. Moore, T.J. Schober, P. Dockery, E.K. Arendt, Cereal Chem. 81(5), 567–575 (2004)
C. Mutlu, S.A. Tontul, M. Erbaş, LWT. 93, 499–505 (2018)
G.M. Weisz, D.R. Kammerer, R. Carle, Food Chem. 115(2), 758–765 (2009)
Z. Zheng, J. Li, J. Li, H. Sun, Y. Liu, Food Hydrocoll. 97, 105222 (2019)
A.G. Göksu, B. Çakır, İ. Gülseren, J. Food Sci. Technol. 60(1), 171–180 (2022)
S. Martini, A. Conte, D. Tagliazucchi, J. Proteom. 208, 103500 (2019)
D. Tagliazucchi, S. Martini, S. Shamsia, A. Helal, A. Conte, Int. Dairy. J. 81, 19–27 (2018)
I.M.E. Lacroix, E.C.Y. Li-Chan, Trends Food Sci. Technol. 54, 1–16 (2016)
Acknowledgements
Hazelnut press cakes were kindly donated by Neva Foods. The original confection was developed by Ms. Özlem Kısa (İZÜ), while a revised version was utilized for hydrolysate bearing confections. This study was partially funded by grants from İZÜ Scientific Research Program, İZÜ-BAP-1000-81 and TAGEM R&D Support Program, TAGEM-21/AR-GE/23.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Güneş, Z.S., Şişman, S., Özarda, Ö. et al. Bioactive, textural and sensory attributes of soft confections enriched with plant protein hydrolysates. Food Measure (2024). https://doi.org/10.1007/s11694-024-02585-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11694-024-02585-9